Abstract
Purpose
Optimal management of colonoscopic perforation (CP) is controversial because early diagnosis and prompt management play critical roles in morbidity and mortality. Herein, we evaluate the outcomes and clinical characteristics of patients with CP according to treatment modality to help establish guidelines for managing CP.
Methods
Our retrospective analysis included 40 CP patients from January 1, 2003, to December 31, 2014. Patients with CP were categorized into 2 groups according to therapeutic modality: operation (surgery) and nonoperation (endo-luminal clip application or conservative treatment) groups.
Results
The postoperative morbidity rate was 40%, and no mortalities were noted. The incidence of abdominal pain and tenderness in patients who received only conservative management was significantly lower than in those who underwent surgery (P < 0.001 and P = 0.004, respectively). Patients tended to undergo surgery more often for diagnosis times longer than 24 hours and for diagnostic CPs. The mean hospital stays for the operation and nonoperation groups were 14.6 ± 7.77 and 5.9 ± 1.62 days, respectively (P < 0.001). Compared to the operation group, the nonoperation group began intake of liquid diets significantly earlier after perforation (3.8 ± 1.32 days vs. 5.6 ± 1.25 days, P < 0.001) and used antibiotics for a shorter duration (4.7 ± 1.29 days vs. 8.7 ± 2.23 days, P < 0.001).
Conclusion
The time of diagnosis and the injury mechanism may be useful indications for conservative management. Nonoperative management, such as endo-luminal clip application, might be beneficial, when feasible, for the treatment of patients with CP.
Keywords: Colonoscopy, Perforation, Management
INTRODUCTION
Colonoscopy is the most effective diagnostic tool for colorectal lesions because it provides direct visualization of the colorectal mucosa; it is also the standard procedure for screening of colorectal cancer [1]. Because early detection and removal of colorectal polyps are crucial to preventing colorectal cancer, the volume of colonoscopy in therapy has increased over the years [2].
Colonoscopy demands a more delicate handling technique than gastroscopy because the colon is a relatively long and redundant hollow viscus, which makes inserting the scope into the ileocecal valve difficult. Therefore, endoscopists need to complete training for a sufficient period and achieve a satisfactory learning curve in order to perform colonoscopy. Although colonoscopy is performed by an experienced endoscopist, the insertion of the scope may be difficult owing to redundancy or acute angulation. In addition, recently, as the volume of colonoscopy has increased, the number of cases of therapeutic procedures, such as an endoscopic mucosal resection (EMR) and an endoscopic submucosal dissection (ESD), has also increased; therefore, procedure-related complications, whether diagnostic or therapeutic, may occur [3,4].
Although colonoscopy is a safe procedure, lethal complications such as perforation and bleeding may occur during the procedure. Colonoscopic perforation (CP) is one of the most serious complications and can lead to leakage of bowel content into the peritoneal cavity and eventually to sepsis. Thus, early diagnosis and prompt management play a critical role in morbidity and mortality due to CP.
A large population-based study reported the incidence of CP to be 0.016%–0.095% [5]. Emergency surgery is the definitive treatment modality for patients with CPs. However, some controversies have surrounded the management of patients with CPs, considering the relatively high rates of postoperative mortality and morbidity [6,7,8,9]. Thus, nonoperative management has been suggested as an alternative treatment modality in selected patients. Endo-luminal clip application and conservative management with intravenous antibiotics are such alternatives for the management of patients with CPs, although the indications for their use are not yet well established [4,10,11]. Therefore, in the present study, we evaluated the clinical characteristics of patients with CPs and assessed the outcomes according to the treatment modality in order to help establish guidelines for the management of patients with CPs in an emergency situation.
METHODS
We conducted a retrospective analysis including 40 CP patients with 14 perforations who were referred from other institutions from January 1, 2003, to December 30, 2014. Colonoscopy was performed by gastroenterologists, gastroenterologist trainees, and surgeons. Data, including patient demographics, indications for colonoscopy, clinical presentations, physical findings, time to diagnosis, diagnostic tool, type of procedure, mechanism of perforation, treatment modality, type of surgery, and clinical outcomes, were collected by reviewing medical charts. The comorbidity status of the patients was described using the guidelines of the American Society of Anesthesiologists. The initial evaluation after perforation included history taking, physical examination, and examination for fever, leukocytosis, and shock. The following definitions were used: fever, temperature > 38.0°C; leukocytosis, white blood cell count > 10,000/mm3; shock, systolic blood pressure < 90 mmHg. Time to diagnosis was divided into 3 periods: during the colonoscopy, < 24 hours after the colonoscopy and > 24 hours after the colonoscopy.
The diagnosis of perforation was made based on clinical evidence, such as a colonic wall defect found during the colonoscopy, or radiologic evidence, such as detection of free air on simple radiography or computed tomography (CT). The mechanism of perforation was classified as trauma-related (direct trauma or torque from the scope) or polypectomy-related (EMR, ESD, and hot biopsy). Based on the treatment modality, the patients were categorized into either an operation or a nonoperation group. The clinical outcomes were evaluated on the basis of when liquid diet was started after surgery or CP, the length of antibiotics use, postoperative complications, and length of hospital stay. Intergroup comparisons were made using the Student t-test for continuous variables and the two-tailed chi-square test for discrete variables. Data with low expected counts were assessed using Fisher exact test. P-values < 0.05 were considered statistically significant.
RESULTS
The frequency of CP at our institute (n = 26)
From the beginning of 2003 to the end of 2014, a total of 31,708 colonoscopies were performed at our institute, and 26 CPs were detected. The number of colonoscopies performed by gastroen-terologists, trainees, and surgeons showed an increasing trend. The overall perforation rate was 0.083% (26 of 31,708), and the perforation rates for diagnostic and therapeutic colonoscopies were 0.060% (10 of 16,705) and 0.107% (16 of 15,003), respectively.
Patients' demographics and characteristics according to injury mechanism
The mean age of the 40 patients with CPs was 61 years (range: 33–81 years), and the male-to-female ratio was 1.1 (21:19). Patient demographics and characteristics according to injury mechanism are presented in Table 1. The type of procedure leading to a CP was diagnostic colonoscopy in 18 patients (45%) and therapeutic colonoscopy in 22 patients (55%). The average age was higher for patients experiencing a diagnostic perforation, and women more frequently experienced perforations caused by diagnostic colonoscopy. The patients with diagnostic perforations had a tendency to have a history of abdominal surgery, but this observation was not statistically significant. The most common perforation site was the sigmoid colon, and a CP of the sigmoid colon was seen more frequently in patients who had undergone diagnostic colonoscopy (14 of 18, 77.8%). Detection of the CP during the procedure was more common for diagnostic colonoscopy. Leukocytosis was more frequently observed in patients with a therapeutic CP. The diagnostic CP patients tended to undergo surgery more frequently than the therapeutic CP patients, but statistical significance was not demonstrated (Table 1).
Table 1. Characteristics and presentations of patients with a colonoscopic perforation according to mechanism.
Values are presented as number (%) or mean±standard deviation unless otherwise indicated.
ASA, American Society of Anesthesiologists; GI, gastrointestinal.
aAbdominal pain, hematochezia. bDiverticulum, colitis.
Clinical presentations and diagnosis of CP
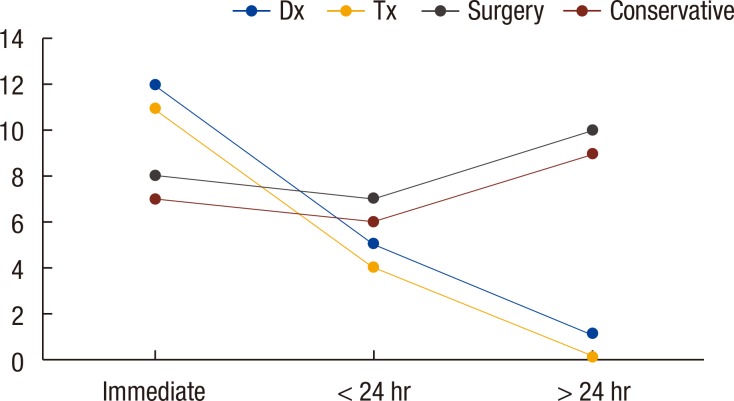
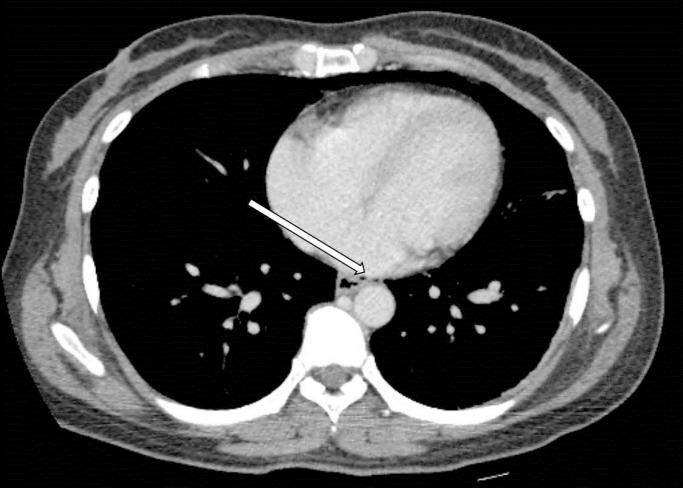
The diagnosis of CP was made within 24 hours of the procedure in 30 patients (75%), of whom 19 (19 of 30, 63.3%) were diagnosed with CP during the procedure. In 10 patients (25%), the diagnosis of CP was made more than 24 hours after the procedure, and in 9 of those patients, CP was related to therapeutic colonoscopy (Fig. 1). Free air was detected on simple abdomen or chest radiography in 26 of the 40 patients with CPs (65%). Ten of the 40 patients (25%) were eventually diagnosed with CP after CT. In one patient, abdominal pain was sustained after colonoscopy. No free air was seen in the simple radiography, and follow-up sigmoidoscopy could not identify the site of perforation due to poor bowel preparation. Finally, a sigmoid mesenteric perforation was diagnosed by using CT. Nine of the 40 patients with CPs (22.5%) had no specific symptoms, such as abdominal pain or distension, after CP. In eight of the nine asymptomatic patients, the perforation was found during the procedure (colonoscopy), and in one of those patients, the endoscopic gross finding showed a suspected malignancy, so abdomino-pelvic CT was obtained after colonoscopy for a cancer work up. Fever, leukocytosis, abdominal distension, tenderness, rebound tenderness, and muscle guarding were noted in 13 (32.5%), 16 (40%), 22 (55%), 27 (67.5%), 9 (22.5%), and 5 patients (12.5%), respectively. Two patients developed shock after perforation, and three patients showed pneumomediastinum (Fig. 2).
Fig. 1. Comparisons of diagnostic (Dx) vs. therapeutic (Tx) colonoscopic perforation and of surgery vs. conservative management according to time.
Fig. 2. Computed tomographic scan of the chest (axial image) shows air in the mediastinum (pneumomediastinum) (arrow).
Management of CP patients
Twenty-five patients (62.5%) were managed operatively and 15 patients (37.5%) nonoperatively (Table 2). When the clinical presentations were compared between the operation and the nonoperation groups, sigmoid colon perforation was frequently noted in the operation group (18 of 25, 72%), but no rectal perforations were noted. On the other hand, in the nonoperation group, sigmoid colon perforations and rectal perforations were observed in 4 (4 of 15, 26.7%) and 6 (6 of 15, 40.0%) of the patients, respectively (P < 0.001), and the incidence of peritoneal signs was significantly higher in the operation group (P < 0.001). Diagnostic CP patients tended to undergo surgery more often, but this observation did not achieve statistical significance, and surgery was more frequently used for treatment when the diagnosis was made more than 24 hours after the colonoscopy.
Table 2. Clinical presentations and outcomes after colonoscopic perforation according to the therapeutic groups (%).
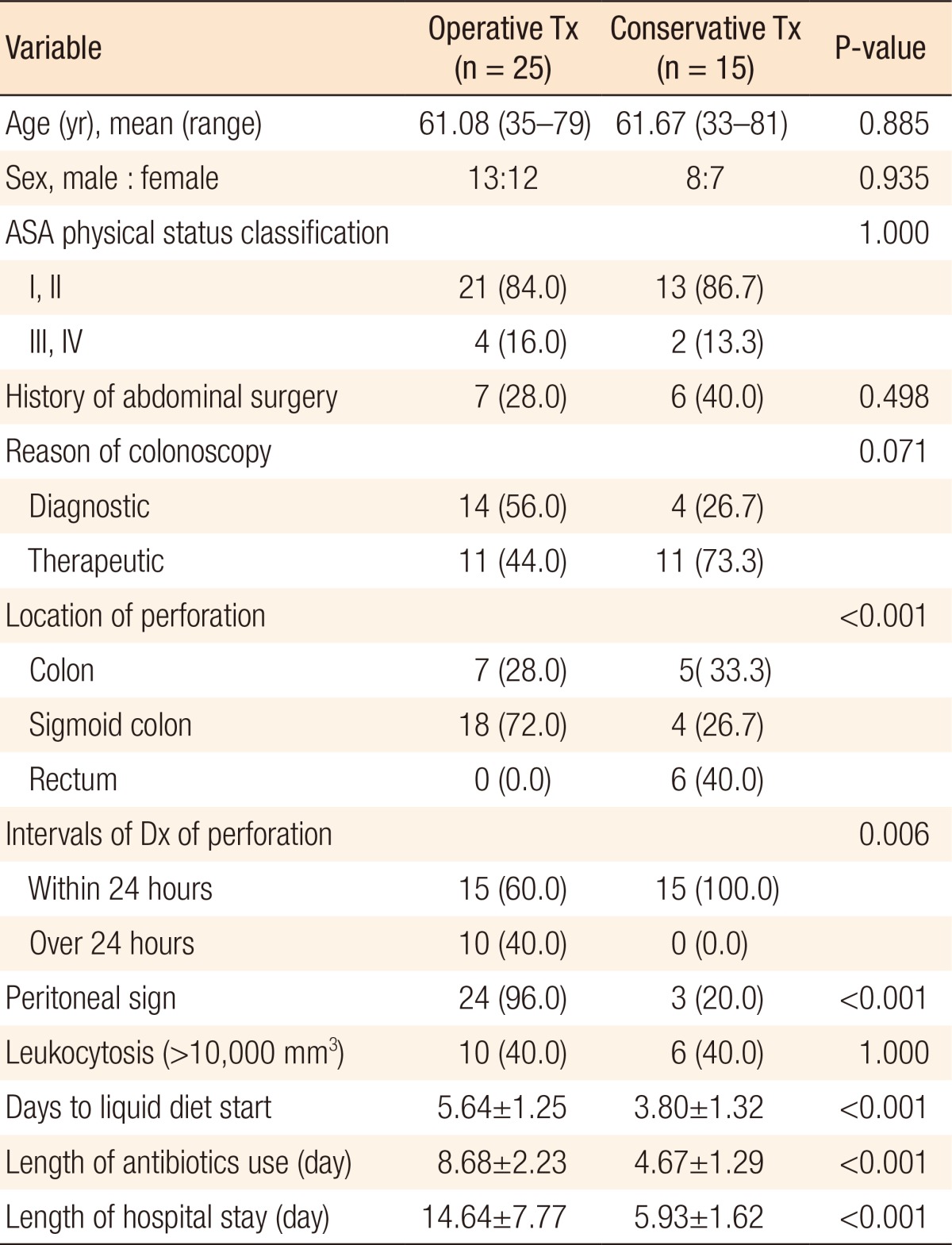
Values are presented as number (%) or mean±standard deviation unless otherwise indicated.
Tx, treatment; ASA, American Society of Anesthesiologists; Dx, diagnosis.
Among the patients who underwent surgery, 21 patients (21 of 25, 84%) showed an antimesenteric perforation and 4 (16%) showed a mesenteric perforation. The sizes of the perforations ranged from 0.2 to 5 cm, with a mean (standard deviation) of 1.55 (±1.38) cm. All patients referred from other institutes (n = 14) underwent surgery. The types of surgery performed in patients with CPs are listed in Table 3. Over half the surgeries performed were primary repair, but bowel resection and anastomosis were performed in 7 patients (7 of 25, 28%). Stoma formation was performed in two patients (2 of 25, 8%) with fecal peritonitis (Hartmann operation and primary repair with ileostomy, respectively).
Table 3. Surgical treatment characteristics according to the time to diagnosis (n = 25).
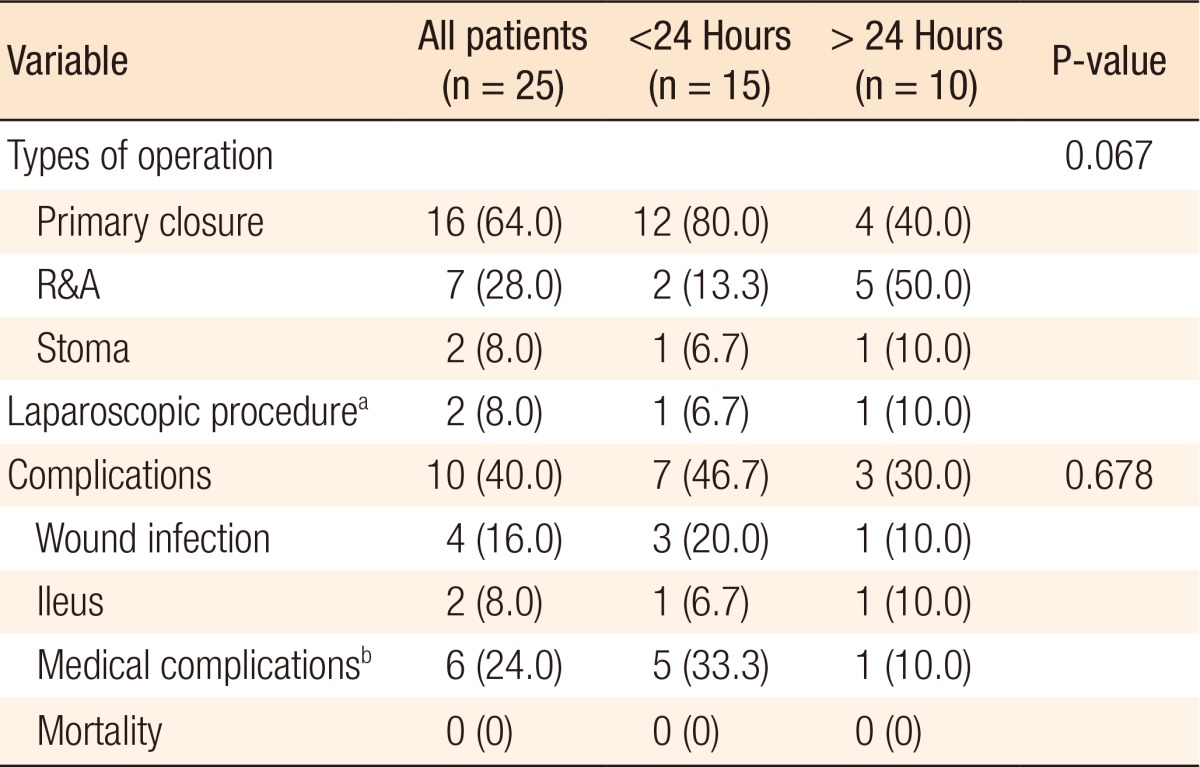
Values are presented as number (%).
R&A, resection and anastomosis.
aLaparoscopic linear stapling repair, laparoscopic segmental resection and anastomosis. bPneumonia, asthma aggravation, pulmonary thromboembolism.
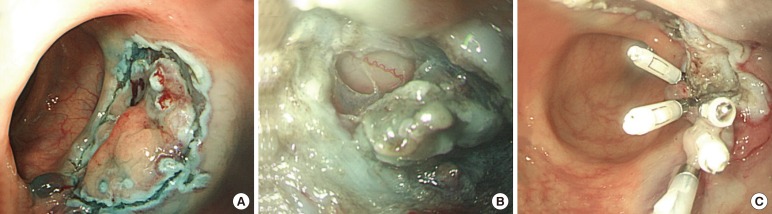
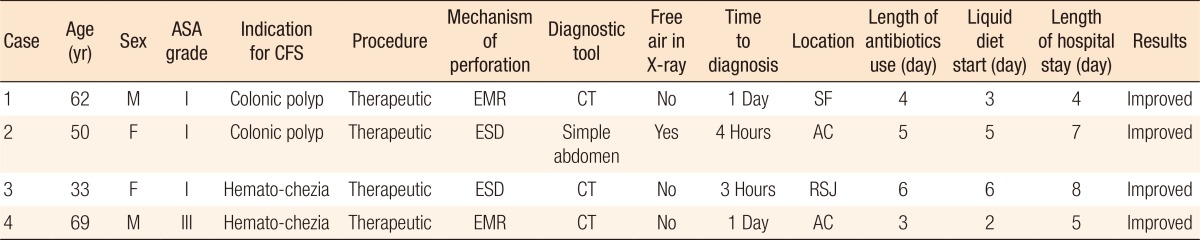
Conservative management with endo-luminal clip application was performed in 11 of the 40 CP patients (27.5%), all of whom were diagnosed during the colonoscopic procedure and for whom the status of bowel preparation was clear. The mean size (range) of the perforation was 0.9 cm (0.2–2.5 cm), and the mean number (range) of applied clips was 5.5 (3–9) (Fig. 3). Conservative management without endo-luminal clip application was performed in 4 of the 40 CP patients (10%). After the diagnosis of CP, the patients were managed with intravenous broad-spectrum antibiotics and no oral intake for 2–6 days (Table 4).
Fig. 3. Endo-luminal clip application. (A) An approximately 2 × 3-cm-sized, flat, nodular lesion at the sigmoid colon is removed by using an endoscopic submucosal dissection (ESD). (B) After ESD, an approximately 0.3-cm-sized perforation is seen at the margin of the ESD. (C) The defect is closed using hemoclips.
Table 4. Clinical features in 4 patients who underwent conservative management.
ASA, American Society of Anesthesiologists; CFS, colonofiberscopy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; CT, computed tomography; SF, splenic flexure; AC, ascending colon; RSJ, rectosigmoid junction.
Outcomes of CP patients
The postoperative morbidity rate was 40% (10 of 25), and no mortalities were noted. Wound infection was the most common complication (4 patients), followed by pneumonia (3 patients), ileus (2 patients), and pulmonary thromboembolism (1 patient). All the complications were managed using supportive care. No endo-luminal clip application- or conservative management-related complications were noted. The overall mean hospital stay for patients with CPs was 11.4 days (range, 2–38 days), and the mean length of intravenous antibiotics use was 7.2 days (range, 2–13 days). Liquid diet was initiated on mean postoperative or postcolorectal perforation day 4.9 (range, day 2 to 8).
To analyze the outcomes according to therapeutic modality, we compared the outcomes of the operation group to those of the nonoperation group who underwent endo-luminal clip application or conservative management. The mean lengths of hospital stay for patients in the operation and the nonoperation groups were 14.6 ± 7.77 days and 5.9 ± 1.62 days, respectively; this difference was statistically significant (P < 0.001). Compared to the operation group, the nonoperation group began intake of liquid diets significantly earlier after perforation (3.8 ± 1.32 days vs. 5.6 ± 1.25 days, P < 0.001) and used antibiotics for a shorter duration (4.7 ± 1.29 days vs. 8.7 ± 2.23 days, P < 0.001) (Table 2).
DISCUSSION
Colonoscopy is a standard tool for screening of colorectal cancer and evaluating various colorectal diseases. Although professionals have achieved more experience in performing the colonoscopic procedure and the incidence of CP has shown a decreasing trend, complications associated with the procedure may be inevitable [7,12]. A CP is one of the most serious complications of colonoscopy and can lead to death. The incidence of CP has been reported to be < 1 per 1,000 cases, and in our retrospective study, the rate of CP was 0.083%, which was consistent with the findings of previous studies [5,6,7,13,14,15].
Old age, female sex, pelvic adhesion, colonic obstruction, therapeutic colonoscopy, and endoscopist's experience are some of the risk factors for perforation [5,6,7,12,14,15,16,17]. Because the incidence of colonic lesions demanding endoscopic intervention, such as polyps, is higher in older patients and the mechanical strength of the colonic wall decreases along the aging process, the incidence of CP could be higher in older patients [3,10,18]. The anatomy of the colon differs between men and women. In women, the colon is longer, and the transverse colon is more mobile; therefore, the insertion of the colonoscope may be more difficult in women [19]. The proportion of females was also significantly higher in diagnostic CP patients in this study. A relatively higher incidence of previous abdominal surgery was noted for patients with diagnostic CPs, but this difference was not statistically significant when compared to patients with therapeutic CPs.
A diagnosis of CP can be made during colonoscopy by direct visualization of the extracolonic luminal structure through the perforation site. The size of the perforation caused by mechanical force during diagnostic procedures, such as antimesenteric tearing by an endoscopic shaft, is larger than that caused by therapeutic procedures such as a polypectomy. The small-sized perforation or a delayed perforation associated with an intervention may not be detected during the procedure. A delayed diagnosis might be associated with the therapeutic procedure [7,10,15,17]. In our study, 9 of 10 patients who were diagnosed with a perforation more than 24 hours after the procedure had a therapeutic colonoscopy-associated perforation. Because of their relatively large sizes, diagnostic CPs were found more frequently during the procedure. Consequently, in the therapeutic CP patients, the time until surgery was delayed, and a high incidence of leukocytosis was noted.
The most common presentation of a CP is abdominal pain during or after colonoscopy. Abdominal pain accompanied by peritoneal irritation signs, such as muscle guarding, tenderness, and rebound tenderness, may be useful for making a diagnosis of perforation. Once peritoneal soilage by colonic content induces diffuse peritonitis, peritoneal irritation signs may be evident. However, if the perforated lesion is covered with omentum or other fat tissue, diffuse peritoneal irritation signs or abdominal pain may not be evident, although localized pain and tenderness may occur [12]. Thus, we assume that the perforation site may have been sealed off by omentum or other fat tissue in patients with a CP who received conservative management. In the present study, one patient had undergone an appendectomy 10 years earlier and had concurrent rectosigmoid junction perforation and bleeding. Postoperative adhesion of the sigmoid colon and cecum may have led to acute angulation of the colon, thereby resulting in perforation and bleeding caused by tearing of the vasa recta during scope insertion.
The most common site of perforation is the sigmoid colon, followed by cecum, which can be explained by the anatomical relation between the fixed rectum and the descending colon [7,8,10,12]. A stretching force can be induced in the antimesenteric side of the sigmoid colon during insertion of a scope to form a sigmoid loop owing to the sharp angulation at the rectosigmoid or sigmoid descending colon junction, and the mobile sigmoid colon. Bowel adhesion caused by a previous pelvic infection or surgery might be a precipitating factor for the perforation [5,7,15]. In this study, the sigmoid colon was the most common perforation site, and the incidence of CPs in the sigmoid colon was higher in diagnostic CP patients than it was in therapeutic CP patients
The morbidity and the mortality rates of CP have been reported to be 31%–48.7% and 8.2%–25.6%, respectively [6,7,8,9,10,15]. In the present study, the morbidity rate was 40%, which was in accordance with previous reports, and no mortalities occurred. Once the diagnosis of CP is made, prompt management should be initiated to prevent perforation-related morbidity and mortality. Although surgical management is a definite treatment modality for a patient with a CP, the relevant postoperative morbidity, mortality, and general anesthesia-related risks are considerable. Thus, the ideal treatment modality for a patient with a CP has been a controversial issue.
Surgical management has been the mainstay for the treatment of patients with CPs and can be performed in a patient with definite peritoneal irritation signs, concurrent colonic lesions such as colorectal cancer, and clinical deterioration after conservative management. The surgical approach is selected according to the degree of peritoneal contamination, the hemodynamic stability, the size of the perforation, the presence of concurrent colorectal lesions, and the surgeon's experience [15]. Primary repair is the simplest surgical method and can be performed under the following conditions: minimal peritoneal contamination, small perforation size, and the absence of a colonic lesion requiring resection. This method can be performed for more than half the CP cases, thereby minimizing the operation time and risk of postoperative leakage [7,8,10,17]. Moreover, primary repair can be performed by a surgeon without much experience with colorectal surgery. Colonic resection and primary anastomosis may be the surgical choice for patients with minimal peritoneal contamination and large perforation size. When severe inflammatory changes occur in the perforated colon and are accompanied by extensive peritoneal contamination, stoma formation may be necessary [12,19]. Hartmann operation is a representative surgical method. Because this operation can be performed safely without a risk of anastomosis site leakage, it has been performed in critically ill patients with a colonic perforation. However, secondary surgery to reverse a colostomy might lead to the use of general anesthesia, which is associated with risks and postoperative morbidity, particularly in elderly patients. Thus, the optimal surgical option for patients with severe peritoneal contamination has been a controversial issue. In our study, primary repair was most commonly performed, and the stoma-forming procedure was performed in 2 patients with fecal peritonitis. Wound infection was the most common postoperative complication, followed by pneumonia, ileus, and pulmonary thromboembolism, which were treated by conservative management without mortality.
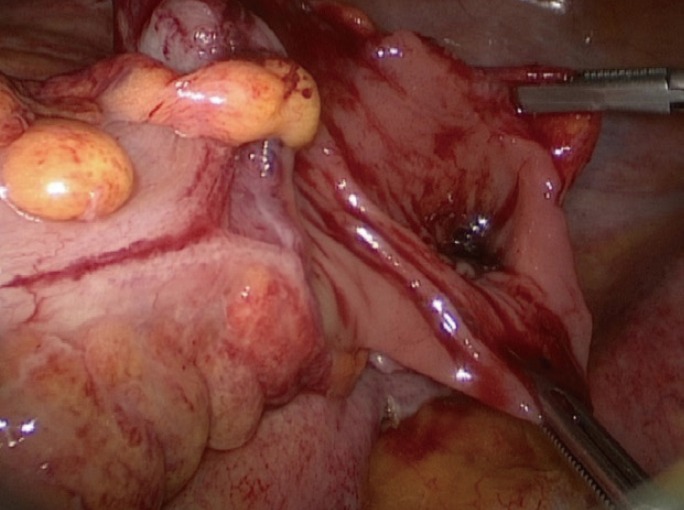
As the advantage of laparoscopic surgery is well known and laparoscopic colorectal surgery is widely performed, the laparoscopic approach has been used for the treatment of patients with a CP [20,21]. Small perforation size, minimal peritoneal contamination, and no colonic lesion requiring resection might be suitable indications for a laparoscopic approach such as an intracorporeal suture or laparoscopic linear stapling repair, although a laparoscopic colonic resection may also be feasible. However, if the perforation site cannot be found or an uncertainty exists regarding secure perforation-site repair, open surgery should be considered. In the present study, only 2 patients were treated by using laparoscopic surgery (laparoscopic linear stapling repair and laparoscopic segmental resection and anastomosis) because of the lack of experience with using laparoscopic surgery to repair a CP (Fig. 4). Although the selection criteria for the laparoscopic approach have not been well established, the incidence of laparoscopic surgery for treating a patient with a CP might be increasing, as is the case with colorectal cancer surgery.
Fig. 4. Laparoscopic operative finding of a sigmoid colon disruption caused by colonoscopy.
Conservative management with intravenous fluid, bowel rest, and intravenous antibiotics may be another option for the treatment of patients with CPs and is reserved for carefully selected patients [4,12,15,22]. Although no definite selection criteria exist for selecting patient with CPs to be treated by using conservative management, patients with clear bowel preparation, minimal peritoneal irritation signs, and a good general condition should be considered. In our study, of the 4 patients treated with conservative management, one patient showed free air on simple radiography, and all patients were discharged from the hospital without any morbidity or mortality. On comparing the clinical presentations between the operation group and the patients who underwent conservative management, the incidence of abdominal pain and tenderness was significantly lower in the patients who underwent conservative management (P < 0.001 and P = 0.004, respectively). Thus, the absence of abdominal pain and tenderness after a CP, along with bowel preparation status, other peritoneal irritation signs, and the general condition of the patient, may be indications for conservative management.
Endo-luminal clip application is a representative endoscopic repair method for the treatment of a patient with an iatrogenic colon perforation. Although apposition of the full layer of the colon is limited by the endo-clip, a previous study in an animal model showed that apposition of the mucosal and the submucosal layers of the colonic wall by using an endo-clip was sufficient for wound healing of the perforation site [23]. Since the first report of endo-luminal clip application by Yoshikane et al., iatrogenic colonic perforations treated by using endoscopic repair have had success rates of 69%–83% [24,25]. The guidelines for endo-luminal clip application have not been well established, and a few studies have suggested that its use may be feasible in patients with a perforation size < 10 mm (or less than the width of the opened branches of the endo-clip), clear bowel preparation status, and a diagnosis of CP made during the colonoscopic procedure [11,12,26]. Although a perforation size of approximately 10 mm is suited for endo-luminal clip application, a larger-sized linear perforation may be an indication, as was the case in our study [25,27]. In our study, endo-luminal clip application was performed by 2 gastroenterologists and did not fail in any of the patients; additionally, no endo-luminal repair-associated complications were noted. The mean size of the perforations was 0.9 cm (0.2–2.5 cm), and the mean number (range) of applied clips was 5.5 (3–9). Endo-luminal clip application has become an alternative treatment modality to conservative or surgical management for patients with a CP. However, because endo-luminal clip application demands a skillful and experienced endoscopist and has a learning curve, endos-copists will have to master the procedure in order to prepare for emergency situations involving patients with CPs.
On comparing the clinical outcomes of the operation and the nonoperation groups, the nonoperation group showed better results, as expected. Although surgical management could provide definite treatment for patients with CPs, when the risk associated with general anesthesia, post-operative complications, legal problems, and hospital cost are taken into account, nonoperative management is logically the preferred modality, if indicated. On the other hand, a failure in conservative management could result in delayed treatment, thereby causing morbidity and mortality. Therefore, surgical repair should be considered unless the perforated section is linear, in which case clip application would be easy, even though the perforation had been detected during diagnostic colonoscopy. When the diagnosis is delayed, we consider the surgical approach to be more favorable. If the perforation is detected during therapeutic colonoscopy, an endo-luminal clip can be applied, and good prognostic results were shown in this study. In the case of delayed diagnosis, careful observation of the symptomatic changes caused by using IV antibiotics could be performed in selected CP patients if the initial symptoms are minimal. However, delayed surgery tends to be an extended, rather than a primary, repair due to peritoneal contamination and soilage. Therefore, an experienced surgeon is needed, the surgeon has to be observant for the patients who CP was suspicious, and the surgery should be performed as soon as possible when peritoneal irritation signs are present.
In conclusion, the treatment of patients with CPs has been a controversial issue because of surgery-associated morbidity and mortality, irrespective of whether it is performed as open surgery or laparoscopic surgery. In this study, well-selected patients who had undergone nonoperative management demonstrated good results. Clinical presentations and physical findings, such as abdominal pain and tenderness, are findings that support a therapeutic decision. Furthermore, in our study, whether the perforation was found during the procedure or within 24 hours of colonoscopy or longer, as well as the injury mechanism, may be important clues for conservative management, and endo-luminal clip application might have an appreciable advantage in the treatment of patients with CPs. However, the comparison of clinical presentations for patients with therapeutic CPs was limited due to the small sample size, so further studies are required to clearly delineate the role of and the indications for endo-luminal clip application and conservative management in patients with CPs.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305–311. doi: 10.1016/S0140-6736(05)17784-8. [DOI] [PubMed] [Google Scholar]
- 2.Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906.e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal CW, Chun YS, Farley DR. Colonoscopic perforations: a retrospective review. J Gastrointest Surg. 2005;9:1229–1235. doi: 10.1016/j.gassur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69(3 Pt 2):654–664. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.van der Sluis FJ, Loffeld RJ, Engel AF. Outcome of surgery for colonoscopic perforation. Colorectal Dis. 2012;14:e187–e190. doi: 10.1111/j.1463-1318.2011.02841.x. [DOI] [PubMed] [Google Scholar]
- 7.Teoh AY, Poon CM, Lee JF, Leong HT, Ng SS, Sung JJ, et al. Outcomes and predictors of mortality and stoma formation in surgical management of colonoscopic perforations: a multicenter review. Arch Surg. 2009;144:9–13. doi: 10.1001/archsurg.2008.503. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal CW, Cullinane DC, Schiller HJ, Sawyer MD, Zietlow SP, Farley DR. Surgical management and outcomes of 165 colonoscopic perforations from a single institution. Arch Surg. 2008;143:701–706. doi: 10.1001/archsurg.143.7.701. [DOI] [PubMed] [Google Scholar]
- 9.La Torre M, Velluti F, Giuliani G, Di Giulio E, Ziparo V, La Torre F. Promptness of diagnosis is the main prognostic factor after colonoscopic perforation. Colorectal Dis. 2012;14:e23–e26. doi: 10.1111/j.1463-1318.2011.02755.x. [DOI] [PubMed] [Google Scholar]
- 10.Lohsiriwat V, Sujarittanakarn S, Akaraviputh T, Lertakyamanee N, Lohsiriwat D, Kachinthorn U. Colonoscopic perforation: a report from World Gastroenterology Organization endoscopy training center in Thailand. World J Gastroenterol. 2008;14:6722–6725. doi: 10.3748/wjg.14.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magdeburg R, Collet P, Post S, Kaehler G. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc. 2008;22:1500–1504. doi: 10.1007/s00464-007-9682-1. [DOI] [PubMed] [Google Scholar]
- 12.Panteris V, Haringsma J, Kuipers EJ. Colonoscopy perforation rate, mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy. 2009;41:941–951. doi: 10.1055/s-0029-1215179. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal CW, Cullinane DC, Schiller HJ, Sawyer MD, Zietlow SP, Farley DR. Surgical management and outcomes of 165 colonoscopic perforations from a single institution. Arch Surg. 2008;143:701–706. doi: 10.1001/archsurg.143.7.701. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo-Zúñiga V, Moreno de Vega V, Doménech E, Mañosa M, Planas R, Boix J. Endoscopist experience as a risk factor for colonoscopic complications. Colorectal Dis. 2010;12(10 Online):e273–e277. doi: 10.1111/j.1463-1318.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 15.Mai CM, Wen CC, Wen SH, Hsu KF, Wu CC, Jao SW, et al. Iatrogenic colonic perforation by colonoscopy: a fatal complication for patients with a high anesthetic risk. Int J Colorectal Dis. 2010;25:449–454. doi: 10.1007/s00384-009-0822-z. [DOI] [PubMed] [Google Scholar]
- 16.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418–3422. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 17.Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007;21:994–997. doi: 10.1007/s00464-007-9251-7. [DOI] [PubMed] [Google Scholar]
- 18.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–236. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 19.Cobb WS, Heniford BT, Sigmon LB, Hasan R, Simms C, Kercher KW, et al. Colonoscopic perforations: incidence, management, and outcomes. Am Surg. 2004;70:750–757. [PubMed] [Google Scholar]
- 20.Hansen AJ, Tessier DJ, Anderson ML, Schlinkert RT. Laparoscopic repair of colonoscopic perforations: indications and guidelines. J Gastrointest Surg. 2007;11:655–659. doi: 10.1007/s11605-007-0137-8. [DOI] [PubMed] [Google Scholar]
- 21.Wullstein C, Köppen M, Gross E. Laparoscopic treatment of colonic perforations related to colonoscopy. Surg Endosc. 1999;13:484–487. doi: 10.1007/s004649901018. [DOI] [PubMed] [Google Scholar]
- 22.Na EJ, Kim KJ, Min YD. Safety of conservative treatment of colonoscopic perforation. J Korean Soc Coloproctol. 2005;21:384–389. [Google Scholar]
- 23.Raju GS, Pham B, Xiao SY, Brining D, Ahmed I. A pilot study of endoscopic closure of colonic perforations with endoclips in a swine model. Gastrointest Endosc. 2005;62:791–795. doi: 10.1016/j.gie.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikane H, Hidano H, Sakakibara A, Ayakawa T, Mori S, Kawashima H, et al. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc. 1997;46:464–466. doi: 10.1016/s0016-5107(97)70045-2. [DOI] [PubMed] [Google Scholar]
- 25.Trecca A, Gaj F, Gagliardi G. Our experience with endoscopic repair of large colonoscopic perforations and review of the literature. Tech Coloproctol. 2008;12:315–321. doi: 10.1007/s10151-008-0442-6. [DOI] [PubMed] [Google Scholar]
- 26.Taku K, Sano Y, Fu KI, Saito Y. Iatrogenic perforation at therapeutic colonoscopy: should the endoscopist attempt closure using endoclips or transfer immediately to surgery? Endoscopy. 2006;38:428. doi: 10.1055/s-2006-925248. [DOI] [PubMed] [Google Scholar]
- 27.Barbagallo F, Castello G, Latteri S, Grasso E, Gagliardo S, La Greca G, et al. Successful endoscopic repair of an unusual colonic perforation following polypectomy using an endoclip device. World J Gastroenterol. 2007;13:2889–2891. doi: 10.3748/wjg.v13.i20.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]