Abstract
Mice may now be the preferred animal model for biomedical research due to its anatomical, physiological, and genetic similarity to humans. However, little is known about accentuated antagonism of chronotropic and dromotropic properties in conscious mice. Accordingly, we describe the complex and interacting influence of the autonomic nervous system on cardiac electrophysiology in conscious mice. Specifically, we report the effects of single and combined cardiac autonomic blockade on measurements of pulse interval (heart rate), atrio-ventricular interval, sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle, and Wenckebach cycle length in conscious mice free of the confounding influences of anesthetics and surgical trauma. Autonomic influences were quantified as the change in parameter induced by its selective blocker (Sympathetic or Parasympathetic Effect) or as the difference between the intrinsic value and the value after a selective blocker (Sympathetic or Parasympathetic Tonus). Sympatho-Vagal Balance (SVB) was assessed as the ratio of control interval to intrinsic interval. SVB suggests slight parasympathetic dominance in the control of cardiac electrophysiology intervals. Furthermore, results documents a complex interaction between the sympathetic and parasympathetic divisions of the autonomic nervous system in the control of cardiac electrophysiology parameters. Specifically, the parasympathetic effect was greater than the parasympathetic tonus in the control of cardiac electrophysiology parameters. In contrast, the sympathetic effect was smaller than the sympathetic tonus in the control of cardiac electrophysiology parameters. Results have important implications because actions of pharmacological agents that alter the autonomic control of cardiac electrophysiology are transformed by these interacting mechanisms.
Keywords: sympathetic, parasympathetic, Wenckebach cycle length, sinus node recovery time, atrio-ventricular interval, electrocardiogram
1. INTRODUCTION
Mammalian heart rate and cardiac electrophysiology are profoundly influenced by the sympathetic and parasympathetic divisions of the autonomic nervous system. Heart rate is slowed by parasympathetic nervous system activity via the muscarinic M2 receptor (Fisher et al. 2004) and elevated by sympathetic nervous system activity via the beta 1-adrenergic receptor (Rohrer et al. 1998). In addition, the sympathetic and parasympathetic divisions of the autonomic nervous system alter heart rate and cardiac electrophysiology through complex and interacting mechanisms.
Sympathetic stimulation has similar effects on both atrial and ventricular electrophysiology and is pro-arrhythmic for both chambers (Shen and Zipes 2014; Kapa et al. 2010). In particular, beta-adrenergic receptor stimulation, by increasing intracellular cAMP levels, increases heart rate, atrial-ventricular (A-V) nodal conduction, and contractile force while shortening atrial and ventricular refractoriness. Furthermore, beta-adrenergic stimulation enhances the development of after-depolarizations and triggered beats (Engel 1978; Schwartz et al. 1993; Wharton et al. 1992; Zipes 1991).
In contrast to sympathetic stimulation which has similar effects on atrial and ventricular electrophysiology, parasympathetic stimulation has opposing effects on these chambers. Specifically, parasympathetic stimulation prolongs ventricular action potential duration and the effective refractory period, (Martins and Zipes 1980; Ng et al. 2001). In contrast, parasympathetic stimulation reduces the atrial effective refractory period (Zipes et al. 1974; Wijffels et al. 1995) while increasing electrophysiological heterogeneity (Fareh et al. 1998) and promoting early afterdepolarization (EAD) (Burashnikov and Antzelevitch 2003). Accordingly, parasympathetic stimulation is pro-arrhythmic in the atria but antiarrhythmic in the ventricles (Wijffels et al. 1995). Furthermore, parasympathetic activation of muscarinic-cholinergic receptor decreases intracellular cAMP levels, heart rate, AV nodal conduction, and contractile force.
There also exists a complex interaction between the sympathetic and parasympathetic divisions of the autonomic nervous system. Early pioneering studies documented that the reduction in heart rate produced by parasympathetic activation was greater during sympathetic stimulation (Rosenblueth and Simeone 1934; Samaan 1935; Warner and Russell 1969; Levy and Zieske 1969a; Stramba-Badiale et al. 1991; Vanhoutte and Levy 1980). This effect was documented to be due, in part, to the fact that efferent parasympathetic stimulation inhibited efferent sympathetic activation at both pre- and post-junctional sites (Vanhoutte and Levy 1980; Takahashi and Zipes 1983; Shen and Zipes 2014) as well as reduced cAMP levels to markedly influence heart rate, ventricular function, intracellular calcium handling, and cardiac electrophysiology (Levy and Zieske 1969b; Brack et al. 2004; Martins and Zipes 1980; Shen and Zipes 2014). Thus, parasympathetic effects became progressively stronger with increasing sympathetic activity. Furthermore, sympathetic effects are substantially smaller in the presence of high parasympathetic activity. This complex interaction has been called accentuated antagonism (Levy and Zieske 1969b) and suggests that changes in cardiac electrophysiology resulting from changes in sympathetic control cannot be interpreted accurately unless concurrent parasympathetic activity is taken into account. Similarly changes in cardiac electrophysiology resulting from changes in parasympathetic activity cannot be interpreted accurately unless concurrent sympathetic activity is taken into account (Rosenblueth and Simeone 1934; Samaan 1935; Warner and Russell 1969; Levy and Zieske 1969a).
In addition to activation of the parasympathetic and sympathetic divisions, the complex and interacting influences on the autonomic nervous system on cardiovascular function can also be studied indirectly by using pharmacological cardiac autonomic blockade (Sayin et al. 2016; Chen et al. 1995a). Results obtained from these studies have been analyzed by a variety of approaches. Comparisons have been made among parasympathetic and sympathetic effects, as well as parasympathetic and sympathetic tonus. A parasympathetic effect is defined as the response to cardiac muscarinic cholinergic receptor blockade (difference between control value and the value after muscarinic cholinergic blockade). A sympathetic effect is defined as the response to cardiac beta1-adrenergic receptor blockade (difference between the control value and the value after beta1-adrenergic receptor blockade). It has been suggested that these effects are difficult to interpret because it is challenging to distinguish the direct result of blockade from the indirect result (Gava et al. 1995; Negrão et al. 1992; Chen and DiCarlo 1997). For example, the heart rate after muscarinic cholinergic receptor blockade (parasympathetic effect) is the result of the direct effect of removal of the parasympathetic influence on the heart as well as the indirect effect of the unopposed sympathetic influence on the heart in response to blockade of the parasympathetic limb. Another potential limitation when using the parasympathetic (or sympathetic) effect is that a possible change in intrinsic heart rate is not considered. Any change in intrinsic heart rate would affect the final heart rate.
In an attempt to reduce the influence of these two suggested limitations, investigators have used parasympathetic and sympathetic tonus (Gava et al. 1995; Negrão et al. 1992; Chen and DiCarlo 1997; Sayin et al. 2016). Parasympathetic tonus is defined as the difference between the intrinsic value and the value after beta1-adrenergic receptor blockade. Sympathetic tonus is defined as the difference between the intrinsic value and the value after muscarinic cholinergic receptor blockade. Thus, both parasympathetic and sympathetic tonus represent the effect of the parasympathetic and sympathetic nervous systems on the heart without the influence of the opposing limb of the autonomic nervous system. By using sympathetic and parasympathetic tonus, investigators are also able to account for any potential change in intrinsic heart rate. However, it is important to note that no consensus exists on the use of these two approaches (Sayin et al. 2016).
This complex interaction between the sympathetic and parasympathetic systems has important implications because actions of pharmacological agents that alter the autonomic nervous system control of cardiac electrophysiology are transformed by these interacting mechanisms (Morady et al. 1988; Mirro et al. 1980). Specifically, agents used in the treatment of cardiovascular disorders have varying effects depending on background levels of autonomic nervous system functioning (Fukudo et al. 1992; Mirro et al. 1980; Hayano et al. 1990). Furthermore, the interacting influences must also be considered in the context of stress and exercise because the high sympathetic activity associated with these conditions is modified by parasympathetic activity (Morady et al. 1988; Mirro et al. 1980).
To address these concepts, we describe for the first time, the complex and interacting effects of the autonomic nervous system on heart rate and cardiac electrophysiology in a conscious, murine model of cardiac electrophysiology (Lujan and DiCarlo 2014). The mouse has significant advantages over other experimental models for the investigation of autonomic control of cardiac electrophysiology (Bryda 2013). The mouse is readily available, inexpensive, has a high throughput, and gives the investigator the ability to create genetically modified models. As a result, conscious mice have replaced many of the other animals, such as dogs, cats and rats in biomedical research because of the many advantages (Bryda 2013; Lujan et al. 2012b, a; Lujan and DiCarlo 2013; Lujan and DiCarlo 2014). However, virtually nothing is known regarding autonomic control of cardiac electrophysiology in conscious mice. Furthermore, when considering accentuated antagonism, investigators must distinguish between chronotropic and dromotropic properties to fully understand cardiac function because each property has its own distinctive relationship with the two divisions of the autonomic nervous system.
Accordingly, using two analytical approaches we investigated the autonomic control of pulse interval (heart rate), atrio-ventricular (AV) interval, sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle (cSNRT), and Wenckebach cycle length (WCL) in a conscious murine model free of the confounding influences of anesthetics and surgical trauma. The approach allows for the accurate documentation of the complex and interacting influence of the autonomic nervous system on cardiac electrophysiology in conscious mice and may be adopted for advancing the concepts and ideas that drive autonomic research.
2. METHODS
2.1 Experimental Subject
All surgical and experimental procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee and conformed to the American Physiological Society Guiding Principles in the Care and Use of Animals. Studies determining the complex and interacting influences of the autonomic nervous system on cardiac electrophysiology parameters were conducted in 8 male C57BL/6J mice (15 weeks of age), a strain commonly used in transgenic studies (Berul et al. 1996).
2.2 Surgical Procedures
Instrumentation
All surgical procedures were performed using aseptic surgical measures. Adult, male C57BL/6 mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and supplemental doses (10–20 mg/kg, i.p.) were administered if the mice regained the blink reflex or responded during the surgical procedures.
The hearts were approached via a left thoracotomy through the second intercostal space. Teflon coated stainless steel wire electrodes (0.003 inch, part no. 316 SS 7/44T, Medwire, Mount Vernon, NY) were sutured 1–2 mm apart with 8.0 silk on the surface of the left ventricle and atrial appendix as previously described in rats (Rodenbaugh et al. 2003a; Rodenbaugh et al. 2003b) and mice (Lujan and DiCarlo 2014). The stimulating wires were tunneled subcutaneously and exteriorized at the back of the neck. Subsequently, a catheter from a telemetry device (Data Sciences International, PA-C10), for recording arterial pressure was inserted into the left carotid artery until the tip reached the aortic arch as previously described in mice (Lujan and DiCarlo 2014). The body of the transmitter was placed subcutaneously on the left side. Next, two catheters for the infusion of cardiac autonomic antagonists (Chen et al. 1995b, 1997; Lujan et al. 2009; Chandler and DiCarlo 1997, 1998; Rodenbaugh et al. 2003a) were placed within the intraperitoneal space via a ventral abdominal approach, tunneled subcutaneously and exteriorized at the back of the neck.
Finally, ECG electrodes (DataSciences International, Small Gauge Lead Coupler Kit: 276-0065-001) were sutured with 6.0 silk subcutaneously in a modified lead II configuration, tunneled subcutaneously and exteriorized at the back of the neck as previously described in mice (Lujan and DiCarlo 2013; Lujan et al. 2012a). The local anesthetic, bupivacaine, was injected (s.q.) at all incision sites. At least 10 days were allowed for recovery. During the recovery periods, the mice were handled, weighed and acclimatized to the laboratory and investigators.
2.3 Experimental Procedures
Conscious, unrestrained mice were studied in their home cages (standard mouse polycarbonate cage, 17 cm W × 27 cm L × 12 cm H) during the light cycle for all experiments. Arterial pressure was recorded via telemetry and the ECG was recorded by taping the leads to single-stranded stainless-steel wires from a miniature electric swivel (Dragonfly Research & Development, Ridgeley, WV). The atrial or ventricular pacing leads were connected and secured with tape to teflon coated stainless steel wire electrodes (0.003 inch, part no. 316 SS 7/44T, Medwire, Mount Vernon, NY). The ECG signals were initially amplified (1,000 times) with a Grass P5 11 differential preamplifier and high-impedance probe (HIP 511GA, Grass Instruments Co., Quincy MA). The low and high pass filters were set at .3 Hz and 10 kHz. The temperature within the cage was monitored and maintained near the thermoneutral zone for mice of approximately 29–31° C (Swoap et al. 2004) by use of a circulating water pad under the cage and a Pelonis® heater. Mice were allowed to adapt to the laboratory environment for approximately two hours to ensure stable hemodynamic conditions.
After the stabilization period, beat by beat, steady-state heart rate, arterial pressure and ECG parameters were recorded over 10–15 s. Subsequently, the atrio-ventricular interval (AV interval), sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle length (cSNRT), and Wenckebach Cycle Length (WCL) were determined as previously described in conscious mice (Lujan and DiCarlo 2014). Briefly, the PowerLab stimulator delivered current via the leads attached to the atrial appendix. The current was recorded via an amp meter (Radio Shack, 22–805) in series with the atrial appendix stimulating electrode. Atrial pacing thresholds were determined and stimulation was performed for 1.0 ms pulse widths at the capture current (approximately 0.002μA).
The AV-interval, SNRT and cSNRT were determined during atrial pacing at a frequency of 8.4, 10 Hz, and 11.6 Hz for approximately 30 second durations (Lujan and DiCarlo 2014). The AV interval was measured as the time from the last paced stimulus to the onset of the QRS complex (Lujan and DiCarlo 2014). The SNRT was measured as the time from the last paced stimulus to the onset of the P wave (Lujan and DiCarlo 2014). To control for differences in sinus rate, SNRT was normalized to resting heart rate by subtracting the sinus cycle length from SNRT (cSNRT = SNRT - sinus cycle length). Sinus cycle length was determined from at least sixty consecutive cycles before the pacing period. A period of at least 60-sec was allowed to elapse between each successive pacing.
The WCL was determined during incremental increases in atrial pacing frequency performed for 1.0 ms pulse widths at twice the capture current (Lujan and DiCarlo 2014). The WCL was defined as the minimum cycle length that fails to conduct through the AV node as indicated by missed ventricular contractions. Missed ventricular contractions were detected by both the ECG and arterial pressure wave form. The WCL is an index of AV nodal conduction where increases in the WCL represent depressions in AV nodal conduction and decreases in WCL represent enhancements in AV nodal conduction.
All the procedures were repeated following beta1-adrenergic receptor blockade (metoprolol 10mg/kg, i.p.). The time elapsed between blocker administration and start of data collection was standardized to 15 minutes. The duration of data collection after blockade was approximately 10 minutes. Following data collection with beta-adrenergic receptor blockade the procedures were repeated with combined autonomic blockade [beta-adrenergic and muscarinic cholinergic receptor blockade (methylatropine 3mg/kg, i.p.)]. Note that the cardiac autonomic antagonists were administered through the chronically implanted i.p. catheters so that the mice were not handled or disturbed during the procedure. Furthermore, supplemental doses of the first antagonist was administered with the second antagonist assuring complete combined blockade. The duration of data collection after double receptor blockade was also approximately 10 minutes.
On an alternate day (>48 h), the mice were treated identically as described above except that the order of blockade was reversed. Intrinsic heart rate was considered to be the heart rate after complete cardiac autonomic blockade (muscarinic cholinergic- and beta-adrenergic-receptor blockades).
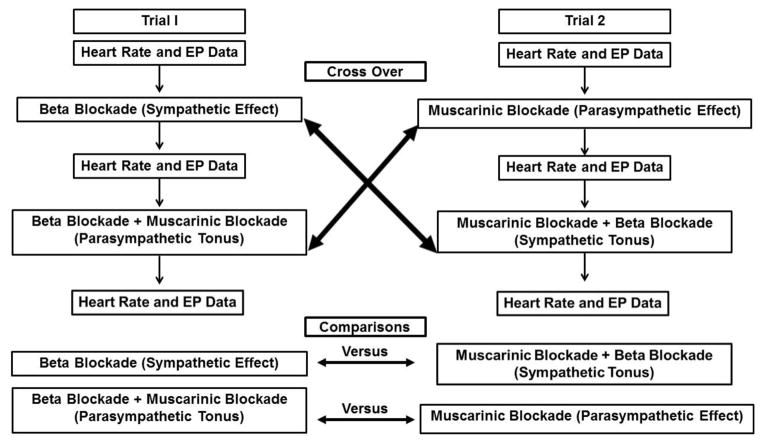
Comparisons were made among parasympathetic and sympathetic effects, as well as parasympathetic and sympathetic tonus. A parasympathetic effect was defined as the response after cardiac muscarinic cholinergic receptor blockade (difference between the control value and the value after muscarinic cholinergic blockade). A sympathetic effect was defined as the response after cardiac beta1-adrenergic receptor blockade (difference between the control value and the value after beta1-adrenergic receptor blockade). Parasympathetic tonus was defined as the difference between the intrinsic value and the value after beta1-adrenergic receptor blockade. Sympathetic tonus was defined as the difference between the intrinsic value and the value after muscarinic cholinergic receptor blockade (Figure 1). Intrinsic values were considered to be the values after complete cardiac autonomic blockade (muscarinic cholinergic- and beta-adrenergic-receptor blockades).
FIGURE 1.
Figure 1 presents a flow diagram of the experimental protocol showing a direct view of the two experimental trials and the point where the crossing between the two trials of the protocol occurred.
2.4 Data Analysis
All physiological recordings were sampled at 4 kHz, and the data were expressed as means ± SE. The final values for AV-interval, SNRT and cSNRT are the maximum value obtained during atrial pacing at frequencies of 8.4 Hz, 10 Hz, and 11.6 Hz. These frequencies were chosen for two reasons. First, these frequencies are within the physiological range (500, 600 and 700 bpm) and were used in a recent study in conscious mice (Lujan and DiCarlo 2014). Second, these frequencies are within the range of previous studies with anesthetized animals [6.67 (400bpm), 8.3 (500 bpm) and 10 Hz (600bpm)] (Berul et al. 1996).
The maximum value obtained during atrial pacing at frequencies of 8.4, 10 Hz, and 11.6 Hz was chosen because of the pioneering work of Berul and colleagues (Berul et al. 1996). These investigators reported the maximum value from all three pacing drives in the calculations of SNRT because this approach is analogous to the methods used in human studies (Mandel et al. 1971).
Control and responses to cardiac autonomic antagonist during the two experimental trials for pulse interval (heart rate), cardiac electrophysiology parameters and mean arterial pressure were compared with a two-way repeated measures ANOVA with post-hoc Student-Newman-Keuls method. Students’ paired t-tests were used to compare parasympathetic effect versus parasympathetic tonus as well as sympathetic effect versus sympathetic tonus. A value of P<0.05 was considered statistically significant.
Sympatho-Vagal Balance was calculated by dividing control intervals by intrinsic intervals for all parameters. Intrinsic intervals were considered the value after combined cardiac autonomic blockade and represents the spontaneous interval in the absence of autonomic modulation. When parasympathetic effect or tonus dominate, control intervals are above intrinsic intervals; however, when sympathetic effect or tonus dominate, control intervals are lower than intrinsic intervals (note that intervals are reciprocals of rate) (Goldberger 1999; Sayin et al. 2016).
3. RESULTS
3.1 Experimental Trials
Figure 1 presents a flow diagram of the experimental protocol showing a direct view of the two experimental trials and the point where the crossing between the two trials of the protocol occurred. This figure provides clarity on how the variations plotted in the figures were computed.
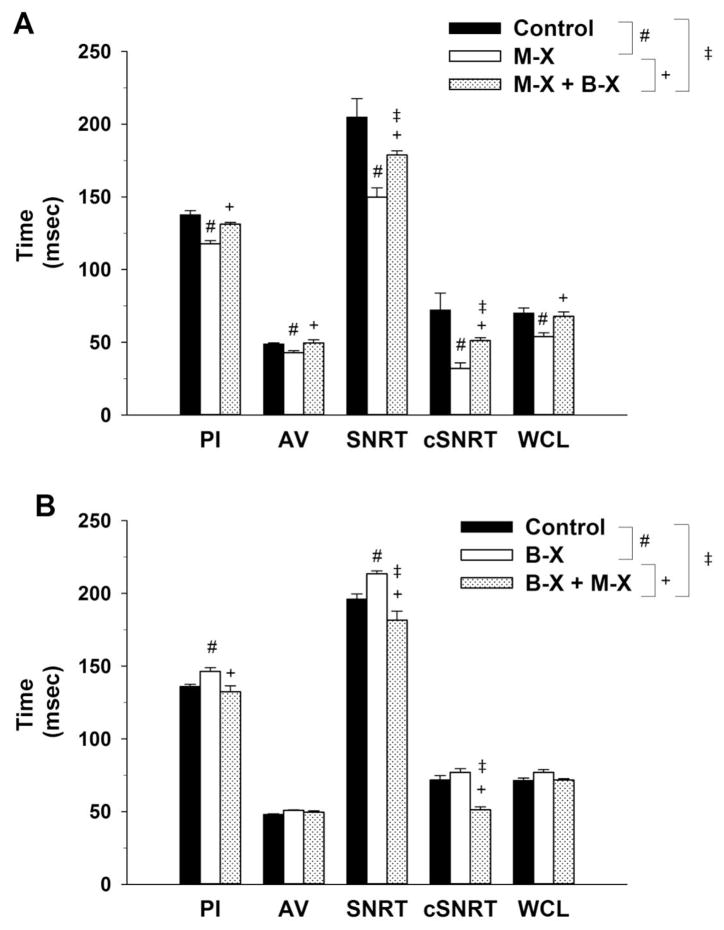
Figure 2, Panel A presents cardiac electrophysiology parameters (PI, AV interval, SNRT, cSNRT and WCL) for the 8 intact, conscious mice before (control) and after Muscarinic Receptor Blockade (M-X) followed by combined Muscarinic Receptor Blockade + Beta Receptor Blockade (M-X + B-X). Panel B presents the change in cardiac electrophysiology parameters (PI, AV interval, SNRT, cSNRT and WCL) for the 8 intact, conscious mice, before (control) and after Beta Receptor Blockade (B-X) followed by combined Beta Receptor Blockade + Muscarinic Receptor Blockade (B-X + M-X). There was no statistical difference between control or intrinsic values for any variable on the two experimental days.
FIGURE 2.
Figure 2, Panel A presents cardiac electrophysiology parameters [pulse interval (PI), atrio-ventricular (AV) interval, sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle (cSNRT) and Wenckebach cycle length (WCL)] for the 8 intact, conscious mice before (control) and after Muscarinic Receptor Blockade (M-X) followed by combined Muscarinic Receptor Blockade + Beta Receptor Blockade (M-X + B-X). Panel B presents the change in cardiac electrophysiology parameters (PI, AV interval, SNRT, cSNRT and WCL) for the 8 intact, conscious mice, before (control) and after Beta Receptor Blockade (B-X) followed by combined Beta Receptor Blockade + Muscarinic Receptor Blockade (B-X + M-X).
3.2 Comparison of Parasympathetic and Sympathetic Effects and Tonus
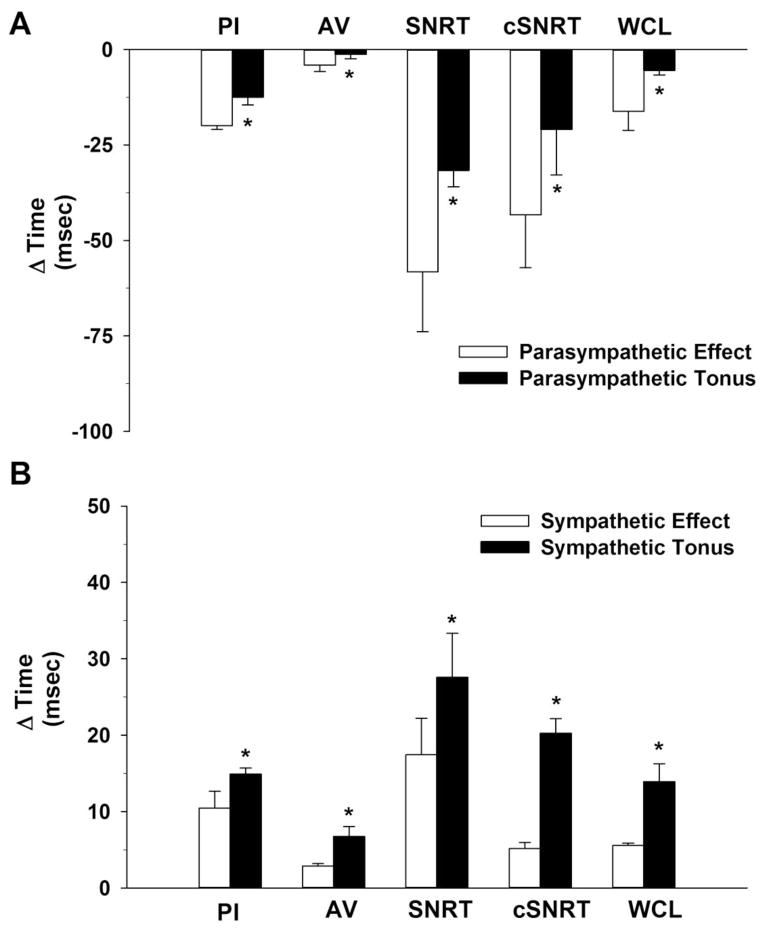
Parasympathetic effect was considered the interval change induced by methyl-atropine alone and the sympathetic effect was considered the interval change induced by metoprolol alone. Parasympathetic tonus was considered the interval change induced by methyl-atropine after metoprolol. The interval change induced by metoprolol after methyl-atropine was considered sympathetic tonus. Figure 3, Panel A compares the parasympathetic effect and parasympathetic tonus on cardiac electrophysiology parameters (PI, AV interval, SNRT, cSNRT and WCL) for the 8 intact, conscious mice. Panel B contrasts and compares the sympathetic effect and sympathetic tonus on cardiac electrophysiology parameters. Figure 3, documents a complex interaction between the sympathetic and parasympathetic divisions of the autonomic nervous system in the control of cardiac electrophysiology parameters. Specifically, the parasympathetic effect was greater than the parasympathetic tonus in the control of cardiac electrophysiology parameters in conscious mice (Panel A). In contrast, the sympathetic effect was smaller than the sympathetic tonus in the control of cardiac electrophysiology parameters in conscious mice. (Panel B).
FIGURE 3.
Figure 3, Panel A contrasts the parasympathetic effect and parasympathetic tonus on cardiac electrophysiology parameters [pulse interval (PI), atrio-ventricular (AV) interval, sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle (cSNRT) and Wenckebach cycle length (WCL)] for the 8 intact, conscious mice. Panel B contrasts the sympathetic effect and sympathetic tonus on cardiac electrophysiology parameters.
*P<0.05, Effect vs Tonus
3.3 Assessment of Sympatho-Vagal Balance
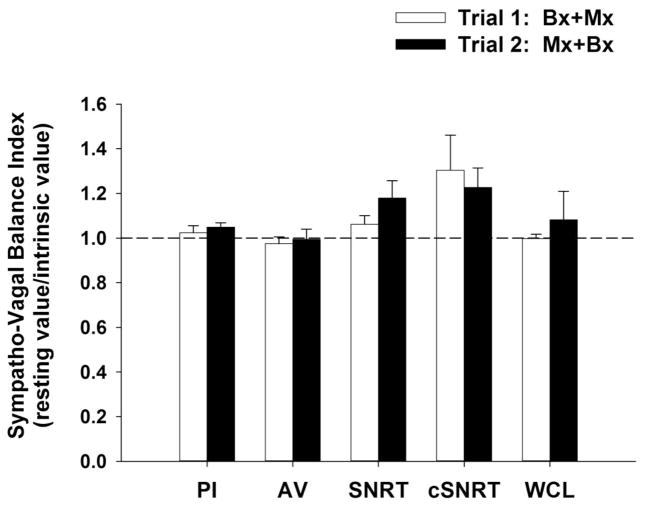
The Sympatho-Vagal balance (SVB) index (ratio of resting interval to intrinsic interval on the two experimental days) for pulse interval, AV interval, SNRT, cSNRT and WCL are presented in Figure 4. The SVB was greater than 1, independent of the order of blockade, indicating a slight parasympathetic dominance in the regulation of PI, SNRT, cSNRT and WCL in conscious mice. In contrast, the SVB for AV interval was less than one and equal to one during experimental trials one and two respectively. Of note, the SBV for heart rate (control heart rate divided by intrinsic heart rate) was less than 1 on both experimental days as rate is the reciprocal of interval, also indicating a slight parasympathetic dominance (Goldberger 1999; Sayin et al. 2016)
FIGURE 4.
Figure 4 presents The Sympatho-Vagal balance (SVB) index (ratio of control interval to intrinsic interval on the two experimental days) for cardiac electrophysiology parameters [pulse interval (PI), atrio-ventricular (AV) interval, sinus node recovery time (SNRT), SNRT corrected for spontaneous sinus cycle (cSNRT) and Wenckebach cycle length (WCL)]. The SVB was greater than 1, independent of the order of blockade, indicating parasympathetic dominance in the regulation of PI, SNRT, cSNRT and WCL in conscious mice.
3.4 Autonomic Blockade Effects on Blood Pressure
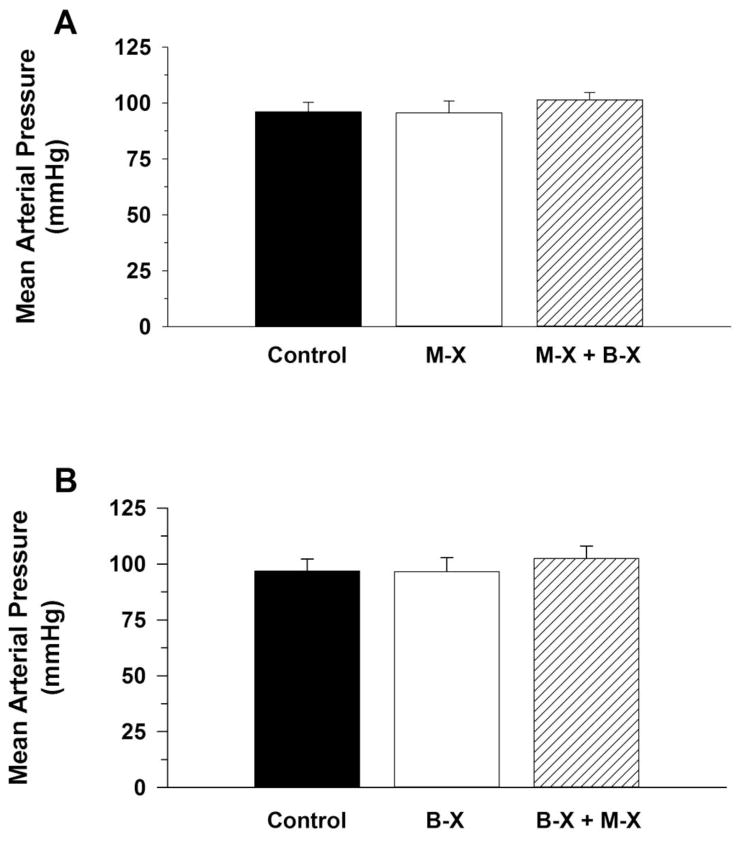
Figure 5, Panel A presents resting mean arterial pressure (MAP) for the 8 intact conscious mice before (control) and after Muscarinic Receptor Blockade (M-X) followed by combined Muscarinic Receptor Blockade + Beta Receptor Blockade (M-X + B-X). Panel B presents resting mean arterial pressure before (control) and after Beta Receptor Blockade (B-X) followed by combined Beta Receptor Blockade + Muscarinic Receptor Blockade (B-X + M-X). Cardiac autonomic blockade, regardless of the order of blockade, did not significantly alter MAP.
FIGURE 5.
Figure 5, Panel A presents resting mean arterial pressure (MAP) for the 8 intact conscious mice before (control) and after Muscarinic Receptor Blockade (M-X) followed by combined Muscarinic Receptor Blockade + Beta Receptor Blockade (M-X + B-X). Panel B presents resting mean arterial pressure before (control) and after Beta Receptor Blockade (B-X) followed by combined Beta Receptor Blockade + Muscarinic Receptor Blockade (B-X + M-X). Cardiac autonomic blockade, regardless of the order of blockade, did not significantly alter MAP.
4. DISCUSSION
Mice may now be the preferred animal model for biomedical research due to its anatomical, physiological, and genetic similarity to humans (Bryda 2013). Other advantages of mice include its small size, ease of maintenance, short life cycle, and abundant genetic resources. Furthermore, when considering accentuated antagonism, investigators must distinguish between chronotropic and dromotropic properties to fully understand autonomic function because each property has its own distinctive relationship with the two divisions of the autonomic nervous system. However, little is known about accentuated antagonism in conscious mice. Accordingly, we administered cardiac autonomic antagonists alone and in combination so that cardiac electrophysiology parameters could be determined under control conditions without antagonists, with either autonomic branch blocked alone, and with both branches blocked (Sayin et al. 2016). In this setting, the effect produced by a selective blocker alone were compared with the effect produced by a selective blocker without the influence of the opposing limb of the autonomic nervous system (Sayin et al. 2016). A comparison of parasympathetic and sympathetic effects alone or the net effect of their interaction documented for the first time accentuated antagonism of cardiac electrophysiology parameters in conscious mice.
Specifically, this study documents a complex and interacting influence of the sympathetic and parasympathetic divisions of the autonomic nervous system on the control of pulse interval (heart rate) and cardiac electrophysiology parameters in intact conscious mice. The procedures conducted in conscious C57BL/6J mice, a strain commonly used in transgenic studies, can be utilized in genetically modified models to enhance our understanding of single gene defects and their autonomic phenotypes in conscious animals (Chien 1995; Curran et al. 1995; Field 1988; Grace and Chien 1995; Wang et al. 1995). Furthermore, the cardiac electrophysiology protocols can be initiated in the conscious state after the resolution of the inflammation that occurs during the initial surgical preparation.
The SVB was greater than 1, independent of the order of blockade, indicating a slight parasympathetic dominance in the regulation of PI, SNRT, cSNRT and WCL in conscious mice. However, until recently (Swoap et al. 2008) it was generally accepted that mice have a resting heart rate between 550–600 beats/min, display low parasympathetic tone, are sympathetically dominate and have an intrinsic heart rate below resting heart rate (Gehrmann et al. 2000; Janssen and Smits 2002). Specifically, early studies reported that the intrinsic heart rate of mice (approximately 500 beats/min) is below the resting heart rate of about 600 beats/min (Janssen and Smits 2002). This has led to the generally accepted concept that the heart rate of mice is dominated by cardiac sympathetic activity at rest (Gehrmann et al. 2000; Janssen and Smits 2002). Subsequent reports have questioned the concept of sympathetic dominance in the control of heart rate at rest in mice (Baudrie et al. 2007; Chen et al. 2005; Pelat et al. 2003). Specifically, investigators (Swoap et al. 2004; Williams et al. 2003; Williams et al. 2002; Wernstedt et al. 2006; Swoap et al. 2008) have documented that the tachycardia and sympathetic dominance of heart rate at rest was associated with mice being housed or studied in environments below their thermoneutral zone (TNZ). The TNZ of mice is 29–30°C (Gordon 1993) and is the range of ambient temperatures in which the mice do not elevate their metabolic rate to maintain core body temperature (Gordon 2012; Jakobson 1981). Studied or housed at temperatures below 29–30°C exposes mice to a significant cold stress (Cannon and Nedergaard 2011) and they respond by enhancing sympathetically mediated non-shivering thermogenesis (Gaskill et al. 2012; Jakobson 1981; Ocloo et al. 2007). Thus environmental conditions of mice are an important determinant of cardiac sympathetic and parasympathetic activity, which in turn has a major impact on heart rate and cardiac electrophysiology (Swoap et al. 2008).
It is now generally accepted that the metabolic and cardiovascular consequences of the cold stress that exist under standard laboratory conditions accounts for the tachycardia and sympathetic dominance of heart rate at rest (Bissonnette et al. 2007; Swoap et al. 2004; Talan et al. 1996; Wernstedt et al. 2006; Williams et al. 2002) and, when mice are studied at thermoneutrality, resting heart rate is below 450 beats/min and cardiac autonomic regulation of heart rate is more similar to that of humans than originally reported. Specifically, the Sympatho-Vagal balance index indicates a slight parasympathetic dominance in the control of resting pulse interval in conscious mice (Figure 4).
This study, for the first time, also documents a complex and interacting influence of the sympathetic and parasympathetic divisions of the autonomic nervous system on the control of pulse interval as well as cardiac electrophysiology parameters in conscious mice such that the response to blockade of one division of the autonomic nervous system was significantly influenced by the presence or absence of the opposing division of the autonomic nervous system (Figure 3). For example, the cardiac electrophysiology responses to muscarinic receptor blockade were greater before beta receptor blockade (Parasympathetic Effect); i.e. in the presence of sympathetic tone (Figure 3, Panel A). Similarly, the cardiac electrophysiology responses to beta receptor blockade (Sympathetic Effect) were smaller before muscarinic receptor blockade; i.e. in the presence of parasympathetic tone (Figure 3, Panel B). This complex interaction has been called accentuated antagonism (Levy and Zieske 1969b). The concept of accentuated antagonism may have originated with the pioneering work of Rosenblueth and Simeone (Rosenblueth and Simeone 1934) who initially documented that the decrease in heart rate produced by parasympathetic (vagal) stimulation was greater when heart rate was increased by sympathetic stimulation. Subsequently, Samaan (Samaan 1935) reported that the cardiac acceleration produced by sympathetic stimulation was attenuated by parasympathetic (vagal) stimulation. These complex and interacting sympathetic and parasympathetic influences on heart rate have been confirmed by many investigators (Warner and Russell 1969; Levy and Zieske 1969a). The current study extends this concept to cardiac electrophysiology parameters in conscious mice.
The concept of Sympatho-Vagal balance has historically been limited to discussions of sympathetic and parasympathetic interactions involving vagal activation and sympathetic withdrawal or sympathetic activation and vagal withdrawal. However, it is clear from recent pioneering studies in humans using indirect, noninvasive, spectral indexes of autonomic function that these interactions are much more complex and interacting (Marchi et al. 2016) as also documented by the complex and interacting influence of the autonomic nervous system in mice.
Parasympathetic activity has been called nature’s beta blocker because of its inhibitory influence on sympathetic activity (Herring and Paterson 2009). The inhibitory influence of parasympathetic activity on sympathetic activity is mediated by a cholinergically induced reduction in norepinephrine release (Burn and Rand 1965; Burn 1967; Lindmar et al. 1968; Haeusler et al. 1968; Loffelholz and Muscholl 1969) as well as a cholinergic reduction in cyclic AMP (adenosine 3′, 5′-monophosphate) (Sutherland et al. 1968; Epstein et al. 1971). Specifically, acetylcholine has been documented to decrease the rate of cyclic AMP formation in cell preparations from canine hearts (Murad et al. 1962). Furthermore, acetylcholine blocked the positive inotropic effects elicited by epinephrine and theophylline by decreasing cyclic AMP (Meester and Hardman 1967).
In addition, many local cardiac neuromodulators intrinsic to sympathetic or parasympathetic neurons impact the complex and interacting influence of the sympathetic and parasympathetic divisions of the autonomic nervous system on heart rate and cardiac electrophysiology (Steele and Choate 1994; Beaulieu and Lambert 1998; Paterson 2001). Specifically, acetylcholine release from parasympathetic terminals activating muscarinic receptors and norepinephrine release from sympathetic terminals activating beta receptors are modified by numerous local cardiac factors (Herring and Paterson 2009). These neuromoduators include neuronal nitric oxide synthase, neuropeptide Y, natriuretic peptides and others (Paterson 2001). Numerous pathological conditions are known to enhance the effect of these neuromodulators on sympathetic and parasympathetic activity. For example, there is evidence that when sympathetic activity is high, the enhanced inhibitory influence of parasympathetic activity is due, at least in part, to accentuated antagonism involving cholinergic-induced formation of nitric oxide (Paterson 2001).
In addition to the peripheral interactions between the sympathetic and parasympathetic divisions of the autonomic nervous system within the heart, complex and interacting influences of the autonomic nervous system on heart rate and cardiac electrophysiology also occurs within the central nervous system. Accordingly, the central nervous system is critical for the autonomic nervous system control of heart rate and cardiac electrophysiology at rest and during physiological and pathophysiological stress. For example, studies document the existence of neuroanatomical interconnections between sympathetic and parasympathetic central neurons which constitute the basis for functional interactions at the central nervous system level (Buijs et al. 2001).
5. CONCLUSION
The Sympatho-Vagal Balance index documents a slight parasympathetic dominance in the regulation of cardiac electrophysiology in conscious mice. Thus, resting cardiac autonomic balance of conscious mice is more similar to that of humans than originally reported. In addition, the concept of accentuated antagonism of heart rate as well as cardiac electrophysiology parameters applies to conscious mice. This is important, especially in view of the ever increasing use of this animal species in cardiovascular research. Furthermore, when considering accentuated antagonism, investigators must distinguish between chronotropic and dromotropic properties because each property has its own distinctive relationship with the sympathetic and parasympathetic divisions of the autonomic nervous system. Understanding the complex and interacting influences of the autonomic nervous system on cardiac electrophysiology has important implications because pharmacological agents used in the treatment of cardiovascular disorders have varying effects depending on background levels of autonomic nervous system functioning (Fukudo et al. 1992; Mirro et al. 1980; Hayano et al. 1990).
Highlights.
The Sympatho-Vagal Balance index documents a slight parasympathetic dominance in the regulation of cardiac electrophysiology in conscious mice.
Thus, resting cardiac autonomic balance of conscious mice is more similar to that of humans than originally reported.
The concept of accentuated antagonism of heart rate as well as cardiac electrophysiology parameters applies to conscious mice.
This is important, especially in view of the ever increasing use of this animal species in cardiovascular research.
Pharmacological agents used in the treatment of cardiovascular disorders have varying effects depending on background levels of autonomic nervous system functioning.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant HL 122223.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heidi L. Lujan, Email: hlujan@med.wayne.edu.
Joshua P. Rivers, Email: jrivers@med.wayne.edu.
Stephen E. DiCarlo, Email: sdicarlo@med.wayne.edu.
References
- Baudrie V, Laude D, Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R904–912. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- Beaulieu P, Lambert C. Peptidic regulation of heart rate and interactions with the autonomic nervous system. Cardiovasc Res. 1998;37(3):578–585. doi: 10.1016/s0008-6363(97)00305-2. [DOI] [PubMed] [Google Scholar]
- Berul CI, Aronovitz MJ, Wang PJ, Mendelsohn ME. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94(10):2641–2648. doi: 10.1161/01.cir.94.10.2641. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ, Maylie J, Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Auton Neurosci. 2007;136(1–2):82–89. doi: 10.1016/j.autneu.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Interaction between direct sympathetic and vagus nerve stimulation on heart rate in the isolated rabbit heart. Experimental physiology. 2004;89(1):128–139. doi: 10.1113/expphysiol.2003.002654. [DOI] [PubMed] [Google Scholar]
- Bryda EC. The Mighty Mouse: the impact of rodents on advances in biomedical research. Missouri medicine. 2013;110(3):207–211. [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431(4):405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107(18):2355–2360. doi: 10.1161/01.cir.0000065578.00869.7c. [DOI] [PubMed] [Google Scholar]
- Burn JH. Release of noradrenaline from the sympathetic postganglionic fibre. British medical journal. 1967;2(5546):197–201. doi: 10.1136/bmj.2.5546.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn JH, Rand MJ. Acetylcholine in adrenergic transmission. Annual review of pharmacology. 1965;5:163–182. doi: 10.1146/annurev.pa.05.040165.001115. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Chandler MP, DiCarlo SE. Sinoaortic denervation prevents postexercise reductions in arterial pressure and cardiac sympathetic tonus. Am J Physiol Heart Circ Physiol. 1997;273:H2738–H2745. doi: 10.1152/ajpheart.1997.273.6.H2738. [DOI] [PubMed] [Google Scholar]
- Chandler MP, DiCarlo SE. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol Reg Integ Comp Physiol. 1998;274:R510–R516. doi: 10.1152/ajpregu.1998.274.2.R510. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, DiCarlo SE. Endurance exercise training-induced resting bradycardia: a brief review. Sports Med Training and Rehab. 1997;8:37–77. [Google Scholar]
- Chen C-Y, DiCarlo SE, Collins HL. Enhanced cardiopulmonary reflex regulation of heart rate during exercise. Med Sci Sport Exerc. 1995a;27(10):1399–1405. [PubMed] [Google Scholar]
- Chen Y, Chandler MP, DiCarlo SE. Acute exercise attenuates cardiac autonomic regulation in hypertensive rats. Hypertension. 1995b;26:676–683. doi: 10.1161/01.hyp.26.4.676. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chandler MP, DiCarlo SE. Daily exercise and gender influence postexercise cardiac autonomic responses in hypertensive rats. Am J Physiol. 1997;272:H1412–H1418. doi: 10.1152/ajpheart.1997.272.3.H1412. [DOI] [PubMed] [Google Scholar]
- Chen Y, Joaquim LF, Farah VM, Wichi RB, Fazan R, Jr, Salgado HC, Morris M. Cardiovascular autonomic control in mice lacking angiotensin AT1a receptors. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R1071–1077. doi: 10.1152/ajpregu.00231.2004. [DOI] [PubMed] [Google Scholar]
- Chien KR. Cardiac muscle diseases in genetically engineered mice: evolution of molecular physiology. Am J Physiol. 1995;269(3 Pt 2):H755–H766. doi: 10.1152/ajpheart.1995.269.3.H755. [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. 0092-8674(95)90358-5 [pii] [DOI] [PubMed] [Google Scholar]
- Engel GL. Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Ann Intern Med. 1978;89(3):403–412. doi: 10.7326/0003-4819-89-3-403. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Levey GS, Skelton CL. Adenyl cyclase and cyclic AMP. Biochemical links in the regulation of myocardial contractility. Circulation. 1971;43(3):437–450. doi: 10.1161/01.cir.43.3.437. [DOI] [PubMed] [Google Scholar]
- Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98(20):2202–2209. doi: 10.1161/01.cir.98.20.2202. [DOI] [PubMed] [Google Scholar]
- Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988;239(4843):1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. Faseb j. 2004;18(6):711–713. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Lane JD, Anderson NB, Kuhn CM, Schanberg SM, McCown N, Muranaka M, Suzuki J, Williams RB., Jr Accentuated vagal antagonism of beta-adrenergic effects on ventricular repolarization. Evidence of weaker antagonism in hostile type A men. Circulation. 1992;85(6):2045–2053. doi: 10.1161/01.cir.85.6.2045. [DOI] [PubMed] [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7(3):e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava NS, Véras-Silvas AS, Negrão CE, Krieger EM. Low-intensity exercise training attenuates cardiac b-adrenergic tone during exercise in spontaneously hypertensive rats. Hypertension. 1995;26:1129–1133. doi: 10.1161/01.hyp.26.6.1129. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279(2):H733–740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- Goldberger JJ. Sympathovagal balance: how should we measure it? Am J Physiol. 1999;276(4 Pt 2):H1273–1280. doi: 10.1152/ajpheart.1999.276.4.H1273. [DOI] [PubMed] [Google Scholar]
- Gordon C. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol. 2012;37:654–685. [Google Scholar]
- Gordon CJ. Temperature regulation in laboratory rodents. Cambridge University Press; New York: 1993. [Google Scholar]
- Grace AA, Chien KR. Congenital long QT syndromes. Toward molecular dissection of arrhythmia substrates. Circulation. 1995;92(10):2786–2789. doi: 10.1161/01.cir.92.10.2786. [DOI] [PubMed] [Google Scholar]
- Haeusler G, Thoenen H, Haefely W, Heuerlimann A. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1968;261:389–411. [PubMed] [Google Scholar]
- Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. Am J Cardiol. 1990;65(1):84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Experimental physiology. 2009;94(1):46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Jakobson M. Physiological adaptability: the response of the house mouse to variations in the environment. Symp Zool Soc London. 1981;47:301–335. [Google Scholar]
- Janssen BJ, Smits JF. Autonomic control of blood pressure in mice: basic physiology and effects of genetic modification. Am J Physiol Regul Integr Comp Physiol. 2002;282(6):R1545–R1564. doi: 10.1152/ajpregu.00714.2001. [DOI] [PubMed] [Google Scholar]
- Kapa S, Venkatachalam KL, Asirvatham SJ. The autonomic nervous system in cardiac electrophysiology: an elegant interaction and emerging concepts. Cardiol Rev. 2010;18(6):275–284. doi: 10.1097/CRD.0b013e3181ebb152. [DOI] [PubMed] [Google Scholar]
- Levy MN, Zieske H. Autonomic control of cardiac pacemaker activity and atrioventricular transmission. J Appl Physiol. 1969a;27(4):465–470. doi: 10.1152/jappl.1969.27.4.465. [DOI] [PubMed] [Google Scholar]
- Levy MN, Zieske H. Effect of enhanced contractility on the left ventricular response to vagus nerve stimulation in dogs. Circ Res. 1969b;24(3):303–311. doi: 10.1161/01.res.24.3.303. [DOI] [PubMed] [Google Scholar]
- Lindmar R, Loffelholz K, Muscholl E. A muscarinic mechanism inhibiting the release of noradrenaline from peripheral adrenergic nerve fibres by nicotinic agents. British journal of pharmacology and chemotherapy. 1968;32(2):280–294. doi: 10.1111/j.1476-5381.1968.tb00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffelholz K, Muscholl E. A muscarinic inhibition of the noradrenaline release evoked by postganglionic sympathetic nerve stimulation. Naunyn-Schmiedebergs Archiv fur Pharmakologie. 1969;265(1):1–15. doi: 10.1007/BF01417206. [DOI] [PubMed] [Google Scholar]
- Lujan HL, Chen Y, DiCarlo SE. Paraplegia Increased Cardiac NGF Content, Sympathetic Tonus and the Susceptibility to Ischemia-Induced Ventricular Tachycardia in Conscious Rats. Am J Physiol Heart Circ Physiol. 2009;296(5):H1364–1372. doi: 10.1152/ajpheart.01286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, DiCarlo SE. Cardiac Output, at Rest and During Exercise, Before and During Myocardial Ischemia, Reperfusion and Infarction in Conscious Mice. Am J Physiol Regul Integr Comp Physiol. 2013;304(4):R286–295. doi: 10.1152/ajpregu.00517.2012. ajpregu.00517.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, DiCarlo SE. Cardiac electrophysiology and the susceptibility to sustained ventricular tachycardia in intact, conscious mice. Am J Physiol Heart Circ Physiol. 2014;306(8):H1213–1221. doi: 10.1152/ajpheart.00780.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Myocardial ischemia, reperfusion, and infarction in chronically instrumented, intact, conscious, and unrestrained mice. Am J Physiol Regul Integr Comp Physiol. 2012a;302(12):R1384–R1400. doi: 10.1152/ajpregu.00095.2012. ajpregu.00095.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol. 2012b;302(1):R68–R74. doi: 10.1152/ajpregu.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel W, Hayakawa H, Danzig R, Marcus HS. Evaluation of sino-atrial node function in man by overdrive suppression. Circulation. 1971;44(1):59–66. doi: 10.1161/01.cir.44.1.59. [DOI] [PubMed] [Google Scholar]
- Marchi A, Bari V, De Maria B, Esler M, Lambert E, Baumert M, Porta A. Calibrated variability of muscle sympathetic nerve activity during graded head-up tilt in humans and its link with noradrenaline data and cardiovascular rhythms. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1134–1143. doi: 10.1152/ajpregu.00541.2015. [DOI] [PubMed] [Google Scholar]
- Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res. 1980;46(1):100–110. doi: 10.1161/01.res.46.1.100. [DOI] [PubMed] [Google Scholar]
- Meester WD, Hardman HF. Blockade of the positive inotropic actions of epinephrine and theophylline by acetylcholine. J Pharmacol Exp Ther. 1967;158(2):241–247. [PubMed] [Google Scholar]
- Mirro MJ, Watanabe AM, Bailey JC. Electrophysiological effects of disopyramide and quinidine on guinea pig atria and canine cardiac purkinje fibers. Dependence on underlying cholinergic tone. Circ Res. 1980;46(5):660–668. doi: 10.1161/01.res.46.5.660. [DOI] [PubMed] [Google Scholar]
- Morady F, Kou WH, Nelson SD, De Buitleir M, Schmaltz S, Kadish AH, Toivonen LK, Kushner JA. Accentuated antagonism between beta-adrenergic and vagal effects on ventricular refractoriness in humans. Circulation. 1988;77(2):289–297. doi: 10.1161/01.cir.77.2.289. [DOI] [PubMed] [Google Scholar]
- Murad F, Chi YM, Rall TW, Sutherland EW. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3′,5′-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962;237:1233–1238. [PubMed] [Google Scholar]
- Negrão CE, Moreira ED, Santos MCLM, Farah VMA, Krieger EM. Vagal function impairment after exercise training. J Appl Physiol. 1992;72:1749–1753. doi: 10.1152/jappl.1992.72.5.1749. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart--a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Experimental physiology. 2001;86(3):319–329. doi: 10.1113/eph8602146. [DOI] [PubMed] [Google Scholar]
- Ocloo A, Shabalina IG, Nedergaard J, Brand MD. Cold-induced alterations of phospholipid fatty acyl composition in brown adipose tissue mitochondria are independent of uncoupling protein-1. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1086–1093. doi: 10.1152/ajpregu.00128.2007. [DOI] [PubMed] [Google Scholar]
- Paterson D. Nitric oxide and the autonomic regulation of cardiac excitability. The G.L. Brown Prize Lecture. Experimental physiology. 2001;86(1):1–12. doi: 10.1113/eph8602169. [DOI] [PubMed] [Google Scholar]
- Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107(19):2480–2486. doi: 10.1161/01.cir.0000065601.83526.3e. [DOI] [PubMed] [Google Scholar]
- Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens. 2003a;25(6):349–358. doi: 10.1081/ceh-120023544. [DOI] [PubMed] [Google Scholar]
- Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol. 2003b;285(6):2605–2613. doi: 10.1152/ajpheart.00319.2003. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Schauble EH, Desai KH, Kobilka BK, Bernstein D. Alterations in dynamic heart rate control in the b1-adrenergic receptor knockout mouse. Am J Physiol. 1998;274:H1184–H1193. doi: 10.1152/ajpheart.1998.274.4.H1184. (Heart Circ Physiol 43) [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Simeone F. The interrelations of vagal and accelerator effects on the cardiac rate. Am J Physiol. 1934;110:42–55. [Google Scholar]
- Samaan A. The antagonistic cardiac nerves and heart rate. J Physiol. 1935;83(3):332–340. doi: 10.1113/jphysiol.1935.sp003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin H, Chapuis B, Chevalier P, Barres C, Julien C. Assessment of cardiac autonomic tone in conscious rats. Auton Neurosci. 2016;194:26–31. doi: 10.1016/j.autneu.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Napolitano C. Role of the Autonomic Nervous System in Sudden Cardiac Death. In: Josephson ME, editor. Sudden cardiac death. Blackwell Scientific Publications; Cambridge,MA: 1993. pp. 16–37. [Google Scholar]
- Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114(6):1004–1021. doi: 10.1161/circresaha.113.302549. [DOI] [PubMed] [Google Scholar]
- Steele PA, Choate JK. Innervation of the pacemaker in guinea-pig sinoatrial node. J Auton Nerv Syst. 1994;47(3):177–187. doi: 10.1016/0165-1838(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Stramba-Badiale M, Vanoli E, De Ferrari GM, Cerati D, Foreman RD, Schwartz PJ. Sympathetic-parasympathetic interaction and accentuated antagonism in conscious dogs. Am J Physiol. 1991;260(2 Pt 2):H335–340. doi: 10.1152/ajpheart.1991.260.2.H335. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Robinson GA, Butcher RW. Some Aspects of the Biological Role of Adenosine 3′,5′-monophosphate (Cyclic AMP) Circulation. 1968;37(2):279–306. doi: 10.1161/01.cir.37.2.279. [DOI] [Google Scholar]
- Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol. 2008;294(4):H1581–1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R391–R396. doi: 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Zipes DP. Vagal modulation of adrenergic effects on canine sinus and atrioventricular nodes. Am J Physiol. 1983;244(6):H775–781. doi: 10.1152/ajpheart.1983.244.6.H775. [DOI] [PubMed] [Google Scholar]
- Talan MI, Kirov SA, Kosheleva NA. Nonshivering thermogenesis in adult and aged C57BL/6J mice housed at 22 degrees C and at 29 degrees C. Experimental gerontology. 1996;31(6):687–698. doi: 10.1016/s0531-5565(96)00095-2. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Levy MN. Prejunctional cholinergic modulation of adrenergic neurotransmission in the cardiovascular system. Am J Physiol. 1980;238(3):H275–281. doi: 10.1152/ajpheart.1980.238.3.H275. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80(5):805–811. doi: 10.1016/0092-8674(95)90359-3. 0092-8674(95)90359-3 [pii] [DOI] [PubMed] [Google Scholar]
- Warner HR, Russell RO., Jr Effect of combined sympathetic and vagal stimulation on heart rate in the dog. Circ Res. 1969;24(4):567–573. doi: 10.1161/01.res.24.4.567. [DOI] [PubMed] [Google Scholar]
- Wernstedt I, Edgley A, Berndtsson A, Faldt J, Bergstrom G, Wallenius V, Jansson JO. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R551–557. doi: 10.1152/ajpregu.00514.2005. [DOI] [PubMed] [Google Scholar]
- Wharton JM, Coleman RE, Strauss HC. The role of the autonomic nervous system in sudden cardiac death. Trends Cardiovas Med. 1992;2:65–71. doi: 10.1016/1050-1738(92)90007-F. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Gagnon SP, Roberts LM, Henderson RP, Overton JM. Cardiovascular and metabolic responses to fasting and thermoneutrality in Ay mice. Physiol Behav. 2003;78(4–5):615–623. doi: 10.1016/s0031-9384(03)00049-0. S0031938403000490 [pii] [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1459–R1467. doi: 10.1152/ajpregu.00612.2001. [DOI] [PubMed] [Google Scholar]
- Zipes DP. The long QT interval syndrome. A Rosetta stone for sympathetic related ventricular tachyarrhythmias [comment] Circulation. 1991;84(3):1414–1419. doi: 10.1161/01.cir.84.3.1414. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res. 1974;8(5):647–655. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]