Abstract
Glypican-3 (GPC3), a member of heparan sulfate proteoglycans, attaches to the cell membrane and is frequently observed to be elevated in hepatocellular carcinoma (HCC). However, GPC3 is not detected in normal liver tissues and benign liver lesions. Consequently, GPC3 is currently being used as a diagnostic biomarker and HCC-specific positron emission computed tomography probe to identify HCCs in normal liver tissues and benign liver lesions. The overexpression of GPC-3 in serum or liver tissue also predicts poor prognosis for HCC patients. In addition, GPC3 promotes HCC growth and metastasis by activating the canonical Wnt and other signaling pathways. Targeting of GPC3, including GC33, HN3 and YP7, might offer new immunotherapeutic tools for HCC treatment.
Keywords: glypican-3, hepatocellular carcinoma, diagnostics, prognosis, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the third leading cause of cancer-related death in the world.1 Although a great progress has been made in HCC treatment, including curative surgery and nonsurgical treatment, the HCC prognosis remains poor.1 The result of treatment depends on the HCC stage at the time of diagnosis. HCC can be cured if diagnosed at an early stage. However, most HCCs are diagnosed at an advanced stage when patients visit physicians with symptoms.
Glypican-3 (GPC3), a member of the heparan sulfate (HS) proteoglycan family, is attached to the cell surface by a glycosyl-phosphatidylinositol (GPI) anchor and can be cleaved off the cell surface. The soluble GPC3 can be detected in serum (sGPC3).2,3 GPC3 is widely expressed in human embryos and plays a significant role in morphogenesis and growth, by mechanisms involving insulin-like growth factor, bone morphogenetic protein (BMP), fibroblast growth factor (FGF) or hedgehog (Hh) signaling pathway.4–6 GPC3 can be detected in the fetal liver from embryonic weeks 18 to 30, but cannot be identified in any normal adult hepatic tissue.4–7 In recent years, extensive research has been carried out on the role of GPC3 in diagnosis, progression and treatment of HCC in vivo and in vitro.8,9 Here, we summarize current evidences for the use of GPC3 as a diagnostic biomarker, its oncogenic function and as a immunotherapeutic target for HCC patients.
GPC3 as a biomarker for diagnosis and prognosis of HCC
In 1997, Hsu et al7 first reported that mRNA and protein levels of GPC3 were upregulated to a greater extent in most HCCs than in normal liver, cholangiocarcinoma and metastatic carcinomas of the liver. Since then, the diagnostic value of GPC3 in HCC has been studied extensively, and increasing studies have confirmed that GPC3 would be a useful serological and immunohistochemical biomarker for HCC. By immunolabeling GPC3 with a monoclonal antibody, Capurro et al8 revealed that 72% of HCCs were GPC3-positive; however, GPC3 was undetectable in normal liver tissues, cirrhosis or benign lesions. Other studies conducted on GPC3 across the world also revealed similar results.9–13 Besides, the membrane-bound GPC3 can also be cleaved off by lipase from the GPI anchor.14 Thus, the diagnostic value of sGPC3 was evaluated later by several studies. Our study also verified that GPC3 was a sensitive and specific biomarker for diagnosis of early HCC due to its high expression in HCC tissue.9 Moreover, we found that sGPC3 was detectable in 48.8% of patients with negative serum α-fetoprotein (AFP), which further confirmed that GPC3 might be a serum marker for HCC and indicated that GPC3 could be used to distinguish AFP-negative HCC from cirrhotic nodules.11 Qiao et al analyzed the serum levels of GPC3, AFP and human cervical cancer oncogene (HCCR) in 189 cases (101 HCC, 40 cirrhosis, 18 hepatitis and 30 control healthy donors).10 They concluded that GPC3 was the best sensitive biomarker of the three biomarkers mentioned earlier, and the combination of these three biomarkers, with a sensitivity of 80.2%, was much higher than AFP alone.11 In recent years, in vivo and in vitro studies displayed the use of Zr-α GPC3, a HCC-specific positron emission computed tomography (PET) probe, in HCC imaging as well as the detection of GPC3 levels by small-animal PET. Those results suggested that the identification of small liver lesions with a HCC-specific PET probe would help physicians to make the differential diagnosis between HCCs and benign lesions in cirrhotic patients so that patient management could be significantly altered.13,14 In addition, extensive studies to determine the prognostic value of GPC3 were carried out in HCC patients.15–19 Increasing evidence revealed that high GPC3 expression was a prominent prognostic factor that predicted a poor outcome for HCCs. Clinicopathological studies on GPC3-Immunohistochemistry stainings revealed that high GPC3 expression was associated with poor postoperative disease-free survival (DFS) and overall survival (OS) and that it also served as an independent risk factor.15–17 Patients with overexpressed GPC3 in HCC tissues showed notably shorter OS and DFS than those with underexpressed GPC3. Several meta-analytic studies also supported the fact that a high GPC3-IHC score was one of the prognostic factors in HCC, as there was a significantly negative correlation between GPC3 expression and OS or DFS of HCC patients.18,19
GPC3-mediated signaling pathway in HCC progression
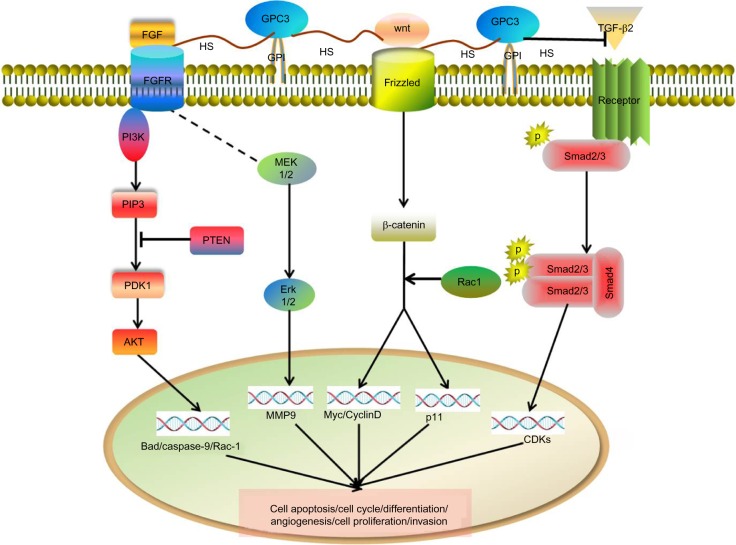
In addition to being a reliable indicator for the diagnosis and prognosis of HCC, GPC3 has a significant role in the progression of HCC (Figure 1). GPC3 functions as a coreceptor/storage site for some ligands, e.g. Wnt and FGF, via its HS side chains, and facilitates ligand and/or its receptors to stimulate the signaling pathways involved in HCC growth and invasion. Several studies showed that GPC3 could promote the growth of hepatoma cells in vivo and in vitro.20–23 Capurro et al suggested that GPC3 promoted the growth of hepatoma cells by stimulating canonical Wnt/β-catenin signaling pathway activity in vivo and in vitro.20 Recently, this GPC3-mediated activation of Wnt signaling pathway in HCC cells has been confirmed by other reports.21,22 Li et al showed that ectopic GPC3 could increase the c-Myc expression, a typical target of the canonical Wnt signaling pathway; c-Myc can also transcriptionally activate GPC3 directly in HCC cells.21 Zittermann et al22 reported that soluble GPC3, which is cleaved off the cell membrane at the GPI anchor domain, could inhibit the in vivo growth of HCC cells by blocking the canonical Wnt signaling pathway in tumors generated by Huh6 and Huh7, and Akt and ERK signaling activation in HepG2- and Huh7-derived tumors, respectively. In other words, this activity can be observed only when GPC3 is attached to the cell membrane. On the other hand, it is possible that GPC3 promotes the growth of HCC cells by mediating other signaling pathways. For example, Sun et al proved that suppression of GPC3 inhibited cell proliferation and enhanced apoptosis via upregulation of TGF-β2 in vivo and in vitro.23 In addition, it has been demonstrated that several FGF members (FGF8, FGF17 and FGF18) were upregulated in the great majority of HCC samples.24
Figure 1.
The diagram of possible GPC3-mediated signaling pathway in HCC progression.
Abbreviations: GPC-3, glypican-3; HCC, hepatocellular carcinoma; P, Phosphorylation.
Metastasis is an important aspect of HCC progression, and epithelial–mesenchymal transition (EMT) is considered as the first step in the metastatic cascade.25 Recently, we have found that the expression of GPC3 in HCC tissue was upregulated during HCC progression from Barcelona Clinic Liver Cancer stage A or B to stage C. The increased expression of GPC3 in tumor tissues was closely related to the level of EMT markers, as well as to the cancer vascular invasion. HepG2 cells, expressing a higher level of GPC3, possessed stronger ability of invasion and exhibited more EMT-like changes than those of HCC cell lines that expressed lower levels of GPC3 (Hep3B and Huh7). Our studies suggested that GPC3 promoted HCC progression and metastasis by inducing EMT in tumor cells, and the ERK signaling pathway is involved in this GPC3-induced process.26 In addition, Ruan et al also reported that GPC3 promoted the metastasis of HCC in vitro and in vivo.27
GPC3 is a new therapeutic target for HCC
Based on the HCC-specific expression of GPC3 in liver, it is an emerging target for liver cancer therapy. A number of studies demonstrated that GPC3 is a potential therapeutic target for HCC.28–30 Currently, antibodies targeting GPC3, including human antibody HN3 and humanized mouse antibodies YP7 and GC33, are in different stages of preclinical or clinical development. GC33, a humanized mouse antibody recognizing a C-terminal domain of GPC3, showed notable cytotoxic activity against GPC3-positive hepatoma cells in vivo by complement-dependent cytotoxicity and/or antibody-dependent cell cytotoxicity.31–34 GC33 exhibited marked effect against metastatic or advanced HCC in a phase I trial,35 and the patients well tolerated a dose escalation of GC33 (2.5–20 mg/kg).36 Currently, more clinical trials for GC33 alone (phase II clinical trials) and GC33 combined with sorafenib, a chemodrug, are recruiting volunteers (phase I clinical trials). A randomized phase II clinical trial on GC33 was conducted in 185 patients with HCC metastasis. In this clinical trial, the dose of GC33 was set at 1,600 mg (intravenous) on days 1 and 8, and then every 2 weeks thereafter. There was no significant change in mean progression-free survival (PFS) between GC33 and placebo groups (2.6 mo vs 1.5 mo, HR = 0.97, P = 0.87).37 However, higher exposure of GC33 with FcgR3A-158V polymorphism or CD16 expression intensity may prolong PFS, and therefore, further studies to analyze the GPC3-positive HCC immuno-microenvironment are necessary.37 YP7, a new humanized mouse anti-GPC3 antibody, has high affinity and recognizes the C-terminal epitope that overlaps the GC33-binding site and displays the capacity of suppressing tumor activity in vivo. HN3, a human single-domain antibody, can also inhibit HCC cell lines and growth of xenograft tumors by binding to the GPC3 N-terminal and C-terminal domains.38 Currently, the GAO study group found that treatment with GPC3 monoclonal antibodies, such as HN3 and YP7, could suppress the growth of HepG2- and Hep3B-generated tumor xenografts.39 Treatment with HN3 showed higher antitumor activity than YP7.40 Both HN3 and YP7 exhibit antitumor activity in vitro and in vivo, but their efficacies in HCC patients need to be determined and require further clinical trials.
GPC3 cannot play a dominant role in the apoptosis of HCC cells, and the fact that the GC33 or HN3 antibody could completely eliminate HCC cells needs further trials for confirmation. In combination with chemotherapy, armed antibodies, such as antibody–drug conjugates, bispecific antibodies and chimeric antigen receptor T-cell adoptive therapy may be better potential ways for HCC therapy.39
Conclusion
GPC3 is overexpressed in most of the HCC tumors, and was also used as an indicator for HCC diagnosis and prognosis. It is used as a potential target for developing therapeutic antibodies for HCC treatment. However, the relationship between GPC3 structure and function remains unclear. In addition, in order to develop GPC3-targeted therapies in HCC treatment, the expression and regulation of GPC3 in HCC need further confirmation.
Acknowledgments
The authors would like to thank Ms Shan Shan Wang for her support in providing a summary of possible signaling networks for GPC3. This study was supported by the Capital Science and Technology Development Fund (2014-1-2181), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (ZYLX201610) and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20151602).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10(3):332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI anchored proteins from the cell surface. Biochem J. 2008;410(3):503–511. doi: 10.1042/BJ20070511. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18(12):1591–1598. doi: 10.1038/modpathol.3800436. [DOI] [PubMed] [Google Scholar]
- 5.Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell. 2008;14(5):700–711. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Liu H, Sun L, Li N, Ding H, Zheng J. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem. 2012;114(6):547–552. doi: 10.1016/j.acthis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57(22):5179–5184. [PubMed] [Google Scholar]
- 8.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125(1):89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16(35):4410–4415. doi: 10.3748/wjg.v16.i35.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao SS, Cui ZQ, Gong L, et al. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58(110–111):1718–1724. doi: 10.5754/hge11124. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Liu H, Shang HW, Li P, Li N, Ding HG. Diagnostic value of glypican-3 in alpha fetoprotein negative hepatocellular carcinoma patients. Afr Health Sci. 2013;13(3):703–709. doi: 10.4314/ahs.v13i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JW, Zuo XL, Wang S. Diagnosis accuracy of serum glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19(19):3655–3673. [PubMed] [Google Scholar]
- 13.Sham JG, Kievit FM, Grierson JR, et al. Glypican-3-targeted Zr PET imaging of hepatocellular carcinoma. J Nucl Med. 2014;55(5):799–804. doi: 10.2967/jnumed.113.132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu D, Qin Y, Wang J, et al. Novel Glypican-3-binding peptide for in vivo hepatocellular carcinoma fluorescent imaging. Bioconjug Chem. 2016;27(3):831–839. doi: 10.1021/acs.bioconjchem.6b00030. [DOI] [PubMed] [Google Scholar]
- 15.Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery. 2013;154(3):536–544. doi: 10.1016/j.surg.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Yorita K, Takahashi N, Takai H, et al. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int. 2011;31(1):120–131. doi: 10.1111/j.1478-3231.2010.02359.x. [DOI] [PubMed] [Google Scholar]
- 17.Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100(8):1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20(20):6336–6344. doi: 10.3748/wjg.v20.i20.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao WK, Qi CY, Chen D, et al. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:104. doi: 10.1186/1471-2407-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65(14):6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Jin R, Zhang X, et al. Oncogenic activation of GPC3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012;56(4):1380–1390. doi: 10.1002/hep.25891. [DOI] [PubMed] [Google Scholar]
- 22.Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126(6):1291–1301. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 23.Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011;13(8):735–747. doi: 10.1593/neo.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauglhofer C, Sagmeister S, Schrottmaier W, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53(3):854–864. doi: 10.1002/hep.24099. [DOI] [PubMed] [Google Scholar]
- 25.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelial–mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int J Oncol. 2015;46(3):1275–1285. doi: 10.3892/ijo.2015.2827. [DOI] [PubMed] [Google Scholar]
- 27.Ruan J, Liu F, Chen X, et al. Inhibition of glypican-3 expression via RNA interference influences the growth and invasive ability of the MHCC97-H human hepatocellular carcinoma cell line. Int J Mol Med. 2011;28(4):497–503. doi: 10.3892/ijmm.2011.704. [DOI] [PubMed] [Google Scholar]
- 28.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129(6):899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Chen Y, Wurmbach E, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131(6):1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhu ZW, Friess H, Wang L, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48(4):558–564. doi: 10.1136/gut.48.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47(3):333–338. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280(10):2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 33.Nakano K, Ishiguro T, Konishi H, et al. Generation of a humanized anti-glypican 3 antibody by CDR grafting and stability optimization. Anticancer Drugs. 2010;21(10):907–916. doi: 10.1097/CAD.0b013e32833f5d68. [DOI] [PubMed] [Google Scholar]
- 34.Nakano K, Orita T, Nezu J, et al. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378(2):279–284. doi: 10.1016/j.bbrc.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68(23):9832–9838. doi: 10.1158/0008-5472.CAN-08-1973. [DOI] [PubMed] [Google Scholar]
- 36.Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19(4):920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 37.Yen CJ, Daniele B, Kudo M, et al. Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma. J Clin Oncol. 2014;32(Suppl 5) abstract 4102. [Google Scholar]
- 38.Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588(2):377–382. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao W, Tang Z, Zhang YF, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 2015;11(6):6536. doi: 10.1038/ncomms7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20(24):6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]