Abstract
The ‘Executive Functions’ (EFs) of inhibitory control, working memory, and cognitive flexibility enable us to think before we act, resist temptations or impulsive reactions, stay focused, reason, problem-solve, flexibly adjust to changed demands or priorities, and see things from new and different perspectives. These skills are critical for success in all life's aspects and are sometimes more predictive than even IQ or socioeconomic status. Understandably, there is great interest in improving EFs. It's now clear they can be improved at any age through training and practice, much as physical exercise hones physical fitness. However, despite claims to the contrary, wide transfer does not seem to occur and ‘mindless’ aerobic exercise does little to improve EFs. Important questions remain: How much can EFs be improved (are benefits only superficial) and how long can benefits be sustained? What are the best methods for improving EFs? What about an approach accounts for its success? Do the answers to these differ by individual characteristics such as age or gender? Since stress, sadness, loneliness, or poor health impair EFs, and the reverse enhances EFs, we predict that besides directly train EFs, the most successful approaches for improving EFs will also address emotional, social, and physical needs.
Keywords: Working memory, Prefrontal cortex, Aerobic exercise, Cognitive training, Stress, Loneliness
There has been great interest in improving executive functions (EFs), accelerating their development, stopping or slowing their decline, and/or remediating deficits. Many different methods have been tried including diverse types of computerized cognitive training (especially working memory training), diverse physical activities (such as aerobic exercise, resistance training, coordinative exercise, yoga, and martial arts) as well as other things such as certain school curricula (including Montessori, Tools of the Mind, Chicago School Readiness Program, and PATHS). Before discussing the pros and cons of different methods that attempt to improve EFs, it would be helpful to briefly explain what is meant by the term, EFs.
1. Executive functions explained
Executive functions (EFs) consist of a family of three, interrelated core skills (inhibitory control, working memory, and cognitive flexibility; Miyake et al., 2000, Diamond, 2013). From those, higher-order EFs are built such as reasoning, problem-solving, and planning (Collins and Koechlin, 2012, Lunt et al., 2012). Inhibitory control involves resisting one's initial impulse or a strong pull to do one thing, and instead act more wisely. Without inhibitory control we would be at the mercy of external stimuli, internal impulses, and habits of thought or action that pull us this way or that. Inhibitory control thus makes it possible for us to choose how we react and to change how we behave rather than being “unthinking” creatures of habit or impulse (Diamond, 2013). It is critical for avoiding social faux pas and for a civil society where people abide by rules and norms. It is difficult to think of any aspect of life where having the presence of mind to wait before speaking or acting, giving a considered response rather than an impulsive one, being able to stay focused despite distraction, and resisting temptations to do inappropriate, ill-advised, self-destructive or illegal things would not be beneficial.
Working memory (WM) involves more than holding information in mind. It involves doing that while performing one or more mental operations. It is needed, for example, for re-ordering the items you are holding in mind or seeing how they relate to one another (‘working with’ the information you are holding in mind; Baddeley and Hitch, 1994, Smith and Jonides, 1999) and also for remembering your question or comment while following an ongoing discussion or for holding in mind what you were about to do when something arises that must be dealt with first (D’Esposito and Postle, 2015). WM is critical for reasoning and problem-solving for they require holding lots of information in mind, exploring their interrelations, and then perhaps dis-assembling those combinations and re-combining the elements in new ways. WM is necessary for making sense of anything that unfolds over time for that always requires holding in mind what happened earlier and relating that to what is happening now (e.g., following a lecture or conversation, relating what you are reading now to what you read earlier, or understanding the relation between a later effect and an earlier cause).
Cognitive flexibility refers to the ability to flexibly adjust to changed demands or priorities, to look at the same thing in different ways or from different perspectives (as required for set shifting or task switching; Allport and Wylie, 2000, Kiesel et al., 2010, Monsell, 2003, Vandierendonck et al., 2010). If one way of solving a problem isn’t working, one needs cognitive flexibility to “think outside the box,” that is, to find other ways of conceiving of the problem or of attacking it. Such flexibility is needed for meeting novel, unanticipated challenges and for seizing opportunities when they unexpectedly arise.
EFs are predictive of achievement, health, wealth, and quality of life throughout life, often more so than IQ or socioeconomic status (SES; e.g., Moffitt et al., 2011, Moffitt, 2012). They are more critical for school readiness than IQ or entry-level reading or math (Alloway et al., 2005, Blair, 2002, Blair and Razza, 2007, Carlson and Moses, 2001, Hughes and Ensor, 2008, Morrison et al., 2010). They are predictive of success throughout the school years from preschool through university (often more so than IQ [Duckworth and Seligman, 2005, Alloway and Alloway, 2010, Borella et al., 2010, Duncan et al., 2007, Fiebach et al., 2007, Gathercole et al., 2004, Loosli et al., 2012, McClelland et al., 2007, Nicholson, 2007, Savage et al., 2006]).
The importance of strong EFs does not stop in childhood. There is abundant evidence that EFs are crucial for success in getting and keeping a job as well as career advancement (Bailey, 2007, Leslie, 1995), making and keeping friends (Hughes and Dunn, 1998), marital harmony (Eakin et al., 2004), weight control (Crescioni et al., 2011), staying out of jail (Moffitt et al., 2011), and resisting substance abuse (Miller et al., 2011). Adults with better EFs also report they are happier and have a better quality of life (Moffitt, 2012).
2. Interventions, programs, and approaches for improving EFs
Only reports on the effectiveness of a method for improving EFs that met certain criteria are discussed in this paper: (a) The report had to be published in English in a peer-reviewed journal. (b) A study could not be solely correlational because causality cannot be determined from correlations. (Too often correlational studies [that find, e.g., students who exercise or play in the orchestra have better EFs than students who do not] are discussed as if they show causality [e.g., that exercise or playing in the orchestra helps produce better EFs]. But, of course, it could be that students with better EFs are more likely to choose to be in the orchestra or are wise enough to exercise more.) (c) The study must not have examined acute effects only (immediate effects of doing an activity only once) because improvements observed there are probably transient, lasting no more than 1–2 h. We do not know what to conclude from that about what sustained benefits one would find from doing the activity repeatedly. (d) The study had to look at benefits other than only improvements on the task practiced during the intervention. There's lots of evidence that if you practice a task or procedure you get better at that. We were interested in whether there was improvement in a basic cognitive ability that generalized at least to similar tasks.
(e) A comparison group had to be included. (To conclude that what individuals did between Times 1 and 2 produced the improvement at Time 2, there needs to be evidence that without that activity there is less improvement at Time 2, even in those who were also tested at both timepoints. Without that there is no way to tell if improvements might have happened anyway from just having taken the assessment measures before (practice effects) or just from normal developmental improvement in the abilities tested].) Including any comparison group is a very minimal requirement because an activity may produce better outcomes than no treatment (business as usual) simply because the activity is something new and different. Therefore a comparison group that engages in another new activity (an active control group) is far better than a no-treatment control group. While any new activity addresses what is known as the Hawthorne effect (McCarney et al., 2007), if participants in the condition of interest expect significant cognitive benefits, but those in the active control group do not, there is still a problem because expectations play a major role in outcomes (Orpen, 1974, Steele and Aronson, 1998). Ideally, those in the active control group should have the same expectations for improvement as those in the condition of interest (Boot et al., 2013, Stothart et al., 2014). To address this, researchers should try to assess the expectations for improvement of participants in, and providers of, the control and experimental conditions, whether and how much participants enjoyed them, and how challenging they found them, as CogMed researchers have started to try to do (Spencer-Smith and Klingberg, 2015). It would be good to know how committed to, or invested in, the program participants were and whether they would like to continue with it after the study ends.
Studies that show improvements from Time 1 to Time 2 among training-group participants but comparable improvements among control-group participants are also fundamentally unconvincing because if there are no significant group differences in the improvements found, one cannot conclude that the training played any role in producing the improvements. This is a danger, for example, when both groups studied receive the same treatment, with one group just receiving a larger dose. If both groups show comparable benefits, even if that treatment might be the best intervention in the world, we cannot conclude it has produced any benefit at all (Diamond, 2014a). One needs to have a group that does not show comparable benefits (i.e., one needs differential improvement [a group × change interaction]).
In all, we found 84 studies that met our criteria. Many different activities (including computerized training, games, aerobics, resistance training, martial arts, yoga, mindfulness, theater, and certain school curricula) have at least one peer-reviewed published report on their efficacy in improving executive functions. Broad reviews include: Bryck and Fisher (2012), Burke (2010), Diamond and Lee (2011), Diamond and Ling (accepted), Greenberg and Harris (2012), Howard-Jones (2014), Muraven (2010), Rabipour and Raz (2012), Riccio and Gomes (2013), and Sedlmeier et al. (2012). Reviews of cognitive training methods that looked at benefits for EFs include: Au et al. (2015), Klingberg (2010), Kueider et al. (2012), Melby-Lervåg and Hulme (2013), Morrison and Chein (2011), Shipstead et al. (2012), Spencer-Smith and Klingberg (2015), Spierer et al. (2013), and von Bastian and Oberauer (2013). Reviews of physical activity programs that examined benefits for EFs include: Angevaren et al. (2008), Audiffren and André (2015), Barenberg et al. (2011), Best (2010), Chaddock et al. (2011), Etnier et al. (2006), Etnier et al. (1997), Fedewa and Ahn (2011), Hillman et al. (2008), Hindin and Zelinski (2012), Kramer and Erickson (2007), Moreau and Conway (2013), Smith et al. (2010), Snowden et al. (2011), Streiner (2009), Tomporowski et al. (2015), Tseng et al. (2011), van Uffelen et al. (2008), and Voss et al. (2011). From the many studies looking at the benefits of an activity, program, or intervention for EFs, some general conclusions and principles have emerged.
3. Conclusions that emerge from the various studies of different methods of trying to improve EFs
1. EF training appears to transfer, but the transfer appears to be narrow. For example, computerized WM training improves WM but not self-control, creativity, or flexibility (e.g., Bergman Nutley et al., 2011, Harrison et al., 2013, Thorell et al., 2009; meta-analysis: Melby-Lervåg and Hulme, 2013). We would not be surprised if WM training improved attention; we would see that as narrow transfer since attention and WM are so closely intertwined that they may not be dissociable (Awh et al., 2000, Awh and Jonides, 2001, Gazzaley and Nobre, 2012, Ikkai and Curtis, 2011, Kane et al., 2007, Nobre and Stokes, 2011, Shipstead et al., 2015). There is some evidence that WM memory reduces inattentiveness in daily living for patients (Spencer-Smith and Klingberg, 2015) but there is a lack of evidence showing that WM training improves attention. (The few studies that have looked have found (a) no benefit of WM training for attention [Richmond et al., 2011, Rueda et al., 2005, Rueda et al., 2012] or (b) improvement in attention after WM training but not better post-test scores than controls [the WM training group simply caught up; Thorell et al., 2009] or (c) better post-test scores but not more improvement in attention than controls [the WM training group started out with somewhat better attention and that difference enlarged slightly after training; Wass et al., 2011]. This deserves further study though. In most cases only one measure of attention was used and what was often assessed was sustained attention, instead of the aspect of attention most aligned with WM (i.e., selective attention).
At one extreme are those who worry that WM training may not really improve WM at all, but simply train task-specific strategies and scanning and response patterns that are helpful on very similar tasks but nothing else (Harrison et al., 2013). At the other extreme are those who read the evidence as showing that WM training can improve intelligence (that is, fluid intelligence, as assessed by the Ravens or BOMAT tests, which are designed to assess reasoning; Jaeggi et al., 2008; meta-analysis: Au et al., 2015). For example, a carefully-done meta-analysis of 20 studies (Au et al., 2015), all of which trained subjects on the N-back task (which requires storage and continual updating of information plus interference control), found small but significant improvements in fluid intelligence. However, another excellent meta-analysis (Melby-Lervåg and Hulme, 2013) found no convincing evidence of generalization of WM training to other cognitive skills (the training produced “short-term, specific training effects that do not generalize.” p. 270)1.
Our take on the evidence thus far is that wide transfer to untrained cognitive skills has not been demonstrated, whether one looks at cognitive training or physical activity training. As we will discuss below, physical exercise that requires no EF skills (e.g., running on a treadmill or riding a stationary bike) does not improve any EF skill. We have seen no study demonstrate transfer after WM training to a test of reasoning controlling for the WM components of the reasoning test. We are also heedful of the points made by Moody (2009) about problems with the way the BOMAT has been administered in some studies of WM training. For example, it makes sense that, if the time allotted for the BOMAT is reduced to 10 min from the usual 45 min, participants would not have time to proceed to the more challenging problems because the more difficult problems only come later in the test. Is how fast one can solve relatively easy visuo-spatial problems really assessing reasoning ability?
People improve on the skills they practice and that transfers to other contexts where those same skills are needed, but people only improve on what they practice; improvement does not seem to transfer to other skills. It is not even clear that training nonverbal WM transfers to verbal WM or that training nonverbal analogical-reasoning transfers to nonverbal gestalt reasoning on Raven's matrices (e.g., Bergman Nutley et al., 2011). To see widespread benefits, diverse skills must be practiced. For that reason, real world activities such as martial arts and certain school curricula (that train diverse executive-function abilities) have shown more widespread cognitive benefits than targeted computerized training (Blair and Raver, 2014, Lakes and Hoyt, 2004, Park et al., 2007, Raver et al., 2011).
Moreau and Conway (2014) make an excellent point: Often WM is trained using N-back or complex span tasks, or minor variations on another test that challenges WM. These tests are repetitive and predictable; the timing of displays, the type of stimuli, and response requirements stay the same. “Intense practice [on these] exacerbates the importance of domain-specific processes, because these tasks, when administered repeatedly, allow honing strategies or skills rather than tapping domain-general processes.” (p. 334). Following this logic, interspersing very different types of WM challenges and reducing the predictability of what will be presented and/or what will be required to generate a correct response should improve both the generalizability and longevity of the effects of cognitive training (echoing findings from the older learning literature for both cognitive skills [Bransford et al., 1979] and motor ones [Kerr and Booth, 1978]).
2. Whether EF gains are seen depends on the amount of time spent practicing. Ericsson's conclusion about the critical importance of practice (with difficulty progressively increasing) for becoming really good at anything (e.g., Ericsson, 2006, Ericsson, 2009, Ericsson et al., 2009, Ericsson and Towne, 2010) appears to apply to improving EF skills just as it does to every other skill Ericsson investigated. For computerized cognitive training, within the range of durations studied (ranging from 2 to 14 weeks), it seems clear that more weeks (longer duration) of training produces better EF outcomes, holding dose (session length) and frequency (number of sessions per week) constant (Basak et al., 2008, Diamond and Ling, accepted, Jaeggi et al., 2008). Among the three mindfulness retreats studied, the one that produced the best EF outcomes lasted the longest (MacLean et al., 2010). For physical activity interventions, we could find only one study that examined the effect of duration: Masley et al. (2009; who studied extremely short durations of 5-7 days versus 3-4 days of a comprehensive program that included exercise) found that more days produced better EF results than fewer days.
Davis et al. (2011) found better EF outcomes from higher versus lower doses of aerobic games (40 min per session versus 20). Liu-Ambrose et al., 2010, Liu-Ambrose et al., 2012 found more improvement in EFs from higher versus lower frequency of resistance training (twice a week versus once). Thus, for duration of training, dose (length of each session), and frequency (how often the sessions occur) the few studies that looked have found more time spent practicing is better. This is not true for studies using the N-back task, however. Stepankova et al. (2014) found that whether subjects trained on the N-back task 2 times a week for 20 sessions or 4 times a week for 40 sessions did not affect their post-test performance on forward or backward digit span or letter-number sequencing (as in Trails-B). Both training groups performed better at post-test than no-treatment controls. In their review of N-back training studies, Au et al. (2015) found a trend for larger benefits from shorter training sessions rather than longer ones. The shortest session length was 18.5 min. They don’t say how long the longest sessions were. Presumably there must be a point where still shorter sessions are disadvantageous and there might be a session length intermediate between 18.5 min and whatever the longer session lengths were that might be better than 18.5 min.
The developers of the preschool and kindergarten curriculum, Tools of the Mind (Tools), initially tried their program as an add-on to existing curricula, so that children did activities designed to improve EFs perhaps an hour a day. The benefits were narrow and specific to the context in which the skills had been practiced. Only when training and practicing of EFs were part and parcel of what the children did all day long in school (whether an activity was language arts or math) were marked benefits seen (Bodrova and Leong, 2007). Clements et al. (2012) replicated the limited benefits from Tools as an add-on, and Diamond et al. (2007) and Blair and Raver (2014) replicated the marked EF benefits when Tools is the all-day curriculum.
Two studies looked at intensity – in one it mattered in the other it didn’t. Chang et al. (2013) found exactly the same EF performance post-intervention whether children did moderate- or low-intensity soccer practice. Moderate-intensity was defined as children reaching 60–70% of their maximal heart rate (the average heart rate was 103.7 ± 8.33 bpm). Low-intensity was defined as children reaching 40–50% of their maximal heart rate (the average heart rate was 140.2 ± 9.53 bpm). Cassilhas et al. (2007) found high-intensity resistance training (with older adults) produced better EF outcomes than low-intensity. The two groups did not differ at post-test on Backward Digit Span or Forward Corsi Block, but the high intensity-group did better at post-test on Backward Corsi Block and Forward Digit Span both in change scores and post-test scores. The high-intensity resistance training group was given loads 80% of the maximum amount of force that that person could generate in one maximal contraction (1 repetition-maximum). The moderate-intensity resistance training group was given loads 50% of the maximum amount of force that that person could generate in one maximal contraction.
3. Whether EF gains are seen depends on the way an activity is presented and conducted (as the song says, “’Tain’t What You Do [It's The Way That You Do It]”). Trulson (1986) deliberately varied the way martial arts were taught and found benefits in one case and effects in the opposite direction in the other case. Personal characteristics of those leading a program can have major effects on how beneficial a program is; this has received little attention in the intervention literature. Whether the person leading a program is committed to it succeeding and believes firmly in its efficacy, similarly whether the local community is supportive of an intervention or has had a say in crafting the intervention, are just some of the many factors that can influence why the “same” intervention might be successful in one instance but not in another. This has certainly played a major role in why Tools of the Mind has been found to be resoundingly successful in some instances (Blair and Raver, 2014, Diamond et al., 2007) but not in others (Farran and Wilson, 2011).
4. EFs need to be continually challenged to see improvements – not just used, but challenged. This is consistent with what Ericsson found in his life's work of studying what makes an expert across many different fields. The answer was always the same – lots and lots of practice and practicing not what is easy but continually pushing to go beyond one's comfort zone or current level of competence (Ericsson et al., 2009, Ericsson and Towne, 2010). That is consistent with what Vygotsky (1986) called the “zone of proximal development,” the zone just beyond what one can do on one's own, but where with a little help from someone else one is able to succeed. Klingberg and colleagues have repeatedly shown that while WM and reasoning can be improved by computer games that keep incrementing difficulty as a person's skill improves (specifically in CogMed®), similar computer games where difficulty does not increase do not produce the same benefits (e.g., Bergman Nutley et al., 2011, Klingberg et al., 2005), though whether that is because they present less challenge, are more boring, or carry less of an expectation of cognitive gain is hotly debated.
5. Those with the poorest EFs consistently gain the most from any program that improves EFs, whether their poorer EFs are associated with ADHD (Holmes et al., 2010, Klingberg et al., 2005), lower SES (Blair and Raver, 2014), the beginnings of cognitive decline with aging (Colcombe and Kramer, 2003, Kramer and Erickson, 2007), or just normal individual variation (Flook et al., 2010, Holmes et al., 2009). Greater benefits to those farthest behind is not due to regression to the mean because control subjects who also had ADHD or mild cognitive decline, or were from similarly economically-disadvantaged families, etc. did not shown similar EF gains.
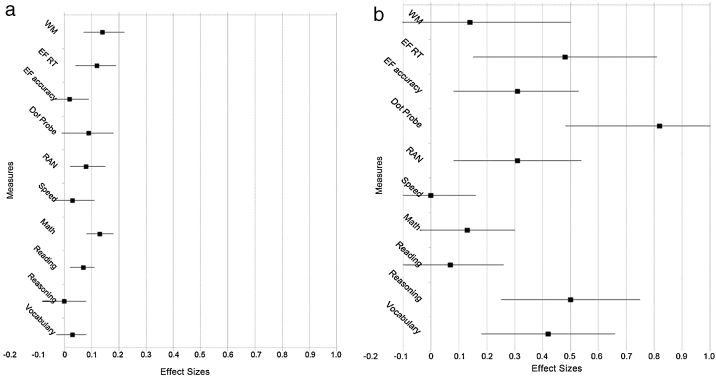
This is seen in particularly stark relief in the study of Tools of the Mind by Blair and Raver (2014). Effect sizes for children from diverse SES backgrounds rarely exceeded 0.1, but effect sizes for lower-income children were as high as 0.8! See Fig. 1. Since those who start further behind on EFs tend to progress more from any EF intervention, EF training might reduce societal disparities in academic achievement and/or health associated with social disparities in EFs (giving those behind a chance to catch-up, at least to some extent).
Fig. 1.
(a) Effect size estimates for main effects for Tools of the Mind versus the control group at the end of kindergarten in Blair and Raver (2014). (b) Effect size estimates for main effects for Tools of the Mind versus the control group at the end of kindergarten in high poverty schools in Blair and Raver (2014). Errors bars represent ± 1 standard error. Legend: RT = reaction time; RAN = Rapid automatic naming.
When it comes to extreme groups, however, such as children with very, very low IQs or adults with severe cognitive decline, cognitive training has not been shown to help them (Colcombe and Kramer, 2003, Söderqvist et al., 2012). Very likely the cognitive training was too demanding for them. They probably needed a simpler training protocol.
6. Once practice ends, benefits diminish. Studies have demonstrated that EF benefits can last for months or even years, but they almost always grow smaller as the time since training increases (Ball et al., 2002, Klingberg et al., 2005, Willis et al., 2006). It would be unrealistic to expect otherwise. If you trained in the gym weekly and were finally able to bench-press 350 lbs., if you did no exercise for 12 months, of course you would no longer be able to bench-press that much after those 12 months. Benefits diminish after practice ends.
7. Often, differences between the treatment and control groups only appear when participants’ EF skills are pushed near their limit. The largest differences between groups are consistently found on the most demanding EF tasks and task conditions (e.g., Davis et al., 2011, Diamond et al., 2007). It is in pushing the limits of participants’ EF skills that group differences emerge.
8. Aerobic exercise, or resistance training, without a cognitive component produces little or no EF benefit. More data supporting this will be cited than for other points because there have been so many and so frequent claims to the contrary (e.g., Hillman et al., 2008).
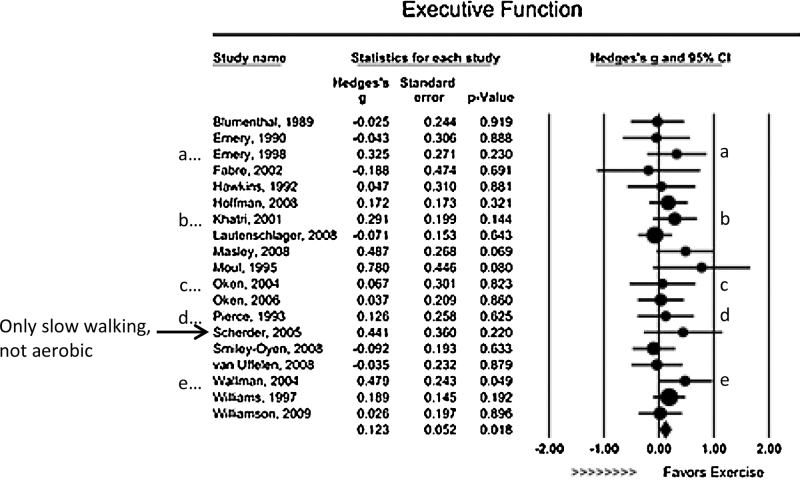
Results of studies of EF benefits from exercise without cognitive demands. Seven studies that met our inclusion criteria examined EF benefits from relatively “mindless” aerobic exercise compared to an active control group. Most were in older adults. Five of the seven studies (71%) found no EF benefit whatsoever (Blumenthal et al., 1989, Fabre et al., 2002, Krafft et al., 2014, Moul et al., 1995, Smiley-Oyen et al., 2008). Consistent with this, two meta-analyses of randomized control trials in adults (mostly older adults) found little or no EF benefits from aerobic activity (Angevaren et al., 2008 [which included 11 studies]; Smith et al., 2010 [which included 17 studies]). See Fig. 2.
Fig. 2.
Results of the meta-analysis by Smith et al. (2010), showing that aerobic exercise produces little to no benefit for EFs. (a) Subjects had chronic obstructive pulmonary disease; (b) Subjects had depression; (c) Subjects had multiple sclerosis; (d) Subjects had hypertension. (e) Subjects had chronic fatigue syndrome.
Only three studies in children that looked at EF benefits from “mindless” aerobic exercise met criteria for inclusion here. All three looked at children of roughly the same age (mean age was between 8 and 10 years across the three studies). Two of the three studies (67%) found no EF benefit at all. The only study of these three that had an active control group had children do aerobics exercise (tag, jump rope, etc.) or sedentary activities (art, board games, etc.) after school for 40 min each school-day for 8 months (Krafft et al., 2014). They found neither more improvement in EFs nor better EF post-test performance from aerobic exercise.
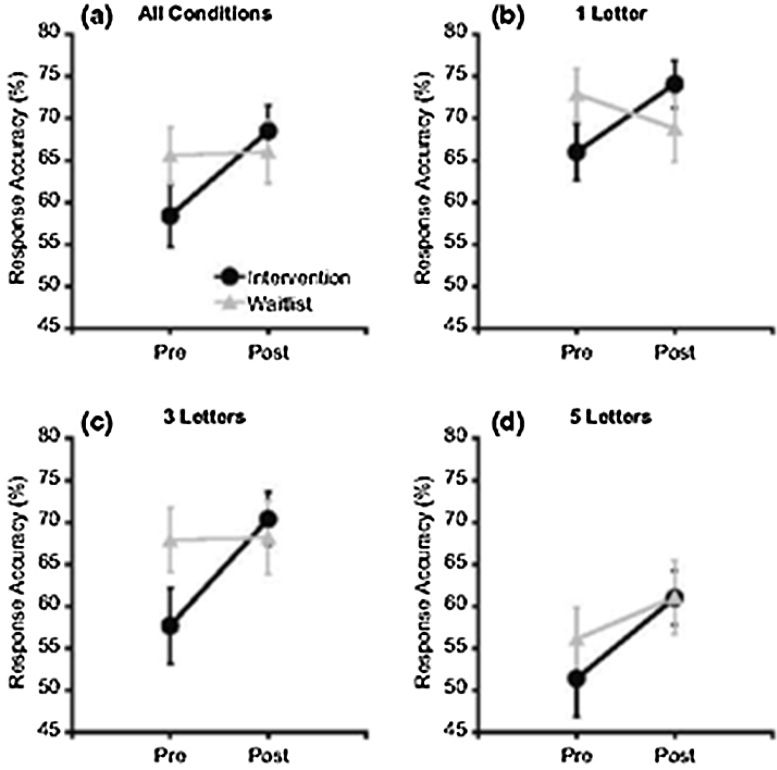
The one study that found any hint of more improvement in EFs and better post-test performance after aerobic exercise found improvement on only one of three EF measures (Tuckman and Hinkle, 1986). They compared aerobic running (which became increasingly more demanding over time by incrementing distance, interval workouts, and relay runs) to standard physical education. After 12 weeks, those who had done aerobic running showed better cognitive flexibility (better performance on an alternate uses task) than did controls. There were no group differences on non-EF tasks (visual-motor coordination or perceptual-motor skills). The third study found that controls started off with somewhat better EFs; the aerobic group simply caught up (Kamijo et al., 2011). See Fig. 3.
Fig. 3.
Results from Kamijo et al. (2011) showing Pre- and Post-Intervention Percentage of Correct Responses on an EF measure (the Sternberg test) in children who did Aerobic Activities for 70 min 5 days a week for an entire school year versus Wait-list Controls. When all conditions were combined, and in the 3- and 5-Letters condition, the control subjects started out better and the aerobic group simply caught up; there was no significant difference in post-test scores. The only significant difference in post-test scores was on the trivially easy 1-Letter condition where controls got worse, perhaps because they were bored.
Consistent with the disappointing effects of just aerobics per se on EFs is the consistent finding that improvements in aerobic fitness are uncorrelated with cognitive improvements (e.g., meta-analysis: Etnier et al., 2006; review: Kramer and Erickson, 2007; also see Blumenthal et al., 1989, Davis et al., 2011, Smiley-Oyen et al., 2008).
On the other hand, however, it is also consistently found that people who are more physically active and have better aerobic fitness have better EFs than those who are more sedentary (children: Hillman et al., 2005, Scudder et al., 2014, Sibley and Etnier, 2003; older adults: Boucard et al., 2012, Colcombe and Kramer, 2003, Voelcker-Rehage et al., 2011; all ages: Etnier et al., 2006, Prakash et al., 2015). Perhaps people need to do aerobic activity for longer (perhaps years versus months) or more times per week than in intervention studies. Perhaps people who freely choose to do aerobic activities enjoy them more than people who are randomly assigned to do them. (There's evidence that any benefit of physical activity for cognition may be proportional to how much joy the physical activity brings [Hill et al., 2010, Raichlen et al., 2012, Heyman et al., 2012, Wolf et al., 2010]. Boring exercise is particularly unlikely to yield cognitive benefits.). It may well be that many of the people who maintain better fitness do so by participating in physical activities that involve cognitive challenges and involve interacting with others (such as ultimate Frisbee, squash, tennis, rock climbing, pickup football, soccer, beach volleyball, social dance or martial arts). It may be that regular exercisers do so mindfully but those new to exercise (assigned to it in an intervention study) do not. Or, perhaps the correlation between better physical and cognitive fitness is due to one or more other variables and not to better fitness per se. Perhaps people who are more physically fit have the good sense to eat better or get more sleep, tend to be healthier in general or tend to be more highly educated. Or, perhaps causality goes in the opposite direction since one probably needs good EFs, especially good inhibitory control and discipline, to maintain a regular exercise regimen. At least the evidence so far seems to indicate that it is not the aerobic fitness itself that is causing the cognitive benefit.
Five studies meeting our criteria for inclusion examined EF benefits from resistance training; all were with older adults. For three of those studies, resistance training was the active control condition, rather than the activity of interest; in none of those studies was any EF benefit observed from resistance training. In the two studies where resistance training was the activity of interest and stretching/toning was the active control condition, a few EF benefits were found for resistance training. For example, in the Liu-Ambrose et al., 2010, Liu-Ambrose et al., 2012 study, controls started out better on the Stroop and Flanker tasks; those in the resistance-training group simply caught up.
Results of studies of EF benefits from exercise that includes cognitive challenges. Contrast the disappointing results for aerobic exercise or resistance training without cognitive components with the generally encouraging results for exercise that requires thought, planning, concentration, problem-solving, WM, and/or inhibitory control. For example, Lakes and Hoyt (2004) randomly assigned kindergarten through Grade 5 classes either to traditional Tae-Kwon-Do martial arts or standard PE. At the end of the school year, children assigned to Tae-Kwon-Do2 showed greater gains than children in standard PE on all dimensions of EFs studied (e.g., cognitive [focused versus distractible], affective [persevere versus quit], and emotion regulation). This generalized to multiple contexts and was found on multiple measures. Chang et al. (2013) claim that whether soccer practice was moderate or low intensity it improved EFs (as assessed by the Flanker task), but lacking a control group it is difficult to interpret their results. Manjunath and Telles (2001) report that 12-year-old girls randomly assigned to yoga (which included mindfulness training in addition to exercises) showed more improvement in EFs and better EF post-test scores (on the Tower of London) than peers randomly assigned to physical exercise.
There is a paucity of studies looking at the benefits of any sport for EFs. Researchers should look into this. Most sports place demands on each of the EFs. Participants need to remember complex movement sequences, mentally work with lots of information, processing in real-time cues such as people's positions and where they will likely go next (for ball sports, cues about the ball's location and trajectory), mentally compare the present situation with past ones, and use that to predict what is likely to happen next or down the line (i.e., they must use WM). Participants need to inhibit attending to distractions and keep their attention focused; they must inhibit a planned action when that is suddenly no longer a good idea and inhibit what might be their first inclination, such as the temptation to try to score oneself rather than passing (i.e., they must use inhibitory control). And, they must use cognitive flexibility: The situation is constantly changing. Participants must quickly and accurately evaluate and respond to those changes, flexibly switching plans in real time, adjusting to the unexpected, adapting to complex and rapidly changing conditions. The situation they are faced with at any moment is often different from anything they have faced before. They can never know for sure what someone else will do; at best they can only predict. Some of this can become automatized and no longer require top-down control, but (a) that is less true for people relatively new to a sport and (b) typically the difficulty of what one is facing keeps increasing. As other players or opponents get better at the sport, the inherent difficulty of what one is faced with increases, providing constant challenge. Ironically, artificial or designer sports, or components of multiple sports in combination, have been studied more than naturally existing sports:
Williams and Lord (1997) found that a regime of aerobic exercise + resistance training + eye-hand and eye-foot coordination produced EF benefits in adults with a mean age of 72 years. Davis et al. (2011), who had children do aerobic games (e.g., basketball and soccer skills, though intensity rather than skill development was emphasized), found that the groups that did 40-minute sessions (43 h total) and 20-min sessions (22 h total) did not differ afterwards in physical fitness (as assessed by treadmill endurance) but the higher-dose group showed significant EF improvements while the lower-dose group did not compared with sedentary peers.
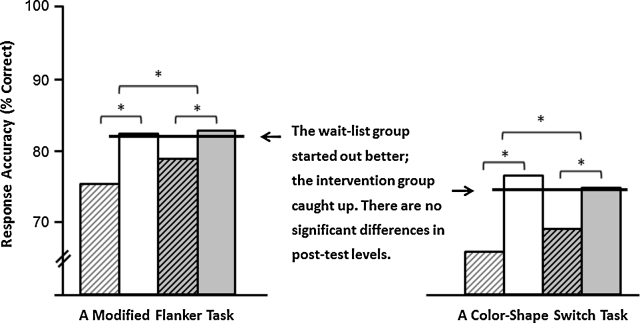
However, although the FITKids Intervention included training in motor skills in addition to aerobic activity, EFs were no better for those participated in the program than for those who were waitlisted. See Fig. 4.
Fig. 4.
Results from Hillman et al. (2014) showing no difference in post-test performance.  Intervention Group pretest score,
Intervention Group pretest score,  Intervention Group post-test score,
Intervention Group post-test score,  Wait-list Control Group pretest score,
Wait-list Control Group pretest score,  Wait-list Control Group post-test score.
Wait-list Control Group post-test score.
Two studies (Moreau et al., 2015, Oswald et al., 2006)) directly tested the benefits of “mindless” physical exercise alone, cognitive training alone, and exercise plus cognitive training. Consistent with what we have said above, neither found EF benefits from physical activity that required no top-down control, and both found significantly better EF benefits from physical activity plus EF challenges than from relatively “mindless” physical activity alone. Although Oswald et al. (2006) also found greater EF benefits from the combined training than from cognitive training alone, Moreau et al. (2015) did not. Moreau et al. found that EF benefits from the combined program were not significantly better than the ones produced by cognitive training alone; adding the physical activity component seemed to produce no additional EF benefit.
Moreau et al. (2015) randomly assigned adults to WM training, aerobic exercise, or training on physical activities with cognitive demands. The WM training was on complex span tasks. Aerobic exercise used treadmills, spinning bikes, and rowing machines. The physical activity with intentional cognitive demands was loosely based on freestyle wrestling; it involved novel motor coordination challenges and cognitive challenges requiring frequent updating, comparing the present situation to past experiences in similar situations, and focused attention and concentration. Difficulty adaptively increased in all three programs over the 8 weeks (24 sessions) the programs were offered. Aerobic exercise alone produced no cognitive gains. WM improved comparably after WM training and after the modified wrestling program that had aerobic, motor skill, and cognitive components.
Oswald et al. (2006) had older adults take part in physical training, cognitive training, combined cognitive and physical training, psychoeducational training, combined psychoeducational and physical training, or no training. Physical training concentrated on balance, eye-hand coordination, motor coordination, and flexibility. It included movements from gymnastics, dance, and yoga and included playing tennis and table tennis. The cognitive training included training and practice on visual search tasks, a maze task and a word-color task (with an emphasis on speed), and lots of memory tasks (e.g., remembering phone numbers, shopping lists, and names) where memory strategies were taught. The psychoeducational intervention involved lectures, group discussions, exercises, and role plays on everyday problems (e.g., avoiding falls, dealing with the death of a loved one, nutrition, and understanding prescription labels). Trainings were administered to small groups of 15–20 persons every week or two for a total of 30 sessions. The cognitive and psychoeducational sessions were 90 min; physical exercise was 45 min; and the combined cognitive (or psychoeducational) training plus physical training was 90 + 45 min (135 min). Five years later, the no-training group showed cognitive declines. Neither physical training, psychoeducational training, nor combined psychoeducational and physical training did anything to alleviate those cognitive declines. (Note that benefits from the combined cognitive and physical training cannot be due to additional time in sessions because combined psychoeducational and physical training included just as much time in sessions yet yielded no EF benefits.) Those who received combined cognitive and physical training or cognitive training alone showed significant cognitive preservation that was still evident 5 years later, and the effect was larger for the combined training than for the cognitive training alone. The combined cognitive and physical training group performed better than any other group 5 years later on reasoning (WAIS-similarities), several memory tasks, and speed of processing; as well as on the color-word task that all 3 groups that received any cognitive training had practiced. (Remember that “performed better” here refers primarily to staving off, or reducing, cognitive decline. The mechanisms for better preserving existing cognitive abilities [protecting them from decline] might well be different from the mechanisms for improving cognitive functions in young adults or enhancing their development during childhood.)
There are now several school programs that integrate physical activity with the teaching of academic subjects. Although there are no studies looking at possible EF benefits from these programs, studies show better academic outcomes from these programs than when academic subjects are taught the traditional way (sitting still). Each program has found that students resoundingly prefer classes with physical activity over traditional classes. Also when there was physical activity, students spent more time on task and were better behaved.
The most heavily researched of these programs is TAKE10!®. Here, children's movements are designed to solidify and concretize academic concepts (e.g., marching in place to a story about exploration, learning multiplication tables by doing jump rope actions, or doing two-part muscle contractions to help students understand word contractions). Kibbe et al. (2011) reviewed 10 years’ of evidence on the effectiveness of TAKE10!® and concluded that the program improved children's academic performance (grades and standardized test performance) and in-class behavior. Other methods of integrating physical activity and academic instruction also report better academic outcomes from the combined movement plus instruction than from traditional teaching methods (Move for Thought [Vazou and Smiley-Oyen, 2014]; movement games [Kubesch and Walk, 2009]; and others [Erwin et al., 2012, Mahar et al., 2006]).
Learning Through the Arts® brings specially-trained artist-educators into schools to partner with teachers in creating and delivering arts-infused lessons. Many of the artists have students do physical activities (e.g., learning math through dance) but other artists do not (e.g., learning history through storytelling). A Canada-wide study found that 6th graders who had participated in the program for three years scored 11 percentage points higher on math tests than did their peers in control groups (Smithrim and Upitis, 2005).
Another hint that maybe being physically active plus cognitively challenged has added value for improving EFs over just being cognitively challenged comes from looking at EF benefits from different activities that older adults have randomly been assigned to do. Training in theater (which involves moving in space and using one's whole body) produced more EF benefits than training in visual arts, digital photography, or quilting (which can be considered more sedentary; Noice et al., 2004, Park et al., 2007).
9. The reason why improvements are found is not always obvious, and sometimes is down-right counter-intuitive. For example, CogMed®, computerized working-memory training, is the method for improving EFs that has been most heavily studied and for which the best evidence of EF improvement exists. Virtually everyone assumed that its success was due to the clever computerized games that it uses. To be certified to administer CogMed®, an individual must get trained in, and commit to, mentoring those doing CogMed®. Many CogMed® studies don’t even mention the mentoring component because it was not considered especially worth noting. However, a recent study by de Jong (2014) found that it is the mentoring that seems to account for the benefits of CogMed® more than the computerized games.
In considering why physical activity might improve EFs, people have considered how aerobic exercise increases blood flow and the amount of oxygen reaching the brain as well as increasing BDNF levels in the brain (Jeon and Ha, 2015, Kimhy et al., 2015, Sabatier and English, 2015, Voss et al., 2013). People have paid little attention to the possibility that the cognitive components of the exercise activity might be the main contributors to any EF benefit (exceptions include: Moreau et al., 2015, Pesce et al., 2013). This is despite many calls to pay attention to the cognitive components (Best, 2010, Moreau and Conway, 2013, Pesce, 2012, Sibley and Etnier, 2003, Tomporowski et al., 2008, Tomporowski et al., 2015). Stress or a depressed mood impairs EFs (Arnsten, 1998, Liston et al., 2009, Schoofs et al., 2009). Lack of sleep impairs EFs (Bernier et al., 2013, Labelle et al., 2015, Saunamäki and Jehkonen, 2007). Exercise reduces stress and depressive feelings (Carmack et al., 1999, Haslacher et al., 2015, Williamson et al., 2001) and improves sleep (Chen et al., 2015, Wachob and Lorenzi, 2015, Yang et al., 2012). However, no study has yet explored whether any EF benefit from exercise might be due to the effects of exercise on reduced stress or improved sleep.
4. A different perspective based on the neurobiology of EFs and prefrontal cortex
EFs depend on prefrontal cortex and other neural regions with which prefrontal cortex is interconnected (Aron et al., 2007, Eisenberg and Berman, 2010, Leh et al., 2010). Prefrontal cortex is the newest area of the brain and the most vulnerable. Prefrontal cortex and EFs suffer first and most if you are stressed, sad, lonely, or not in good physical health. Conversely, your ability to reason, exercise good self-control, and flexibly adapt to change (that is, your EFs) are better when you are more relaxed and un-stressed, feel emotionally and socially nourished, and are in good health. (Evidence for these deleterious and salutary effects on EFs is provided below.)
Since stress, sadness, loneliness, and poor health impair EFs, and the reverse enhances EFs, we predict that approaches that will be found most successful at improving EFs will be those that not only directly train and challenge EFs but also indirectly support EFs by working to reduce things that impair them and enhance things that support them. If a program focuses only on training EFs and does nothing to decrease how stressed an individual feels, increase joy, enhance feelings of social connectedness and social support, improve sleep, or increase physical fitness, then unmet emotional, social, or physical needs, we predict, will work to oppose any improvement in EFs from the program. Similarly, we also predict that one should be able to see some EF improvement from just decreasing felt stress, providing a sense of belonging or social inclusion, and/or improving physical health – without any direct training of EFs – just as directly training EFs, without addressing emotional, social, or physical well-being should also produce some limited EF improvement.
Effect of stress on EFs. Our brains work better when we are not in a stressed emotional state and that is particularly true for prefrontal cortex and EFs (Arnsten, 1998). One reason stress impairs EFs first and most is because even mild stress overwhelms prefrontal cortex (but not other brain regions) with excess dopamine (Cerqueira et al., 2007, Roth et al., 1988). The norepinephrine system in prefrontal cortex (through heightened α1 adrenoceptor stimulation) also contributes to prefrontal cortex dysfunction when we are stressed (Birnbaum et al., 1999). In response to stress the adrenal cortex releases cortisol. In primates (including humans) there are more glucocorticoid receptors in prefrontal cortex than anywhere else in the brain (Sánchez et al., 2000), thus the effect of cortisol's release in response to stress is most pronounced in prefrontal. In addition, stress can disrupt the functional connectivity between prefrontal cortex and other brain regions (Liston et al., 2009).
Stress can make anyone look like he or she has an EF disorder, such as ADHD, when that is not the case. You may have noticed that when you are stressed you cannot think as clearly or exercise as good self-control.
Effect of depressed mood or affect on EFs. When we are sad we have worse attentional control (Desseilles et al., 2009, von Hecker and Meiser, 2005); when we are happy we have better attentional control (Gable and Harmon-Jones, 2008). People show better cognitive flexibility when they are happy. The most heavily researched predictor of creativity in social psychology is mood (Hirt et al., 2008). The most robust finding is that a happy mood leads to greater creativity (Ashby et al., 1999). It enables people to work more flexibly (Murray et al., 1990) and see potential relatedness among unusual and atypical members of categories (Isen et al., 1985, Isen et al., 1987). In short, people are better able to “think outside the box” when they are happier. (It's not that happier people are more creative than sadder people, but that in general a given individual tends to be more creative when he or she is happier than when he or she is more miserable or dejected.)
Effect of loneliness on EFs. We are fundamentally social. We need to feel we are not alone. We thrive when we know beyond a shadow of a doubt that there are people who care about us, believe in us, and are there for us. We are often happiest when we feel part of a group working toward a common goal. When we are lonely our EFs suffer (e.g., Cacioppo and Patrick, 2008, Campbell et al., 2006). When we feel socially supported we show better EFs (Cacioppo and Patrick, 2008, Tangney et al., 2004). Feeling excluded or as if you do not belong impairs prefrontal cortex functioning, selective attention, and reasoning (Baumeister et al., 2005, Campbell et al., 2006, Twenge et al., 2002). Conversely, Ybarra et al. (2011) found that just basic getting-to-know-you interactions (even without any cooperative goal) boosted EFs.
When some subjects were chosen randomly to be warned to anticipate being alone and others were told they should expect close relationships throughout their lives, all performed comparably on simple memorization questions that do not require EFs, but on logical reasoning problems (which require EFs), those told to expect that they would be lonely performed worse (even worse than a third group of subjects told unrelated bad news; Baumeister et al., 2002). Other researchers have not tried to manipulate feelings of social isolation or support; they merely give subjects a questionnaire when they come in the lab that includes questions about whether they feel socially supported or isolated. Campbell et al. (2006), for example, found that prefrontal cortex functioned less efficiently in those who reported that they felt lonely or isolated.
Effect of sub-optimal health on EFs. We function better after a good night's sleep. Lack of sleep impairs EFs (e.g., Bernier et al., 2013, Saunamäki and Jehkonen, 2007) and can make you look like you have an EF impairment, like ADHD, when you do not. It has been estimated that perhaps 25% of those diagnosed with ADHD, do not have ADHD but have sleep apnea instead (Beebe and Gozal, 2002, Naseem et al., 2001, Youssef et al., 2011).
Seligman (personal communication) once said, “I never let anyone with a cold do anything important.” That is probably wise advice. Prefrontal cortex does not function as well and executive functioning is of poorer quality when you have an infection (Abdel Rahman et al., 2014, Carson et al., 2006). This appears to be due, not to the infection itself, but to the immune response that the body mounts in response to the infection (Jing et al., 2013, Smith et al., 2007).
As mentioned above, people who are not as physically fit do not have as good EFs as people who are fit (e.g., Etnier et al., 2006). Similarly, people who are very overweight tend to have worse EFs than people who are more trim (e.g., Blanco-Gómez et al., 2015, Lokken et al., 2009).
Importance of self-confidence and feelings of self-efficacy for improving EFs. We know of no data on the importance of believing that you can succeed through effort in improving your EFs or in how much your EFs improve. However, feeling confident in your ability to succeed, believing that through effort you can improve, and treating errors and failed attempts as learning opportunities or as simply what happens when you push past your comfort zone, venturing beyond what you already know is important for success at so many other things (Bandura, 1994, Bandura, 2006, Blackwell et al., 2007, Dweck, 2002, Dweck, 2006, Murphy and Dweck, 2010), that we predict it may also be key for improving EFs. There is also plenty of evidence that our expectations about whether or not we can do something has a huge effect on whether we succeed in doing it (Aronson et al., 1999, Good et al., 2008, Steele and Aronson, 1998). Certainly that must be true for success in any program that has improving EFs as its goal.
5. Burning questions that are calling out for answers from research
It is clear that EFs can indeed be improved and that is true at all ages from infants (e.g., Kovács and Mehler, 2009) through elders (e.g., Williams and Lord, 1997). However we do not know how much EFs can be improved. Does training simply nudge EFs slightly higher? Are benefits closely tied to specific types of tasks or contexts or do they generalize further than that? How long do benefits last? Are improvements just superficial and ephemeral, yielding no enduring benefits? There's evidence that they can last at least 6 months or a year, and one tantalizing finding that benefits can still be seen 5 years later (Oswald et al., 2006) but we know so little about what to expect more than a year after training. Except for Oswald et al., no study that met our inclusion criteria has looked at whether EF benefits last even one month after any form of physical activity (whether the activity was aerobic exercise, resistance training, yoga, martial arts, or anything else). What factors affect how long benefits last? Presumably using and challenging the target skills needs to be embedded in one's regular routine or periodic booster or refresher sessions are needed. Is doing the former or the latter more effective? No one has looked at whether booster or refresher sessions help, much less when to give them or what the correct durations or frequencies should be.
What are the best methods for improving EFs? What about an approach accounts for its success? That is so important and we know so little about it. Studies are also sorely needed that systematically vary dose, frequency, and duration. There are so few studies that have done that to date. Studies are needed that vary other characteristics of the activities. For example, for physical activities, other characteristics would include whether they are solitary or not, whether and how much the activity places demands on EFs, whether and how much the activity also trains and challenges motor skills (such as eye-hand coordination or bimanual coordination), and how much participants enjoy the activity (see evidence above that enjoying an activity may be critical for reaping cognitive benefits from it). Moreau and Conway (2014) hypothesize that programs (whether they be cognitive training or physical activities) that are characterized by complexity, novelty, and diversity (variety) will be the most successful at improving EFs. That hypothesis deserves experimental testing. We don’t know if it matters whether EF challenges are an intrinsic characteristic of a physical activity, separate and occur at times other than when physical activity is done, or are separate but done at the same time as the physical activity (e.g., reading or doing cognitive work while riding a stationary bicycle or running on a treadmill).
An open question is compliance differences across intervention methods. Training studies have done surprisingly little to assess compliance with the exception of CogMed studies and investigations of physical activity programs that have participants wear heartrate and/or motor activity monitors. Of course, assigning participants to an experimental group does not guarantee that participants will show up for their sessions. Researchers should record and report not just attrition rates, but also compliance rates.
Au et al. (2015) add another interesting dimension into the mix. They found an inverse relationship between the size of the extrinsic reward for participating in training (i.e., the amount of money subjects were offered for participating) and how much N-back training improved reasoning. That is consistent with an older literature on the negative effects of extrinsic rewards (even gold stars or stickers) on performance and enjoyment of the activity (e.g., Deci et al., 1999, Lepper et al., 1973). It deserves to be followed up.
Do the answers to any of these questions differ by type of program, EF component (e.g., WM or inhibitory control) or any subject characteristics (such as age, gender, SES level, or ethnic group)? For example, might aerobic exercise be more cognitively beneficial for younger children (say, 3-4 years old) than any study of EF benefits from aerobic exercise have looked?
Will the predictions we stated in the previous section be supported or disconfirmed? Will supporting other aspects of an individual (emotional, social, and physical) be key to seeing benefits last? It is likely that if stress builds up in a person's life (especially if he or she has not been helped to develop ways to handle stress), or a person does not have experiences that bring him or her joy and pride, or the person feels alone, or if he or she is not getting enough sleep or is unhealthy, that person's EFs will suffer even if an intervention temporarily improved them (Diamond, 2012, Diamond, 2014b). These assumptions should be tested.
Some benefits only show up later (and are not evident immediately after training) or are much larger later than right after training (e.g., Blair and Raver, 2014, Holmes et al., 2009). We have known for roughly 50 years that strategies that produce the best longer-term outcomes often do not produce the best short-term ones, and conversely training strategies that produce the best immediate gains often do not produce the best longer-term benefits (Bjork, 2001, Rosenbaum et al., 2001). For example, using the same materials leads to better performance just after training but worse performance later, whereas variable training produces worse immediate results but better long-term results (Bransford et al., 1979, Shea and Morgan, 1979). Extrapolating from this, it is likely that training diverse EF skills will take longer to produce benefits than training just one skill. Consistent with that, when some children were trained only on WM, some only on reasoning, and some on both, all for the same length of time, those trained on just one skill showed greater immediate improvements in that skill than those trained on both (Bergman Nutley et al., 2011). If the goal is lasting benefits to more diverse EF skills, then we probably need to be patient. The training will probably need to continue for longer and we will likely need to wait until longer after the training to see the full benefits. These assumptions should be tested.
There are many activities worth investigating for their possible benefits to EFs. Diamond, 2010, Diamond, 2012, Diamond, 2014b has predicted that many activities not yet studied might well improve EFs, such as band, orchestra or choir, social, communal dance (such as rueda or any folk dance), drumming circle, youth circus, caring for an animal, filmmaking, capoeira, martial arts not yet investigated (like aikido), and team sports (such as basketball, soccer, crew, synchronized swimming, or track and field) because each of these tax and challenge EFs, provide joy and build self-confidence, create feelings of belonging to a group with an important shared goal, and make demands on the body, keeping one fit. It is probably critical that the individual deeply enjoys and is committed to whatever the activity it is. That is needed to sustain hours and hours of practice and the effort needed to keep pushing oneself to improve. Many of these activities also incorporate Moreau and Conway's (2014) principles of complexity, novelty, and diversity.
In summary, the interesting question is no longer whether EFs can be improved. They can, at every age from infants through elders and via diverse approaches. We do not know how much they can be improved, however, or how long benefits last and what determines how much EFs improve or whether benefits last. Some claims clearly seem exaggerated. Physical activity (without cognitive challenge, that brings little joy, and lacks any social component) appears not to improve EFs. Despite claims by commercial computerized training programs, wide transfer does not seem to occur; when it has been reported, replication attempts have often failed. People improve on what they practice, but only on what they practice. That generalizes to other contexts where those same skills are needed. From the rich research literature to date, several general principles have emerged, such as that transfer definitely occurs but it is narrow (see other principles above).
Since (a) stress, sadness, loneliness, and poor physical health (e.g., not enough sleep) impair EFs (indeed their detrimental effects are also evident at the physiological and neuroanatomical level in prefrontal cortex) and since (b) EFs are better when we are less stressed, happier, well rested, and feel there are people who we can share experiences with, who care about us, and who we can turn to for support, it follows, we hypothesize, that while training and challenging EFs is necessary for improving them, benefits will be greater if emotional, social, and physical needs are also addressed. Each aspect of ourselves affects, and is affected by, the other aspects. The different parts of the human being are fundamentally interrelated (Diamond, 2000, Diamond, 2007, Diamond, 2014b). We also hypothesize that key to EF benefits continuing to last is that challenges to EFs and that good emotional, social, and physical health continue.
Author note
AD gratefully acknowledges financial support from NIDA R01 # DA037285 and a Canada Research Chairs award (CRC -950-27472).
An earlier version of the work reported here was presented as a Keynote Address by AD at the FLUX Integrative Developmental Cognitive Neuroscience Conference, Los Angeles, CA (Sept. 12, 2014).
A more detailed and in-depth report of the review summarized here can be found in:
Diamond, A. & Ling, D. (accepted). Review of the evidence on, and fundamental questions surrounding, efforts to improve executive functions (including working memory. To appear in M. Bunting, J. Novick, M. Dougherty, & R. W. Engle (Eds.), An integrative approach to cognitive and working memory training: Perspectives from psychology, neuroscience, and human development. Oxford, UK: Oxford University Press.
Footnotes
Karbach and Verhaeghen's (2014) meta-analysis was not discussed here because, although it included studies that looked at the effects of extended practice, it included them together with studies that looked at acute effects from a single training session (and such studies are explicitly outside of what we are discussing here).
Each Tae-Kwon-Do session began with three questions that emphasized self-monitoring and planning: (a) Where am I (i.e., focus on the present moment)? (b) What am I doing? (c) What should I be doing? The later two questions directed children to select specific behaviors, compare their current behavior to their goal, and prepare concrete plans to improve their behavior. Being traditional Tae-Kwon-Do, the values of respect, humility, responsibility, honor, perseverance, discipline, and self-control were emphasized.
References
- Abdel Rahman T.T., Abdel Guaad M.A., Mortagy A.K. Assessment of executive functions in chronic Hepatitis C virus infected patients. Egypt. J. Hosp. Med. 2014;55:257–260. [Google Scholar]
- Alloway T.P., Alloway R.G. Investigating the predictive roles of working memory and IQ in academic attainment. J. Exp. Child Psychol. 2010;106:20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Alloway T.P., Gathercole S.E., Adams A.M., Willis C., Eaglen R., Lamont E. Working memory and phonological awareness as predictors of progress towards early learning goals at school entry. Br. J. Dev. Psychol. 2005;23:417–426. [Google Scholar]
- Allport A., Wylie G. Task switching, stimulus-response bindings, and negative priming. In: Monsell S., Driver J., editors. Control of Cognitive Processes: Attention and Performance XVII. MIT Press; Cambridge, MA: 2000. pp. 35–70. [Google Scholar]
- Angevaren M., Aufdemkampe G., Verhaar H.J.J., Aleman A., Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008;3(3):1–70. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. The biology of being frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Behrens T.E., Smith S., Frank M.J., Poldrack R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson J., Lustina M.J., Good C., Keough K., Steele C.M., Brown J. When white men can’t do math: necessary and sufficient factors in stereotype threat. J. Exp. Soc. Psychol. 1999;35:29–46. [Google Scholar]
- Ashby F., Isen A., Turken A. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Audiffren M., André N. The strength model of self-control revisited: linking acute and chronic effects of exercise on executive functions. J. Sport and Health Sci. 2015;4(1):30–46. [Google Scholar]
- Au J., Sheehan E., Tsai N., Duncan G.J., Buschkuehl M., Jaeggi S.M. Improving fluid intelligence with training on working memory: a meta-analysis. Psychon. Bull. Rev. 2015;22:366–377. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- Awh E., Anllo-Vento L., Hillyard S.A. The role of spatial selective attention in working memory for locations: evidence from event-related potentials. J. Cogn. Neurosci. 2000;12:840–847. doi: 10.1162/089892900562444. [DOI] [PubMed] [Google Scholar]
- Awh E., Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cog. Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Baddeley A.D., Hitch G.J. Developments in the concept of working memory. Neuropsychology. 1994;8:485–493. [Google Scholar]
- Bailey C.E. Cognitive accuracy and intelligent executive function in the brain and in business. Ann. N.Y. Acad. Sci. 2007;1118:122–141. doi: 10.1196/annals.1412.011. [DOI] [PubMed] [Google Scholar]
- Ball K., Berch D.B., Helmers K.F., Jobe J.B., Leveck M.D., Marsiske M., Willis S.L. Effects of cognitive training interventions with older adults: a randomized controlled trial. J. Am. Med. Assoc. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy. In: Corsini R.J., editor. second ed. vol. 3. Wiley; New York, NY: 1994. pp. 368–369. (Encyclopedia of Psychology). [Google Scholar]
- Bandura A. Guide for constructing self-efficacy scales. In: Pajares F., Urdan T., editors. vol. 5. Information Age Publishing; Greenwich, CT: 2006. pp. 307–337. (Self-Efficacy Beliefs of Adolescents). [Google Scholar]
- Barenberg J., Berse T., Dutke S. Executive functions in learning processes: do they benefit from physical activity? Educ. Res. Rev. 2011;6:208–222. [Google Scholar]
- Basak C., Boot W.R., Voss M.W., Kramer A.F. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol. Aging. 2008;23:765–777. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.F., DeWall C.N., Ciarocco N.J., Twenge J.M. Social exclusion impairs self-regulation. J. Pers. Soc. Psychol. 2005;88:589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F., Twenge J.M., Nuss C.K. Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. J. Pers. Soc. Psychol. 2002;83:817–827. doi: 10.1037//0022-3514.83.4.817. [DOI] [PubMed] [Google Scholar]
- Bergman Nutley S., Söderqvist S., Bryde S., Thorell L.B., Humphreys K., Klingberg T. Gains in fluid intelligence after training non-verbal reasoning in 4-year-old children: a controlled, randomized study. Dev. Sci. 2011;14:591–601. doi: 10.1111/j.1467-7687.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- Beebe D.W., Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Bernier A., Beauchamp M.H., Bouvette-Turcot A.A., Carlson S.M., Carrier J. Sleep and cognition in preschool years: specific links to executive functioning. Child Dev. 2013;84:1542–1553. doi: 10.1111/cdev.12063. [DOI] [PubMed] [Google Scholar]
- Blanco-Gómez A., Ferré N., Luque V., Cardona M., Gispert-Llauradó M., Escribano J., Canals-Sans J. Being overweight or obese is associated with inhibition control in children from six to ten years of age. Acta Paediatr. 2015;104:618–625. doi: 10.1111/apa.12976. [DOI] [PubMed] [Google Scholar]
- Birnbaum S., Gobeske K.T., Auerbach J., Taylor J.R., Arnsten A.F.T. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Best J.R. Effects of physical activity on children's executive function: contributions of experimental research on aerobic exercise. Dev. Rev. 2010;30:331–351. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork R.A. How to succeed in college: learn how to learn. APS Obs. 2001;14(3):9. [Google Scholar]
- Blackwell L., Trzesniewski K., Dweck C.S. Implicit theories of intelligence predict achievement across an adolescent transition: a longitudinal study and an intervention. Child Dev. 2007;78:246–263. doi: 10.1111/j.1467-8624.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Blair C. School readiness: integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. Am. Psychol. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C. Closing the achievement gap through modification of neurocognitive and neuroendocrine function: results from a cluster randomized controlled trial of an innovative approach to the education of children in kindergarten. PLoS ONE. 2014;9(11):e112393. doi: 10.1371/journal.pone.0112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Razza R.P. Relating effortful control, executive function, and false-belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal J.A., Emery C.F., Madden D.J., George L.K., Coleman R.E., Riddle M.W., Williams R.S. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J. Gerontol. 1989;44(5):M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- Bodrova E., Leong D.J. second ed. Merrill/Prentice Hall; New York, NY: 2007. Tools of the Mind: The Vygotskian Approach to Early Childhood Education. [Google Scholar]
- Boot W.R., Simons D.J., Stothart C., Stutts C. The pervasive problem with placebos in Psychology: why active control groups are not sufficient to rule out placebo effects. Perspect. Psychol. Sci. 2013;8:445–454. doi: 10.1177/1745691613491271. [DOI] [PubMed] [Google Scholar]
- Borella E., Carretti B., Pelgrina S. The specific role of inhibition in reading comprehension in good and poor comprehenders. J. Learn. Disabil. 2010;43:541–552. doi: 10.1177/0022219410371676. [DOI] [PubMed] [Google Scholar]
- Boucard G.K., Albinet C.T., Bugaiska A., Bouquet C.A., Clarys D., Audiffren M. Impact of physical activity on executive functions in aging: a selective effect on inhibition among old adults. J. Sport Exerc. Psychol. 2012;34:808–827. doi: 10.1123/jsep.34.6.808. [DOI] [PubMed] [Google Scholar]
- Bransford J.D., Franks J.J., Morris C.D., Stein B.S. Some general constraints on learning and memory research. In: Cermak L.S., Craik F.I.M., editors. Levels of Processing in Human Memory. Lawrence Erlbaum Associates Inc; Hillsdale, NJ: 1979. pp. 331–354. [Google Scholar]
- Burke C.A. Mindfulness-based approaches with children and adolescents: a preliminary review of current research in an emergent field. J. Child Fam. Stud. 2010;19:133–144. [Google Scholar]
- Bryck R.L., Fisher P.A. Training the brain: practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am. Psychol. 2012;67:87–100. doi: 10.1037/a0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J., Patrick W. W. W. Norton & Co., Inc; New York, NY: 2008. Loneliness: Human Nature and the Need for Social Connection. [Google Scholar]
- Campbell W.K., Krusemark E.A., Dyckman K.A., Brunell A.B., McDowell J.E., Twenge J.M., Clementz B.A. A magnetoencephalography investigation of neural correlates for social exclusion and self-control. Soc. Neurosci. 2006;1:124–134. doi: 10.1080/17470910601035160. [DOI] [PubMed] [Google Scholar]
- Carlson S.M., Moses L.J. Individual differences in inhibitory control and children's theory of mind. Child Dev. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carmack C., de Moor C., Boudreaux E., Amaral-Melendez M., Brantley P. Aerobic fitness and leisure physical activity as moderators of the stress-illness relation. Ann. Behav. Med. 1999;21:251–257. doi: 10.1007/BF02884842. [DOI] [PubMed] [Google Scholar]
- Carson P.J., Konewko P., Wold K.S., Mariani P., Goli S., Bergloff P., Crosby R.D. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin. Infec. Dis. 2006;43:723–730. doi: 10.1086/506939. [DOI] [PubMed] [Google Scholar]
- Cerqueira J.J., Mailliet F., Almeida O.F., Jay T.M., Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L., Pontifex M.B., Hillman C.H., Kramer A.F. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J. Int. Neuropsychol. Soc. 2011;17:975–985. doi: 10.1017/S1355617711000567. [DOI] [PubMed] [Google Scholar]
- Chang Y.K., Tsai Y.J., Chen T.T., Hung T.M. The impacts of coordinative exercise in executive function in kindergarten children: an ERP study. Exp. Brain Res. 2013;225:187–196. doi: 10.1007/s00221-012-3360-9. [DOI] [PubMed] [Google Scholar]
- Chen L.J., Fox K.R., Ku P.W., Chang Y.W. Effects of aquatic exercise on sleep in older adults with mild sleep impairment: a randomized controlled trial. Int. J. Behav. Med. 2015;2015(May) doi: 10.1007/s12529-015-9492-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Clements D.H., Sarama J., Layzer C. Washington, DC; 2012. Presentation in Session on “The Efficacy of an Intervention Synthesizing Scaffolding Designed to Promote Self Regulation with an Early Mathematics Curriculum: Effects on Executive Function” at Society for Research on Educational Effectiveness (SREE) Conference on Understanding Variations in Treatment Effects March 8, 2012. [Google Scholar]
- Colcombe S.J., Kramer A.F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Collins A., Koechlin E. Reasoning, learning, and creativity: frontal lobe function and human decision-making. PLoS Biol. 2012;10(3):e1001293. doi: 10.1371/journal.pbio.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescioni A.W., Ehrlinger J., Alquist J.L., Conlon K.E., Baumeister R.F., Schatschneider C., Dutton G.R. High trait self-control predicts positive health behaviors and success in weight loss. J. Health Psychol. 2011;16:750–759. doi: 10.1177/1359105310390247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.L., Tomporowski P.D., McDowell J.E., Austin B.P., Miller P.H., Yanasak N.E., Naglieri J.A. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci E.L., Koestner R., Ryan R.M. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol. Bull. 1999;125:627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- de Jong P. Workshop on Enhancing Executive Functions in Education in Nijmegen. May 20, 2014 Nijmegen, NL; May 20, 2014. Effects of training working memory in adolescents with a below average IQ. [Google Scholar]
- D’Esposito M., Postle B.R. The cognitive neuroscience of working memory. Ann. Rev. Psychol. 2015;66:115–140. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desseilles M., Balteau E., Sterpenich V., Dang-Vu T.T., Darsaud A., Vandewalle G., Schwartz S. Abnormal neural filtering of irrelevant visual information in depression. J. Neurosci. 2009;29:1395–1403. doi: 10.1523/JNEUROSCI.3341-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Diamond A. Interrelated and interdependent. Dev. Sci. 2007;10:152–158. doi: 10.1111/j.1467-7687.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The evidence base for improving school outcomes by addressing the whole child and by addressing skills and attitudes, not just content. Early Educ. Dev. 2010;21:780–793. doi: 10.1080/10409289.2010.514522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Activities and programs that improve children's executive functions. Curr. Dir. Psychol. Sci. 2012;21:335–341. doi: 10.1177/0963721412453722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Ann. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Whether coordinative (soccer) exercise improves executive functioning in kindergarten children has yet to be demonstrated. Exp. Brain Res. 2014;232:1. doi: 10.1007/s00221-014-3920-2. [DOI] [PubMed] [Google Scholar]
- Diamond A. Want to optimize executive functions and academic outcomes? Simple, just nourish the human spirit. Minn. Symp. Child Psychol. 2014;37:203–230. [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Lee K. Interventions and programs demonstrated to aid executive function development in children 4–12 years of age. Science. 2011;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A., Ling, D., (accepted). Fundamental questions surrounding efforts to improveexecutive functions (including working memory). In: Bunting, M., Novick, J., Dougherty, M., Engle, R.W. (Eds.), An Integrative Approach to Cognitive andWorking Memory Training: Perspectives from Psychology, Neuroscience, and Human Development. Oxford University Press, New York, NY.
- Dweck C.S. Beliefs that make smart people dumb. In: Sternberg R.J., editor. Why Smart People Can be so Stupid. Yale University Press; New Haven, CT: 2002. pp. 22–41. [Google Scholar]
- Dweck C.S. Random House; New York, NY: 2006. Mindset: The New Psychology of Success. [Google Scholar]
- Duckworth A.L., Seligman M.E.P. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol. Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Dowsett C.J., Claessens A., Magnuson K., Huston A.C., Klebanov P., Japel C. School readiness and later achievement. Dev. Psychol. 2007;43:1428–1446. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Eakin L., Minde K., Hechtman L., Ochs E., Krane E., Bouffard R., Looper K. The marital and family functioning of adults with ADHD and their spouses. J. Atten. Disord. 2004;8:1–10. doi: 10.1177/108705470400800101. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.P., Berman K.F. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson K.A. The influence of experience and deliberate practice on the development of superior expert performance. In: Ericsson K.A., Charness N., Feltovich P., Hoffman R.R., editors. Cambridge Handbook of Expertise and Expert Performance. Cambridge U. Press; Cambridge, UK: 2006. pp. 685–706. [Google Scholar]
- Ericsson K.A. Paper presented at the Proceedings of the International Symposium on Performance Science 2009. Utrecht, The Netherlands; 2009. Discovering deliberate practice activities that overcome plateaus and limits on improvement of performance. [Google Scholar]
- Ericsson K.A., Nandagopal K., Roring R.W. Toward a science of exceptional achievement: attaining superior performance through deliberate practice. Ann. N.Y. Acad. Sci. 2009;1172(1):199–217. doi: 10.1196/annals.1393.001. [DOI] [PubMed] [Google Scholar]
- Ericsson K.A., Towne T.J. Expertise Wiley Interdisciplinary Reviews. Cog. Sci. 2010;1:404–416. doi: 10.1002/wcs.47. [DOI] [PubMed] [Google Scholar]
- Erwin H., Fedewa A.L., Ahn S. Student academic performance outcomes of a classroom physical activity intervention: a pilot study. Int. Electron. J. Element. Educ. 2012;5:109–124. [Google Scholar]
- Etnier J.L., Nowell P.M., Landers D.M., Sibley B.A. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Etnier J.L., Salazar W., Landers D.M., Petruzzello S.J., Han M., Nowell P. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J. Sport Exerc. Psychol. 1997;19:249–277. [Google Scholar]
- Fabre C., Chamari K., Mucci P., Masse-Biron J., Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 2002;23:415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- Farran D.C., Wilson S.J. Paper presented at the Peabody Res. Inst. Colloq. Series. Nov 2011, Nashville, TN; 2011. Is self regulation malleable? Results from an evaluation of the tools of the mind curriculum. [Google Scholar]
- Fedewa A.L., Ahn S. The effects of physical activity and physical fitness on children's achievement and cognitive outcomes: a meta-analysis. Res. Q. Exerc. Sport. 2011;82:521–535. doi: 10.1080/02701367.2011.10599785. [DOI] [PubMed] [Google Scholar]
- Fiebach C.J., Ricker B., Friederici A.D., Jacobs A.M. Inhibition and facilitation in visual word recognition: prefrontal contribution to the orthographic neighborhood size effect. NeuroImage. 2007;36:901–911. doi: 10.1016/j.neuroimage.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Flook L., Smalley S.L., Kitil J.M., Galla B.M., Kaiser-Greenland S., Locke J., Kasari C. Effects of mindful awareness practices on executive functions in elementary school children. J. Appl. Sch. Psychol. 2010;26:70–95. [Google Scholar]
- Gable P.A., Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychol. Sci. 2008;19:476–482. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Pickering S.J., Knight C., Stegmann Z. Working memory skills and educational attainment: evidence from National Curriculum assessments at 7 and 14 years of age. Appl. Cog. Psychol. 2004;18:1–16. [Google Scholar]
- Gazzaley A., Nobre A.C. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C., Aronson J., Harder J.A. Problems in the pipeline: stereotype threat and women's achievement in high-level math courses. J. Appl. Dev. Psychol. 2008;29:17–28. [Google Scholar]
- Greenberg M.T., Harris A.R. Nurturing mindfulness in children and youth: current state of research. Child Dev. Perspect. 2012;6:161–166. [Google Scholar]
- Harrison T.L., Shipstead Z., Hicks K.L., Hambrick D.Z., Redick T.S., Engle R.W. Working memory training may increase working memory capacity but not fluid intelligence. Psychol. Sci. 2013;24:2409–2419. doi: 10.1177/0956797613492984. [DOI] [PubMed] [Google Scholar]
- Haslacher H., Michlmayr M., Batmyagmar D., Perkmann T., Ponocny-Seliger E., Scheichenberger V., Winker R. Physical exercise counteracts genetic susceptibility to depression. Neuropsychobiology. 2015;71:168–175. doi: 10.1159/000381350. [DOI] [PubMed] [Google Scholar]
- Heyman E., Gamelin F.X., Goekint M., Piscitelli F., Roelands B., Leclair E., Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans: possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Titterness A.K., Morrish A.C., Carrier E.J., Lee T.T., Gil-Mohapel J., Christie B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Castelli D.M., Buck S.M. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med. Sci. Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Pontifex M.B., Castelli D.M., Khan N.A., Raine L.B., Scudder M.R., Kamijo K. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134:e1063–e1071. doi: 10.1542/peds.2013-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindin S.B., Zelinski E.M. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: a meta-analysis. J. Am. Geriatr. Soc. 2012;60(1):136–141. doi: 10.1111/j.1532-5415.2011.03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt E.R., Devers E.E., McCrea S.M. I want to be creative: exploring the role of hedonic contingency theory in the positive mood-cognitive flexibility link. J. Pers. Soc. Psychol. 2008;94:214–230. doi: 10.1037/0022-3514.94.2.94.2.214. [DOI] [PubMed] [Google Scholar]
- Holmes J., Gathercole S.E., Dunning D.L. Adaptive training leads to sustained enhancement of poor working memory in children. Dev. Sci. 2009;12:F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Holmes J., Gathercole S.E., Place M., Dunning D.L., Hilton K.A., Elliott J.G. Working memory deficits can be overcome: impacts of training and medication on working memory in children with ADHD. Appl. Cog. Psychol. 2010;24:827–836. [Google Scholar]
- Howard-Jones P. Education Endowment Foundation; Millbank, UK: 2014. Neuroscience and Education: A Review of Educational Interventions and Approaches Informed by Neuroscience. [Google Scholar]
- Hughes C., Dunn J. Understanding mind and emotion: longitudinal associations with mental-state talk between young friends. Dev. Psychol. 1998;34:1026–1037. doi: 10.1037//0012-1649.34.5.1026. [DOI] [PubMed] [Google Scholar]
- Hughes C., Ensor R. Does executive function matter for preschoolers’ problem behaviors? J. Abnorm. Child Psychol. 2008;36:1–14. doi: 10.1007/s10802-007-9107-6. [DOI] [PubMed] [Google Scholar]
- Ikkai A., Curtis C.E. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49:1428–1434. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isen A.M., Daubman K.A., Nowicki G.P. Positive affect facilitates creative problem solving. J. Pers. Soc. Psychol. 1987;52:1122–1131. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- Isen A.M., Johnson M.M., Mertz E., Robinson G.F. The influence of positive affect on the unusualness of word associations. J. Pers. Soc. Psychol. 1985;48:1413–1426. doi: 10.1037//0022-3514.48.6.1413. [DOI] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Jonides J., Perrig W.J. Improving fluid intelligence with training on working memory. Proc. Nat. Acad. Sci. U.S.A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.K., Ha C.H. Expression of brain-derived neurotrophic factor, IGF-1 and cortisol elicited by regular aerobic exercise in adolescents. J. Phys. Ther. Sci. 2015;27:737–741. doi: 10.1589/jpts.27.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Zhang H., Wolff A.R., Bilkey D.K., Liu P. Altered arginine metabolism in the hippocampus and prefrontal cortex of maternal immune activation rat offspring. Schizophr.Res. 2013;148(1–3):151–156. doi: 10.1016/j.schres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Pontifex M.B., O’Leary K.C., Scudder M.R., Wu C.-T., Castelli D.M., Hillman C.H. The effects of an afterschool physical activity program on working memory in preadolescent children. Dev. Sci. 2011;14:1046–1058. doi: 10.1111/j.1467-7687.2011.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M.J., Brown L.H., McVay J.C., Silvia P.J., Myin-Germeys I., Kwapil T.R. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol. Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Karbach J., Verhaeghen P. Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychol. Sci. 2014;25:2027–2037. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr R., Booth B. Specific and varied practice of motor skill. Percept. Mot. Skills. 1978;46:395–401. doi: 10.1177/003151257804600201. [DOI] [PubMed] [Google Scholar]
- Kibbe D.L., Hackett J., Hurley M., McFarland A., Schubert K.G., Schultz A., Harris S.E. Ten Years of TAKE 10!®: integrating physical activity with academic concepts in elementary school classrooms. Prev. Med. 2011;52(1):S43–S50. doi: 10.1016/j.ypmed.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Kiesel A., Steinhauser M., Wendt M., Falkenstein M., Jost K., Phillip A., Koch I. Control and interference in task switching—a review. Psychol. Bull. 2010;136:849–874. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Kimhy D., Vakhrusheva J., Bartels M.N., Armstrong H.F., Ballon J.S., Khan S., Sloan R.P. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with Schizophrenia: a single-blind, randomized clinical trial. Schizophr. Bull. 2015;41:859–868. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn. Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Fernell E., Olesen P., Johnson M., Gustafsson P., Dahlstrom K., Westerberg H. Computerized training of working memory in children with ADHD—a randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kovács A.M., Mehler J. Cognitive gains in 7-month-old bilingual infants. Proc. Nat. Acad. Sci. U.S.A. 2009;106:6556–6560. doi: 10.1073/pnas.0811323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft C.E., Schwarz N.F., Chi L., Weinberger A.L., Schaeffer D.J., Pierce J.E., McDowell J.E. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity. 2014;22:232–242. doi: 10.1002/oby.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A.F., Erickson K.I. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn. Sci.s. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kubesch S., Walk L. Körperliches und kognitives training exekutiver funktionen in kindergarten und schule. Physical and cognitive training of executive functions in preschool and schoolSportwissenschaft. 2009;39:309–317. [Google Scholar]
- Kueider A.M., Parisi J.M., Gross A.L., Rebok G.W. Computerized cognitive training with older adults: a systematic review. PLoS ONE. 2012;7:e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M.A., Dang-Vu T.T., Petit D., Desautels A., Montplaisir J., Zadra A. Sleep deprivation impairs inhibitory control during wakefulness in adult sleepwalkers. J. Sleep Res. 2015;(Jun) doi: 10.1111/jsr.12315. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lakes K.D., Hoyt W.T. Promoting self-regulation through school-based martial arts training. J. Appl. Dev. Psychol. 2004;25:283–302. [Google Scholar]
- Leh S.E., Petrides M., Strafella A.P. The neural circuitry of executive functions in healthy subjects and Parkinson's Disease. Neuropsychopharmacology. 2010;35:70–85. doi: 10.1038/npp.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper M.R., Greene D., Nisbett R.E. Undermining children's intrinsic interest with extrinsic reward: a test of the “overjustification” hypothesis. J. Pers. Soc. Psychol. 1973;28:129–137. [Google Scholar]
- Leslie J.B. Career derailment: are you at risk? Any of nine fatal flaws can derail even the most successful executive. Healthcare Executive. 1995;10(6):6–9. [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Nat. Acad. Sci. U.S.A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T., Nagamatsu L.S., Graf P., Beattie B.L., Ashe M.C., Handy T.C. Resistance training and executive functions: a 12-month randomized controlled trial. Arch. Intern. Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T., Nagamatsu L.S., Voss M.W., Khan K.M., Handy T.C. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol. Aging. 2012;33:1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Lokken K.L., Boeka A.G., Austin H.M., Gunstad J., Harmon C.M. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg. Obesity Related Dis. 2009;5:547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Loosli S.V., Buschkuehl M., Perrig W.J., Jaeggi S.M. Working memory training improves reading processes in typically developing children. Child Neuropsychol. 2012;18(1):62–78. doi: 10.1080/09297049.2011.575772. [DOI] [PubMed] [Google Scholar]
- Lunt L., Bramham J., Morris R.G., Bullock P.R., Selway R.P., Xenitidis K., David A.S. Prefrontal cortex dysfunction and ‘Jumping to Conclusions’: bias or deficit? J. Neuropsychol. 2012;6(1):65–78. doi: 10.1111/j.1748-6653.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- MacLean K.A., Ferrer E., Aichele S.R., Bridwell D.A., Zanesco A.P., Jacobs T.L., Saron C.D. Intensive meditation training improves perceptual discrimination and sustained attention. Psychol. Sci. 2010;21:829–839. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar M.T., Murphy S.K., Rowe D.A., Golden J., Shields A.T., Raedeke T.D. Effects of a classroom-based program on physical activity and on-task behavior. Med. Sci. Sports Exerc. 2006;38:2086–2094. doi: 10.1249/01.mss.0000235359.16685.a3. [DOI] [PubMed] [Google Scholar]
- Manjunath N.K., Telles S. Improved performance in the Tower of London test following yoga. Indian J. Physiol. Pharmacol. 2001;45:351–354. [PubMed] [Google Scholar]
- Masley S., Roetzheim R., Gualtieri T. Aerobic exercise enhances cognitive flexibility. J. Clin. Psychol. Med. Sett. 2009;16:186–193. doi: 10.1007/s10880-009-9159-6. [DOI] [PubMed] [Google Scholar]
- McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med. Res. Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M.M., Cameron C.E., Connor C.M., Farris C.L., Jewkes A.M., Morrison F.J. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Dev. Psychol. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M., Hulme C. Is working memory training effective?. A meta-analytic review. Dev. Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Miller H.V., Barnes J.C., Beaver K.M. Self-control and health outcomes in a nationally representative sample. Am. J. Health Behav. 2011;35(1):15–27. doi: 10.5993/ajhb.35.1.2. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., Dickson N., Hancox R.J., Harrington H., Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Nat. Acad. Sci. U.S.A. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E. Childhood self-control predicts adult health, wealth, and crime. Multi-Disciplinary Symposium Improving the Well-Being of Children and Youth; Copenhagen; 2012. [Google Scholar]
- Monsell S. Task switching. Trends Cogn. Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Moody D.E. Can intelligence be increased by training on a task of working memory? Intelligence. 2009;37:327–328. [Google Scholar]
- Moreau D., Conway A.R.A. Cognitive enhancement: a comparative review of computerized and athletic training programs. Int. Rev. Sport Exerc. Psychol. 2013;6:155–183. [Google Scholar]
- Moreau D., Conway A.R.A. The case for an ecological approach to cognitive training. Trends Cogn. Sci. 2014;18:334–336. doi: 10.1016/j.tics.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Moreau D., Morrison A.B., Conway A.R.A. An ecological approach to cognitive enhancement: complex motor training. Acta Psychol. 2015;157:44–55. doi: 10.1016/j.actpsy.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Morrison A.B., Chein J.M. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon. Bull. Rev. 2011;18(1):46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Morrison F.J., Ponitz C.C., McClelland M.M. Self-regulation and academic achievement in the transition to school. In: Calkins S.D., Bell M., editors. Child Development at the Intersection of Emotion and Cognition. American Psychological Association; Washington, DC: 2010. pp. 203–224. [Google Scholar]
- Moul J.L., Goldman B., Warren B. Physical activity and cognitive performance in the older population. J. Aging Phys. Activ. 1995;3:135–145. [Google Scholar]
- Muraven M. Building self-control strength: practicing self-control leads to improved self-control performance. J. Exp. Soc. Psychol. 2010;46:465–468. doi: 10.1016/j.jesp.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.C., Dweck C.S. A culture of genius: how an organization's lay theories shape people's cognition, affect, and behavior. Pers. Soc. Psychol. Bull. 2010;36:283–296. doi: 10.1177/0146167209347380. [DOI] [PubMed] [Google Scholar]
- Murray N., Sujan H., Hirt E.R., Sujan M. The influence of mood on categorization: a cognitive flexibility interpretation. J. Pers. Soc. Psychol. 1990;59:411–425. [Google Scholar]
- Naseem S., Chaudhary B., Collop N. Attention Deficit Hyperactivity Disorder in adults and obstructive sleep apnea. Am. Coll. Chest Phys. 2001;119:294–296. doi: 10.1378/chest.119.1.294. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Beyond IQ: youngsters who can focus on the task at hand do better in math. Sci. Am. 2007;(March) [Google Scholar]
- Nobre A.C., Stokes M.G. Attention and short-term memory: crossroads. Neuropsychologia. 2011;49:1391–1392. doi: 10.1016/j.neuropsychologia.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Noice H., Noice T., Staines G. A short-term intervention to enhance cognitive and affective functioning in older adults. J. Aging Health. 2004;16:562–585. doi: 10.1177/0898264304265819. [DOI] [PubMed] [Google Scholar]
- Orpen C. The effect of expectations of managerial job performance: a four-year longitudinal study with South African business managers. J. Soc. Psychol. 1974;93(1):135–136. [Google Scholar]
- Oswald W.D., Gunzelmann T., Rupprecht R., Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: the SimA study in a 5-year perspective. Eur. J. Aging. 2006;3:179–192. doi: 10.1007/s10433-006-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.C., Gutchess A.H., Meade M.L., Stine-Morrow E.A.L. Improving cognitive function in older adults: nontraditional approaches. J. Gerontol., Ser. B: Psychol. Sci. Soc. Sci. 2007;62:45–52. doi: 10.1093/geronb/62.special_issue_1.45. [DOI] [PubMed] [Google Scholar]
- Pesce C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J. Sport Exerc. Psychol. 2012;34:766–786. doi: 10.1123/jsep.34.6.766. [DOI] [PubMed] [Google Scholar]
- Pesce C., Crova C., Marchetti M., Struzzolino I., Masci I., Vannozzi G., Forte R. Searching for cognitively optimal challenge point in physical activity for children with typical and atypical motor development. Mental Health Phys. Activ. 2013;6:172–180. [Google Scholar]
- Prakash R.S., Voss M.W., Erickson K.I., Kramer A.F. Physical activity and cognitive vitality. Ann. Rev. Psychol. 2015;66:769–795. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- Rabipour S., Raz A. Training the brain: fact and fad in cognitive and behavioral remediation. Brain Cogn. 2012;79:159–179. doi: 10.1016/j.bandc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Raichlen D.A., Foster A.D., Gerdeman G.L., Seillier A., Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner's high’. J. Exp. Biol. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- Raver C.C., Jones S.M., Li-Grining C., Zhai F., Bub K., Pressler E. CSRP's impact on low-income preschoolers’ preacademic skills: self-regulation as a mediating mechanism. Child Dev. 2011;82:362–378. doi: 10.1111/j.1467-8624.2010.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio C.A., Gomes H. Interventions for executive function deficits in children and adolescents. Appl. Neuropsychol.: Child. 2013;2:133–140. doi: 10.1080/21622965.2013.748383. [DOI] [PubMed] [Google Scholar]
- Richmond L.L., Morrison A.B., Chein J.M., Olson I.R. Working memory training and transfer in older adults. Psychol. Aging. 2011;26:813–822. doi: 10.1037/a0023631. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A., Carlson R.A., Gilmore R.O. Acquisition of intellectual and perceptual-motor skills. Ann. Rev. Psychol. 2001;52:453–470. doi: 10.1146/annurev.psych.52.1.453. [DOI] [PubMed] [Google Scholar]
- Roth R.H., Tam S.Y., Ida Y., Yang J.X., Deutch A.Y. Stress and the mesocorticolimbic dopamine systems. Ann. N.Y. Acad. Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Checa P., Cómbita L.M. Enhanced efficiency of the executive attention network after training in preschool children: immediate changes and effects after two months. Dev. Cogn. Neurosci. 2012;2:S192–S204. doi: 10.1016/j.dcn.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proc. Nat. Acad. Sci. U.S.A. 2005;102:14931–14935. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M.J., English A.W. Pathways mediating activity-induced enhancement of recovery from peripheral nerve injury. Exerc. Sport Sci. Rev. 2015;43:163–171. doi: 10.1249/JES.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M.M., Young L.J., Plotsky P.M., Insel T.R. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunamäki T., Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol. Scand. 2007;115(1):1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- Savage R., Cornish K., Manly T., Hollis C.P. Cognitive processes in children's reading and attention: the role of working memory, divided attention, and response inhibition. Br. J. Psychol. 2006;97:365–385. doi: 10.1348/000712605X81370. [DOI] [PubMed] [Google Scholar]
- Schoofs D., Wolf O.T., Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav. Neurosci. 2009;123:1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Scudder M.R., Lambourne K., Drollette E.S., Herrmann S.D., Washburn R.A., Donnelly J.E., Hillman C.H. Aerobic capacity and cognitive control in elementary school-age children\ Med. Sci. Sports Exerc. 2014;46:1025–1035. doi: 10.1249/MSS.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlmeier P., Eberth J., Schwarz M., Zimmermann D., Haarig F., Jaeger S., Kunze S. The psychological effects of meditation: a meta-analysis. Psychol. Bull. 2012;138:1139–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Shea J.B., Morgan R.L. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J. Exp. Psychol.: Hum. Learn. Mem. 1979;5:179–187. [Google Scholar]
- Shipstead Z., Harrison T.L., Engle R.W. Working memory capacity and the scope and control of attention. Atten. Percept. Psychophys. 2015;77:1863–1880. doi: 10.3758/s13414-015-0899-0. [DOI] [PubMed] [Google Scholar]
- Shipstead Z., Hicks K.L., Engle R.W. Cogmed working memory training: does the evidence support the claims? J. Appl. Res. Mem. Cogn. 2012;1:185–193. [Google Scholar]
- Sibley B.A., Etnier J.L. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr. Exerc. Sci. 2003;15:243–256. [Google Scholar]
- Smiley-Oyen A.L., Lowry K.A., Francois S.J., Kohut M.L., Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann. Behav. Med. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh-Bohmer K., Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smithrim K., Upitis R. Learning through the arts: lessons of engagement. Can. J. Educ. 2005;28:109–127. [Google Scholar]
- Snowden M., Steinman L., Mochan K., Grodstein F., Prohaska T.R., Thurman D.J., Anderson L.A. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practices and research. J. Am. Geriatr. Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- Söderqvist S., Bergman Nutley S., Ottersen J., Grill K.M., Klingberg T. Computerized training of non-verbal reasoning and working memory in children with intellectual disability. Front. Hum. Neurosci. 2012;6(271):1–8. doi: 10.3389/fnhum.2012.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Smith M., Klingberg T. Benefits of a working memory training program for inattention in daily life: a systematic review and meta-analysis. PLoS ONE. 2015;10(3):e0119522. doi: 10.1371/journal.pone.0119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer L., Chavan C.F., Manuel A.L. Training-induced behavioural and brain plasticity in inhibitory control. Front. Hum. Neurosci. 2013;7:427. doi: 10.3389/fnhum.2013.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C.M., Aronson J. How stereotypes influence the standardized test performance of talented African American students. In: Jencks C., Phillips M., editors. The Black-White Test Score Gap. Brookings Institution; Washington, DC: 1998. pp. 401–427. [Google Scholar]
- Stepankova H., Lukavsky J., Buschkuehl M., Kopecek M., Ripova D., Jaeggi S.M. The malleability of working memory and visuospatial skills: a randomized controlled study in older adults. Dev. Psychol. 2014;50:1049–1059. doi: 10.1037/a0034913. [DOI] [PubMed] [Google Scholar]
- Stothart C.R., Simons D.J., Boot W.R., Kramer A.F. Is the effect of aerobic exercise on cognition a placebo effect? PLoS ONE. 2014;9(10):e109557. doi: 10.1371/journal.pone.0109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner D.L. The effects of exercise programs on cognition in older adults: a review. Clin. J. Sport Med. 2009;19(5):438. doi: 10.1097/JSM.0b013e3181b9d86a. [DOI] [PubMed] [Google Scholar]
- Tangney J.P., Baumeister R.F., Boone A.L. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J. Pers. 2004;72:271–322. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Thorell L.B., Lindqvist S., Bergman N., Bohlin G., Klingberg T. Training and transfer effects of executive functions in preschool children. Dev. Sci. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Tomporowski P.D., Davis C., Miller P.H., Naglieri J.A. Exercise and children's intelligence, cognition, and academic achievement. Educ. Psychol. Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomporowski P.D., McCullick B., Pendleton D.M., Pesce C. Exercise and children's cognition: the role of exercise characteristics and a place for metacognition. J. Sport Health Sci. 2015;4(1):47–55. [Google Scholar]
- Trulson M.E. Martial arts training: a novel “cure” for juvenile delinquency. Hum. Relations. 1986;39:1131–1140. [Google Scholar]
- Tseng C.N., Gau B.S., Lou M.F. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J. Nurs. Res. 2011;19:119–130. doi: 10.1097/JNR.0b013e3182198837. [DOI] [PubMed] [Google Scholar]
- Tuckman B.W., Hinkle J.S. An experimental study of the physical and psychological effects of aerobic exercise on schoolchildren. Health Psychol. 1986;5:197–207. doi: 10.1037//0278-6133.5.3.197. [DOI] [PubMed] [Google Scholar]
- Twenge J.M., Catanese K.R., Baumeister R.F. Social exclusion causes self-defeating behavior. J. Pers. Soc. Psychol. 2002;83:606–615. doi: 10.1037//0022-3514.83.3.606. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A., Liefooghe B., Verbruggen F. Task switching: interplay of reconfiguration and interference control. Psychol. Bull. 2010;136:601–626. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- van Uffelen J.G.Z., Chinapaw M.J.M., Hopman-Rock M., van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin. J. Sport Med. 2008;18:486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- Vazou S., Smiley-Oyen A.L. Moving and academic learning are not antagonists: acute effects on executive function and enjoyment. J. Sport Exerc. Psychol. 2014;36:474–485. doi: 10.1123/jsep.2014-0035. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C., Godde B., Staudinger U.M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011;5:26–37. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bastian C.C., Oberauer K. Distinct transfer effects of training different facets of working memory capacity. J. Mem. Lang. 2013;69(1):36–58. [Google Scholar]
- von Hecker U., Meiser T. Defocused attention in depressed mood: evidence from source monitoring. Emotion. 2005;5:456–463. doi: 10.1037/1528-3542.5.4.456. [DOI] [PubMed] [Google Scholar]
- Voss M.W., Erickson K.I., Prakash R.S., Chaddock L., Kim J.S., Alves H., Kramer A.F. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Nagamatsu L.S., Liu-Ambrose T., Kramer A.F. Exercise, brain, and cognition across the lifespan. J. Appl. Physiol. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L.S. MIT Press; Cambridge, MA: 1986. Thought and Language. [Google Scholar]
- Wachob D., Lorenzi D.G. Brief report: influence of physical activity on sleep quality in children with Autism. J. Autism Dev. Disord. 2015;45:2641–2646. doi: 10.1007/s10803-015-2424-7. [DOI] [PubMed] [Google Scholar]
- Wass S., Porayska-Pomsta K., Johnson M.H. Training attentional control in infancy. Curr. Biol. 2011;21:1–5. doi: 10.1016/j.cub.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Lord S.R. Effects of group exercise on cognitive functioning and mood in older women. Aust. N. Z. J. Public Health. 1997;21:45–52. doi: 10.1111/j.1467-842x.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Williamson D., Dewey A., Steinberg H. Mood change through physical exercise in nine-to ten-year-old children. Percept. Mot. Skills. 2001;93(1):311–316. doi: 10.2466/pms.2001.93.1.311. [DOI] [PubMed] [Google Scholar]
- Willis S.L., Tennstedt S.L., Marsiske M., Ball K., Elias J., Koepke K.M. Long-term effects of cognitive training on everyday functional outcomes in older adults. J. Am. Med. Assoc. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S.A., Bick-Sander A., Fabel K., Leal-Galicia P., Tauber S., Ramirez-Rodriguez G., Kempermann G. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010;8(1):12–25. doi: 10.1186/1478-811X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.Y., Ho K.H., Chen H.C., Chien M.Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J. Physiother. 2012;58:157–163. doi: 10.1016/S1836-9553(12)70106-6. [DOI] [PubMed] [Google Scholar]
- Ybarra O., Winkielman P., Yeh I., Burnstein E., Kavanagh L. Friends (and sometimes enemies) with cognitive benefits: what types of social interactions boost cognitive functioning? Soc. Psychol. Pers. Sci. 2011;2:253–261. [Google Scholar]
- Youssef N.A., Ege M., Angly S.S., Strauss J.L., Marx C.E. Is obstructive sleep apnea associated with ADHD? Ann. Clin. Psychiatry. 2011;23:213–224. [PubMed] [Google Scholar]