Abstract
Rewards influence responses to acute painful stimuli, but the relationship of chronic pain to hedonic or motivational aspects of reward is not well understood. Here, we independently evaluated hedonic qualities of sweet or bitter tastants as well as motivation to seek food reward in rats with experimental neuropathic pain induced by L5/6 spinal nerve ligation (SNL). Hedonic response was measured by implantation of intraoral catheters to allow passive delivery of liquid solutions and “liking/disliking” responses were scored according to a facial reactivity scale. SNL rats did not differ from controls in either “liking” or “disliking” reactions to intraoral sucrose or quinine, respectively, at post-surgery day 21, suggesting no differences in perceived hedonic value of sweet or bitter tastants. To assess possible motivational deficits during acute and chronic pain, we employed fixed- and progressive-ratio response paradigms of sucrose pellet presentation in rats with transient inflammatory or chronic neuropathic pain. Assessment of response acquisition and break points under the progressive ratio schedule revealed no differences between sham and SNL rats for up to 120 days post-injury. However, rats with inflammation showed decrements in lever pressing and break points on post-CFA days 1 and 2 that normalized by day 4, consistent with transient ongoing pain. Thus, while acute, ongoing inflammatory pain may transiently reduce reward motivation, we did not detect influences of chronic neuropathic pain on hedonic or motivational responses to food rewards. Adaptations that allow normal reward responding to food regardless of chronic pain may be of evolutionary benefit to promote survival.
Keywords: hedonic response, facial reactivity, motivation, liking, food reward, operant responding
Introduction
Competing motivations including those induced by rewards such as palatable food can alter the experience of pain, allowing for optimal behavioral decisions that maximize benefit to the organism [17; 37]. As predicted by the motivation-decision model [16], a decreased pain response to a nociceptive stimulus is observed in the setting of available rewards [22]. Conversely, acute painful stimuli could diminish the value of rewards [3]. However, the relationship between reward value and chronic pain is not well understood.
Reward is a complex psychophysical construct comprised of multiple domains. Berridge and colleagues have dissociated hedonic pleasure (i.e., “liking”) both mechanistically and anatomically from reward-associated motivation (i.e., “wanting”) [10]. Motivational qualities of rewards are thought to be mediated by dopaminergic transmission in mesolimbic pathways while hedonic qualities are likely to be associated with the release of endogenous opioids in brain regions including the orbitofrontal cortex, the anterior cingulate cortex, the amygdala and the nucleus accumbens (NAc) [5–7; 35]. These reward-associated areas also show significant overlap with brain circuits important for pain and the reward of pain relief [3; 22; 28], providing a neural basis for interaction between pain and reward. Recent questionnaire-based studies in patients with chronic pain reported reduced reward responsiveness [14] and higher anhedonia scores in a subset of patients with breakthrough pain [27]; however, the potential influence of pain medications on these outcomes makes interpretation difficult. Whether there is a direct causal link between chronic pain and hedonic or motivational deficits remains unclear.
Experimental inflammatory or neuropathic pain can diminish some forms of reward in rodents including that resulting from intracranial self-stimulation or from morphine [21; 30; 32]. However, pain-induced alterations in responses to natural food rewards, including sucrose preference, have been variable, with some studies reporting decreased sucrose consumption [1; 23; 24; 42; 46; 49] or no change [39; 48]. Although no deficits were observed in sucrose preference in mice with either sciatic nerve injury-induced neuropathic pain or CFA-induced inflammation, decreased motivation for food reward using operant responding has been observed [39]. Whether hedonic responses to food rewards may be suppressed by pain has not been investigated preclinically. In the present study, we independently assessed possible effects of neuropathic pain on hedonic and motivational responses to food rewards using a facial reactivity score following passive oral delivery of tastants and single lever progressive ratio operant responding. To our knowledge this is the first use of the taste reactivity paradigm to assess the effects of aversive states on hedonic impact independently of motivation or incentive salience.
2. Materials and Methods
2.1 Animals
Male, Sprague Dawley (Harlan Laboratories Inc., Indianapolis, IN, USA) rats weighing 250–350 g were housed in climate-controlled rooms on a 12 h light/dark cycle with food and water provided ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Arizona or the IACUC committee at Eli Lilly and were in accordance with the National Institute of Health and the International Association for the Study of Pain. Animals were monitored throughout the duration of the study to reduce any unnecessary stress and/or pain.
2.2 Surgical Procedures
2.2.1 Intraoral catheter implantation
Bilateral intraoral catheters were implanted for taste reactivity as previously described [36]. Catheters were constructed using a 4-inch length of PE100 tubing, with the distal end of the PE tubing flared using a cautery device (Bovie Medical Corporation). A 6 mm plastic washer with a 3 mm hole in its center was threaded on to the tubing and placed against the flared end. The hub of a 14 mm 20-gauge steel needle was removed and the needle was connected to the opposing end of the tubing. Rats were anesthetized by intraperitoneal (i.p.) administration of ketamine/xylazine (80/12 mg/kg; Western Medical Supply/Sigma) and placed in a stereotaxic holder (Stoelting). The skull was exposed and four screws were placed adjacent to one another on either side of the interaural line to anchor the intraoral catheter. The needle end of the catheter was inserted in front of the first molar into the soft flesh where the skin and jaw connect. The needle was then used to guide the catheter underneath the skin anterior to the zygomatic arch and extruded up to the exposed skull. The tubing was pulled flush against the cheek bone, then trimmed to leave 10 mm of tubing visible. The steel tubing of the needle was bent at 90 degrees to form an “L” shape and cemented to the skull using dental cement leaving the open end of the steel tubing available through the acrylic. To prevent debris and particulates from accruing in the catheter, a 5 mm section of PE 100 tubing, heat sealed at one end, was fitted over the exposed end of the steel tubing. Immediately following catheter implantation rats underwent spinal nerve ligation (SNL) or sham surgery.
2.2.2 Catheter Maintenance
On post-surgery day 7 and 14 rats were anesthetized with isoflurane (2% mixed with oxygen; 2 l/min). Once the animal was deeply anesthetized, the position of the intraoral catheter was checked by lightly pressing a spatula on the tongue and pressing the bottom jaw open. If the catheter was positioned properly then the rats received an intraoral injection of 0.5 ml of water to keep the catheter patent and to verify that the injection was resulting in fluid entering the mouth.
2.2.3 Spinal Nerve Ligation (SNL)
The L5/L6 surgical procedure was used to produce experimental neuropathic pain as previously described by Kim and Chung [20]. For the taste reactivity experiments (Fig. 1 and 2), rats were anesthetized with 2% isoflurane oxygen mixture and the lumbar vertebrae exposed. The L5 and L6 spinal nerves were tightly ligated with 4-O silk suture and the wound closed. Sham-operated rats were prepared in the same manner as the SNL rats but without ligation of the L5/L6 spinal nerves. For the operant experiments (Fig. 3 and 4), the SNL and sham animals were purchased from Charles River Laboratories; animals were acclimated in the housing facility for a minimum of 7 days prior to experimentation. All rats were monitored for any signs of motor impairments and assessed for overall health.
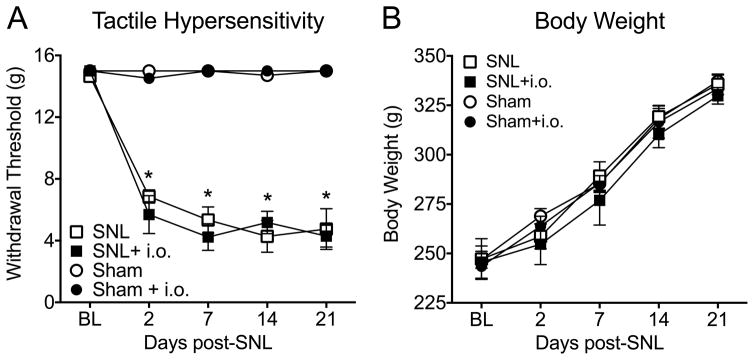
Figure 1. Intraoral catheterization does not alter tactile hypersensitivity or body weight in rats.
(A) Both SNL catheterized and non-catheterized rats displayed a significant reduction in paw withdrawal thresholds compared to sham rats starting on day 2 post-surgery (*p<0.0001). Sham rats in both groups had no significant change in tactile hypersensitivity thresholds compared to baseline (n=8–13). (B) Rats with and without intraoral catheterization did not display any alterations in weight gain over the time course, indicating that the catheterization did not impede food acquisition (n=8–12). All data are graphed as mean ± SEM.
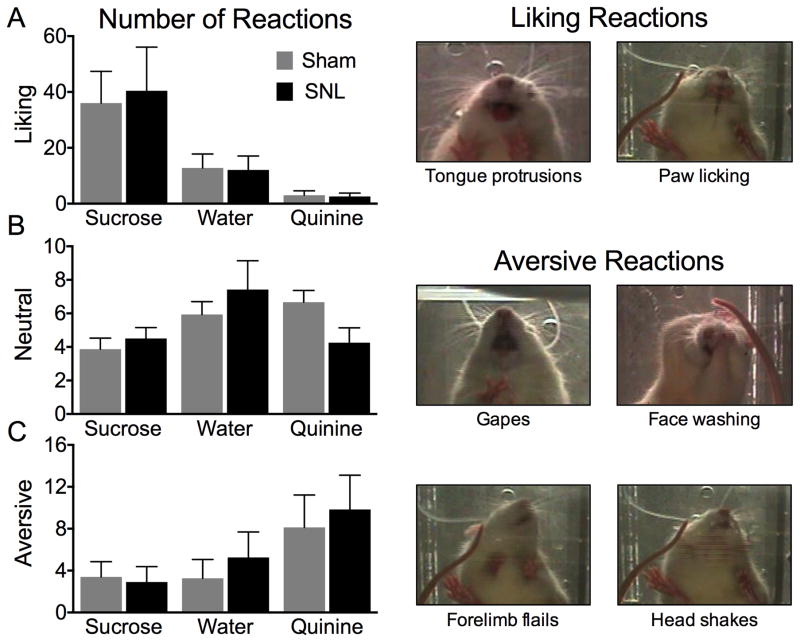
Figure 2. Neuropathic pain does not alter the hedonic or aversive impact of orally administered tasting substances.
Facial reactions to sweet (1% sucrose) neutral (water) and bitter (0.3 mM quinine) tastants in sham and SNL rats were scored according to the facial reactivity scale of Berridge [4]. Representative examples of “liking” and “aversive” reactions are shown on the right. (A) SNL and sham rats showed greater levels of “liking” reactions to sucrose compared to water and quinine. No difference in “liking” reactivity was detected between SNL or sham rats for each of the substances (n=11–13). (B) Neutral responses to intraoral sucrose, water, or quinine did not differ between SNL and sham rats (n=11–13). (C) Both cohorts of rats displayed the greatest amount of “aversion” reactions to quinine compared to the other substances, however, there was no difference in “aversive” reactivity between SNL or sham rats for each of the substances (n=11–13). All data are graphed as mean ± SEM.
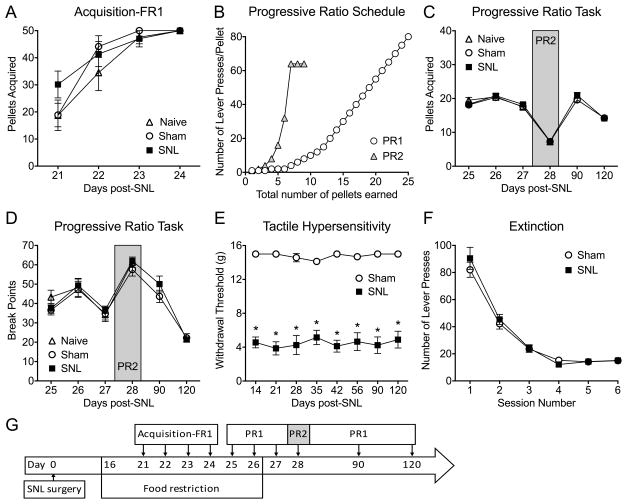
Figure 3. Neuropathic pain produced no significant differences in operant lever pressing tasks.
Naïve, sham-operated and SNL-operated groups of rats were trained to lever press for sucrose pellets according to schedules outlined in the time-line (E). (A) No significant difference in the number of lever presses during the FR1 acquisition phase was detected in SNL, sham, or naïve rats (n=12–16). (B) Graphical representation demonstrating differences in the number of lever presses required to earn a reward on the two progressive ratio schedules (PR1 and PR2). (C) Naïve, sham, and SNL rats all exhibited similar numbers of lever presses during each session of the progressive ratio task (n=12–16). (D) Sham or SNL surgery did not alter the last completed ratio (break point) on the progressive ratio task (n=12–16). (E) Tactile paw withdrawal responses of rats investigated in A, C and D were measured throughout the duration of the experiment (days 14 – 120). Compared to sham controls, SNL rats exhibited significantly decreased tactile withdrawal thresholds at all time points that were tested, confirming the presence of mechanical allodynia (n=12–16, *p<0.0001). (F) SNL did not alter the number of lever presses during the extinction trials (n=16). (G) Time line for experiments in Figs. A,C,D,E. Data are graphed as means ± SEM.
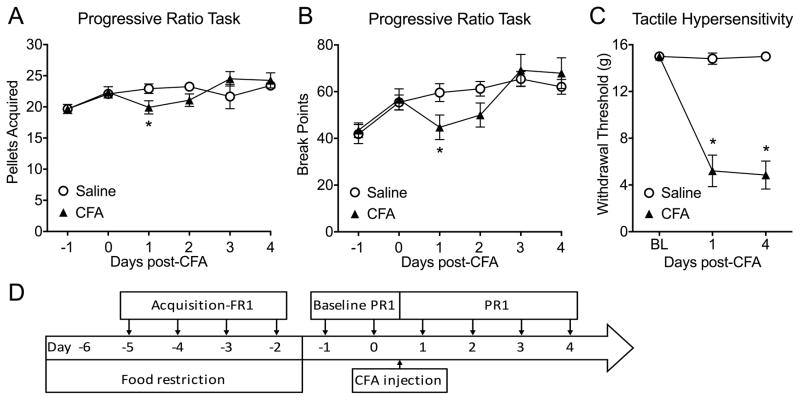
Figure 4. Inflammatory pain produced a transient significant difference in lever presses and break points in a progressive ratio task.
Rats were trained on an FR1 schedule to lever press for sucrose pellets for 4 consecutive days. Then, baseline progressive ratio responses (PR1) were recorded. After that, saline or CFA was injected in the left hind paw and animals were tested in the progressive ratios task (PR1), as shown in the timeline (D). (A) CFA produced a significant difference in lever presses during the progressive ratio task at day 1 (n=12, *p=0.0317). (B) The break point for CFA-treated rats was significantly decreased compared to saline-treated rats on day 1 (n=12, *p=0.0324). (C) CFA-injected rats displayed a significant reduction in paw withdrawal thresholds compared to saline treated rats at day 1 and day 4 post-CFA (n=12, *p<0.0001). Data are graphed as mean ± SEM.
2.3 Tasting Substances
Sucrose (1%; Sigma) or quinine hydrochloride (0.3 mM; Tocris) were dissolved in water to the final concentration and administered via intraoral catheters. Dustless Precision Pellets (Bioserv, 45 mg) were used for the operant experiments.
2.2.4 Complete Freund’s Adjuvant (CFA)
Rats were briefly anesthetized with isoflurane and received an intraplantar injection of complete Freund’s adjuvant (CFA; 100 μl, s.c., EMD Millipore, Temecula, CA, USA) into the left hindpaw. Control rats received an equivolume saline injection. Rats were tested 1, 2, 3, and 4 days following injection.
2.4 Behavioral Testing
2.4.1 Von Frey Testing
Rats were placed in suspended chambers with wire mesh bottoms for 30 min to habituate prior to testing. A series of calibrated von Frey filaments were applied perpendicular to the plantar surface of the ipsilateral hindpaw until the filament buckled. The up-down method was used to determine the 50% withdrawal threshold with the Dixon nonparametric test as described previously [11].
2.4.2 Taste Reactivity
2.4.2.1 Apparatus
A multi-chamber acrylic glass apparatus (4.5 × 6.5 × 5.5 inches per chamber) with a removable cover with breathing holes was used for this study. Behavior was recorded by a Sony digital camera pointed at a mirror positioned 2 inches directly underneath the apparatus.
2.4.2.2 Habituation to Intraoral Injection
On days 19 and 20 post-intraoral catheterization and SNL (or sham) surgery, rats were placed into the apparatus and allowed to habituate for 15 minutes. Following the acclimation period, PE100 tubing (24 inches) was connected to the end of the anchored intracranial steel tubing and threaded through one of the breathing holes. A 20-gauge needle attached to a 3 ml syringe was filled with water and connected to the tubing followed by filling the tubing with 354 μl of water (354 μl is required to fill 24 inches of PE100 tubing with an inner diameter of 0.86 mm). Afterwards, 1 ml of water was injected into the oral cavity of the rat. Injection of 1 ml into the rat’s mouth occurred over a 1 min time course (rate of 17 μl/s).
2.4.2.3 Testing
Testing for taste reactivity to sweet and bitter substances occurred on day 21 post-intraoral catheterization. As during the conditioning period, rats were placed into a multi-chamber apparatus and allowed to habituate for 15 minutes. PE100 tubing (24-inches) was connected to the steel tubing, and a 3-ml syringe was attached to the opposite end of the PE 100 tubing via a 20-gauge needle. Different PE100 tubing and a 3-ml syringe were used for different tastants to avoid mixing of solutions. Rats were then injected intraorally for one minute in the following sequence: sucrose (1%), water, and quinine (0.3 mM), with 1 ml flush with water immediately following each intraoral injection and 15 min separation in between injections. The dead volume of the intraoral catheter is approximately 15 μl, thus results in dilution of the final tastant concentration with water by about 1.5%. The doses of sucrose and quinine were selected because they elicit a moderate number of “liking” and “disliking” reactions [34]. During each 60-second injection the rats’ responses were video recorded. The 60 s segments of the video were cut by a different investigator prior to scoring of facial reactions to insure blinding. Rats were allowed to move freely throughout the duration of the experiment.
2.4.2.4 Taste Reactivity Scoring
Hedonic, neutral, and aversive reactions to intraorally administered substances were scored by a trained observer (AO) who was blinded to the experimental conditions in accordance with previously published rating scales [4]. The speed of the video footage capturing the facial and body responses of the rats was reduced to 1/10th actual speed to allow for accurate scoring. Hedonic, or “liking,” responses included rhythmic midline tongue protrusions, lateral tongue protrusions, and paw licks. Unpleasant, or “aversive,” responses included head shakes, gapes, face washes, forelimb flails, and chin rubs. Neutral responses included passive dripping of solution out of the mouth, ordinary grooming, and rhythmic mouth movements (representative examples of “liking” and “aversive” reactions are shown in Fig. 2).
A time bin scoring procedure was used so that taste reactivity components with distinct frequencies and durations were balanced in their contributions to the final affective hedonic or aversive total scores [4]. Tongue protrusions, gapes, chin rubs, forelimb flails, and head shakes typically occur as individual events and were therefore scored with 1 point each time that they occurred. Paw licks, rhythmic mouth movements, passive dripping of solution, and face washes typically occur for longer durations, and were scored as follows: 1 point if they occurred between 0–5 seconds, 2 points if they occurred between 5–10 seconds, and an additional point for each successive 5-second time frame.
2.4.3 Operant Responding
Neuropathic pain study
On day 16 post-surgery, food deprivation was implemented and rats received 85–90% of their normal free-feed (10–15 grams); the amount of food delivered was determined on a sliding scale based upon established need for animals of different weights as directed by veterinary guidelines. Food was given immediately following operant sessions after rats were returned to their home cages. The operant experiments consisted of a fixed-ratio (FR) training (acquisition) phase and a progressive-ratio (PR) testing phase (the experimental time-line is shown in Fig. 3G). On post-operative days 21–23 the rats were placed in 2-lever operant responding chambers (Med Associates, VT) and underwent training for 90 minutes. One lever was inactive during this period and the other lever delivered 1 sucrose pellet for each lever press (i.e., FR1 schedule). The active lever was counterbalanced such that half of the rats in each group had the active lever on the right side and the other half had it on the left side. The maximal number of pellets delivered in a single session was 50. On post-operative day 24, rats were again allowed to lever press for food; however, the session length was decreased to 30 minutes. Rats that did not complete the task (did not acquire 50 pellets) were excluded from the experiment (occurred in <5% of rats; no difference in the number of eliminated rats was observed between groups). Progressive ratio testing began on post-operative day 25 with the reinforcement schedule set such that the number of lever presses required to receive a pellet increased as each ratio (each pellet delivery) was completed. The initial progressive ratio schedule 1 (PR1) was as follows: (1,1,1,2,2,2,4,6,8,10,12,15,20,25,30,35,40,45,50,55,60,65,70,75,80). At the highest ratio, rats had to press the lever 80 times to obtain a food reward. Two operant sessions were given under these conditions, then food restriction was removed and rats received an additional operant session using the PR1 reinforcement schedule. On day 28, the reinforcement schedule was made more challenging by increasing the ratios more rapidly. The progressive ratio schedule 2 (PR2) for day 28 was as follows: (1,2,4,8,16,32,64). Rats were tested on day 90 and day 120 post-surgery using the PR1 schedule.
Assessment of extinction
Extinction was assessed in a separate group of rats. On day 16 post SNL or sham surgery, the same food deprivation protocol as in the progressive ratio experiment was implemented and on day 21, rats received the same acquisition training for the first 4 sessions. Then the reinforcement frequency was changed to an FR4 schedule, (i.e., rats received a pellet only once for every 4 presses). Following 4 days of FR4 training, an extinction paradigm (lever does not administer pellets at all) was assessed on 6 consecutive days (sessions). Rats were food-restricted as described above for every session during this experiment.
CFA study
Five days prior to beginning training, animals received food restriction to 85–90% of their free-feed based on body weight (10–15 g) (the experimental time-line is shown in Fig. 4D). Then the rats were trained to lever press for food using fixed ratio 1 schedule (FR1) as described above. On the 4th day of FR1 training the session length was decreased to 30 minutes. Rats that did not complete the task (did not receive 50 pellets) were excluded from the experiment (occurred in <5% of rats). Free access to food was then made available for the rest of the experiment. The next two consecutive days, the rats underwent pre-CFA baseline progressive ratio (PR1) testing. After the second baseline test the rats received intraplantar injection of CFA or saline (day 0). The rats underwent the same PR1 progressive ratio testing on post-CFA days 1, 2, 3, and 4.
2.5 Statistical Analysis
All statistical analyses were conducted with GraphPad Prism 6. Evoked tactile hypersensitivity was analyzed using two-way ANOVA with Sidak’s multiple comparisons post-hoc test. Taste reactivity scores and operant experiments containing 3 groups were analyzed using the two-way ANOVA. Operant experiments containing 2 groups were analyzed using 2-tailed unpaired T tests. Significance was set at p<0.05 for all tests.
3. Results
3.1 von-Frey and Body Weight in animals with neuropathic injury
SNL significantly decreased withdrawal threshold on the ipsilateral hindlimb at days 2–21 post SNL compared to sham operated rats, confirming the development of chronic neuropathic pain (Fig. 1A, *p<0.05). Intraoral catheters did not alter body weight at any time point suggesting that this procedure did not interfere with the ability of rats to consume food (Fig. 1B).
3.2 Taste Reactivity
To investigate if the presence of chronic neuropathic pain influences hedonic food responses, facial responses to sweet (1% sucrose), neutral (water), or bitter (0.3 mM quinine) tastants were assessed. The number of “liking” reactions in response to intraoral administration of sucrose, water, or quinine was not significantly different between SNL and sham rats (Fig. 2A). As expected, the number of “liking” reactions was significantly different for the different tastants (interaction: p=0.942, F(2,75)=0.06; tastant: p=0.0002, F(2,75)=9.73; group: p=0.872, F(1,75)=0.026; two-way ANOVA). SNL did not alter the number of “neutral” reactions compared to sham rats in response to intraoral administration of 1% sucrose, water, or 0.3 mM quinine (Fig. 2B); however, the effect of tastants was significantly different (interaction: p=0.097, F(2,75)=2.41; tastant: p=0.033, F(2,75)=3.56; group: p=0.896, F(1,75)=0.017; two-way ANOVA). Additionally, SNL rats did not show a significant difference in the number of “aversive” reactions in response to intraoral administration of 1% sucrose, water, or 0.3 mM quinine (Fig. 2C). Different tastants had significantly different effect on the number of “aversive” reactions (interaction: p=0.851, F(2,75)=0.162; tastant: p=0.038, F(2,75)=3.41; group: p=0.583, F(1,75)=0.304; two-way ANOVA). Collectively, no changes in passive “liking” or “disliking” responses to sweet or bitter tastes were observed at 21 days after SNL injury.
3.3 Operant Lever Pressing Acquisition, Progressive Ratio, Extinction
Next, we investigated if chronic pain may influence motivation to obtain food using operant lever pressing tests. All SNL, sham, and naive rats increased lever pressing for sucrose pellets during the FR1 acquisition task; however, no significant differences were observed between the groups in total number of sucrose pellets received (Fig. 3A, interaction: p=0.288, F(6,160)=1.24; time: p<0.0001, F(3,160)=33.93; group: p=0.251, F(2,160)=1.39). Rats were then tested on two different progressive ratio schedules, PR1 and PR2 (Fig. 3B) with or without food restriction (see the time line in Fig. 3G). No significant differences were observed between SNL, sham, and naive rats in total number of sucrose pellets received on any of the days that were tested up to 4 months after injury (Fig. 3C, interaction: p=0.877, F(5,174)=0.359; time: p<0.0001, F(5,174)=131.2; group: p=0.224, F(1,174)=1.49). The break points (the number of lever presses for the last completed ratio) for the SNL, sham, and naïve groups were also not significantly different (Fig. 3D, interaction: p=0.861, F(5,174)=0.382; time: p<0.0001, F(5,174)=38.76; group: p=0.153, F(1,174)=2.06). Tactile hypersensitivity was present throughout the experiment in rats with SNL injury (Fig. 3E). In a separate cohort of rats, the number of lever presses during extinction trials was assessed. SNL and sham rats decreased lever pressing over time, but no significant differences between SNL and sham-operated rats were observed (Fig. 3F, interaction: p=0.734, F(5,174)=0.556; time: p<0.0001, F(5,174)=119.1; group: p=0.460, F(1,174)=0.548).
Operant responding of rats with chronic neuropathic pain was compared to rats with transient inflammatory pain using an equivalent progressive ratio scheme (Fig. 4D). In contrast to SNL rats, CFA treated rats showed differential effects on the number of pellets earned (Fig. 4A, interaction: p=0.042, F(3,88)=2.858). On post-CFA day 1, CFA treated rats earned significantly fewer pellets (Fig. 4A, p=0.0317) and showed suppressed break points (Fig. 4B, p=0.0324) compared to saline-treated animals. By post-CFA days 3 and 4, however, injured rats earned a similar number of pellets and exhibited similar break points as their saline-treated controls (Fig. 4A, and 4B). CFA elicited significantly decreased withdrawal thresholds at all time points (Fig. 4C, *p<0.0001).
4. Discussion
This study assessed independently the impact of neuropathic pain on hedonic and motivational responses to food rewards. Using a facial reactivity scale, we showed that the number of “liking” and “disliking” responses to sweet or bitter tastants was not altered in rats with spinal nerve ligation, suggesting no effect of chronic neuropathic pain on hedonic value. Using a progressive ratio response paradigm to obtain sucrose pellets, we demonstrated no deficit in motivation to obtain a reward up to 4 months post-SNL. In contrast, rats with inflammatory pain showed significant but transient reductions in motivation at times that likely reflect the presence of ongoing pain. These data suggest differential effects of acute inflammatory and chronic neuropathic pain on motivation to obtain food reward.
The impact of chronic pain can be understood within the context of the biopsychosocial model as a disorder comprised not only of biological influences, but also of psychological and social factors that interact to promote disease and illness [15]. Multiple biological factors might contribute to suffering including neural adaptations in reward circuits [2; 41] that could suppress the value of appetitive rewards, alter reward motivation, or both. However, these factors are rarely evaluated independently. Data demonstrating hedonic or motivational deficits in chronic pain patients are scarce. Moreover, because of large variability among patient populations and because of the powerful effects of analgesic drugs on brain circuits, comprehensive evaluations of the effects of pain on reward deficits in humans are difficult and the outcomes of these investigations have been indecisive. For example, in patients with trigeminal neuralgia, myofacial pain dysfunction syndrome, and temporomandibular joint arthritis, multiple clinical variables were found to correlate with depression, but not with anhedonia [25]. Patients with chronic low back pain (CLBP) did not show altered ratings of pleasure or aversion to sweet, salty, or bitter tastants [43]. One study in CLBP patients found no change in response to sweet solutions but detected a significant decrease in pleasure from high fat pudding [18]. Completion of goal-oriented tasks and the desire to participate in activities were diminished in patients with neuropathic pain, suggesting possible deficits in motivation, but contributions of pain-related decreased physical mobility could not be ruled out [26; 29].
Several animal studies addressed potential reward deficits in pain under controlled experimental conditions; however, no studies directly investigated the effect of pain on hedonic quality of food reward. Berridge and colleagues have developed and validated a facial reactivity scale to assess hedonic liking in rodents independently of motivation [4; 8]. Research with human infants, monkeys, and rats show that the facial reactions to diverse tasting stimuli are conserved across mammalian species and reflect hedonic impact rather than changes in sensation or reflexes [4; 9; 44]. We used the facial reactivity scale to assess the number of “liking” reactions in response to intraoral sucrose solution and found no difference between SNL and sham rats, suggesting that neuropathic pain does not affect the hedonic component of reward, i.e., does not produce anhedonia. Furthermore, we saw no differences in the number of “disliking” reactions to 0.3 mM quinine. Thus, under the conditions of this study, SNL does not alter the affective spectrum in response to sweet or bitter taste. We note the possibility that variability of the responses observed could prevent detection of subtle changes in hedonic response. These results are consistent with data demonstrating that patients with chronic pain report the same degree of pleasure as healthy controls in response to consumption of a sweet solution [18; 43].
A recent study used an operant responding paradigm to investigate potential motivational deficits in mice with inflammatory (CFA) or neuropathic (SNI) pain [39]. A motivational deficit was demonstrated only when the animals were required to exert more effort to earn a food pellet (i.e., when the task was increased from an FR1 to an FR6 schedule) [39]. We evaluated the impact of SNL with a FR1 as well as two different progressive ratio schedules (PR1 and PR2, see Methods) and extended the observation time period up to 4 months following SNL. We did not detect any differences in food responding in SNL, sham, and naïve rats even at these prolonged time points. The reasons for the differences observed between these studies may be related to animal species (mice vs. rats) or different operant procedures (FR6 vs. PR reinforcement schedules or nose poke vs. lever press). Also noteworthy is that Schwartz and colleagues trained mice to acquire food pellets before pain induction, while our study started training at day 21 post-surgery. We also investigated whether food restriction may influence motivation to lever press for sucrose pellets. We saw a significant reduction in break points for all groups on day 27 when the rats had free access to food compared with day 26 when their food was restricted. However, there was no difference between the groups. It should be noted that changing learning conditions in later stages of training may not reveal motivational deficits under the new conditions, because lever pressing may have already gained a habitual component which is less susceptible to reward devaluation [45]. Schwartz and colleagues used food-restricted mice throughout the experiment [39].
In contrast to SNL-induced chronic pain, a small, but significant, decrease in lever pressing was observed in CFA-treated animals on day 1 post injury, confirming that the assay and conditions that we used are sufficiently sensitive to capture possible treatment-induced differences. Our data showed that the CFA-induced decrease in motivational response to food is transient and recovers at 3–4 days post-injury. Based on our previous report, CFA produces long-lasting evoked hypersensitivity, but ongoing pain, assessed by motivation to seek a context associated with pain relief, could only be demonstrated at day 1, but not day 4, following CFA [31]. The transient nature of ongoing pain found in animals is consistent with the presence of throbbing pain that persists for about 30 h after accidental injection of CFA in a human, in spite of much longer evoked hypersensitivity [19]. Thus, the diminished motivation to work for food pellets correlated temporally with the presence of ongoing pain, but not evoked hypersensitivity. In contrast, the previous study found significant difference in rewards earned in mice 7–21 days post CFA [39]. This discrepancy could reflect species or experimental differences.
Notably, SNL-induced ongoing pain has been demonstrated in our laboratory for time periods up to 60 days [47]. While ongoing pain was likely present in the SNL rats that were studied, this could not be verified in these rats. We found no differences between SNL and sham-operated groups in food responses up to 120 days post-surgery. Reasons for the differences between CFA and SNL rats could include the relative intensity of ongoing pain in these two models or possible compensatory adaptations occurring at later time points following neuropathic pain that result in normalization of reward responding. According to the motivation-decision model [16], pain demands motivational responses to be transiently directed toward healing and protection of the injured site from further damage. In chronic pain however, adaptations that allow normal reward responding are evolutionarily beneficial as continuing diversion from food consumption jeopardizes survival. It remains to be seen whether differences between SNL and sham-operated rats would be observed at the early, acute stage of pain induction.
Notably, no differences were observed in animals with neuropathic pain in extinction from operant responding for food pellets. Operant extinction responding may occur as a result of multiple mechanisms including the formation of new memories (new learning) and re-learning that overcome established reinforced behaviors [12]. Chronic pain itself involves learning (memory traces of pain) [38] and may be associated with neural adaptations in the brain regions important for cognitive functions, memory and decision-making [33]. Due to the overlap between neural circuitry of pain and reward, chronic pain could interfere with food reward learning, including the acquisition, consolidation, maintenance, or extinction of reward seeking. Our findings suggest that ongoing neuropathic pain does not impair the ability of animals to acquire or extinguish food reward memories.
In summary, we used multiple approaches to investigate the influence of experimental chronic neuropathic pain on food-mediated reward responding including independent evaluation of hedonic response to passive delivery of sweet or bitter solution and motivational drive to food reward. We were not able to identify differences in either parameter in SNL animals. In contrast, we found motivational deficits in a rat model of transient inflammatory pain. Preclinical studies of chronic pain have demonstrated significant impact on brain structure and function, particularly at long post-injury time points [13; 40]. Our findings suggest that neuropathic pain-induced changes in the brain do not significantly influence hedonic value for sweet or bitter tastants or motivation to obtain food reward. It is possible that compensatory mechanisms that restore normal reward function may occur to insure survival. Further work will be required to determine if chronic pain may alter hedonic impact or motivation under other conditions. Nevertheless, the current findings are largely consistent with clinical experience and reveal surprisingly little effect of chronic pain on food-associated pleasure and motivation.
Acknowledgments
The authors thank both the University of Arizona and Eli Lilly and Company for their collaborative support and funding of this work. A.O. is a recipient of a LIFA award from Eli Lilly and Company. This work was partially funded by DA034975 from the NIH-NIDA.
Footnotes
Conflict of Interest: We have no conflicts of interest to declare.
References
- 1.Amorim D, David-Pereira A, Pertovaara A, Almeida A, Pinto-Ribeiro F. Amitriptyline reverses hyperalgesia and improves associated mood-like disorders in a model of experimental monoarthritis. Behav Brain Res. 2014;265:12–21. doi: 10.1016/j.bbr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci Lett. 2012;520(2):182–187. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 7.Berridge KC. Wanting and Liking: Observations from the Neuroscience and Psychology Laboratory. Inquiry (Oslo) 2009;52(4):378. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bimpisidis Z, De Luca MA, Pisanu A, Di Chiara G. Lesion of medial prefrontal dopamine terminals abolishes habituation of accumbens shell dopamine responsiveness to taste stimuli. Eur J Neurosci. 2013;37(4):613–622. doi: 10.1111/ejn.12068. [DOI] [PubMed] [Google Scholar]
- 10.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34(12):4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Clem RL, Schiller D. New Learning and Unlearning: Strangers or Accomplices in Threat Memory Attenuation? Trends Neurosci. 2016 doi: 10.1016/j.tins.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrov EL, Tsuda MC, Cameron HA, Usdin TB. Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J Neurosci. 2014;34(37):12304–12312. doi: 10.1523/JNEUROSCI.0312-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elvemo NA, Landro NI, Borchgrevink PC, Haberg AK. Reward responsiveness in patients with chronic pain. Eur J Pain. 2015;19(10):1537–1543. doi: 10.1002/ejp.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 16.Fields HL. A Motivation-Decision Model of Pain: The Role of Opioids. In: Dostrovsky HFEKJO, editor. Proceedings of the 11th World Congress on Pain; 2006; pp. 449–459. [Google Scholar]
- 17.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci. 2009;29(41):13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geha P, Dearaujo I, Green B, Small DM. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain. 2014;155(4):712–722. doi: 10.1016/j.pain.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Gould HJ., 3rd Complete Freund’s adjuvant-induced hyperalgesia: a human perception. Pain. 2000;85(1–2):301–303. doi: 10.1016/s0304-3959(99)00289-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 21.Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Jr, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain. 2014;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 23.Liu YT, Shao YW, Yen CT, Shaw FZ. Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiol Behav. 2014;131:105–110. doi: 10.1016/j.physbeh.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Experimental gastritis leads to anxiety- and depression-like behaviors in female but not male rats. Behav Brain Funct. 2013;9:46. doi: 10.1186/1744-9081-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marbach JJ, Lund P. Depression, anhedonia and anxiety in temporomandibular joint and other facial pain syndromes. Pain. 1981;11(1):73–84. doi: 10.1016/0304-3959(81)90140-8. [DOI] [PubMed] [Google Scholar]
- 26.Mauskopf J, Austin R, Dix L, Berzon R. The Nottingham Health Profile as a measure of quality of life in zoster patients: convergent and discriminant validity. Qual Life Res. 1994;3(6):431–435. doi: 10.1007/BF00435395. [DOI] [PubMed] [Google Scholar]
- 27.Narayana A, Katz N, Shillington AC, Stephenson JJ, Harshaw Q, Frye CB, Portenoy RK. National Breakthrough Pain Study: prevalence, characteristics, and associations with health outcomes. Pain. 2015;156(2):252–259. doi: 10.1097/01.j.pain.0000460305.41078.7d. [DOI] [PubMed] [Google Scholar]
- 28.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17(10):1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(Suppl 1):S9–S27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- 30.Niikura K, Narita M, Narita M, Nakamura A, Okutsu D, Ozeki A, Kurahashi K, Kobayashi Y, Suzuki M, Suzuki T. Direct evidence for the involvement of endogenous beta-endorphin in the suppression of the morphine-induced rewarding effect under a neuropathic pain-like state. Neurosci Lett. 2008;435(3):257–262. doi: 10.1016/j.neulet.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 31.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience. 2009;161(3):671–679. doi: 10.1016/j.neuroscience.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 36.Phillips MI, Norgren R. A Rapid Method for Permanent Implantation of an Intraoral Fistula in rats. Behavioral Research Methods and Instruments. 1970;2(3) [Google Scholar]
- 37.Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1–2):140–147. doi: 10.1016/j.pain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Sandkuhler J, Lee J. How to erase memory traces of pain and fear. Trends Neurosci. 2013;36(6):343–352. doi: 10.1016/j.tins.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345(6196):535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47(3):1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi M, Wang JY, Luo F. Depression shows divergent effects on evoked and spontaneous pain behaviors in rats. J Pain. 2010;11(3):219–229. doi: 10.1016/j.jpain.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Small DM, Apkarian AV. Increased taste intensity perception exhibited by patients with chronic back pain. Pain. 2006;120(1–2):124–130. doi: 10.1016/j.pain.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 45.Thrailkill EA, Bouton ME. Contextual control of instrumental actions and habits. J Exp Psychol Anim Learn Cogn. 2015;41(1):69–80. doi: 10.1037/xan0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115(4):812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, King T, De Felice M, Guo W, Ossipov MH, Porreca F. Descending Facilitation Maintains Long-Term Spontaneous Neuropathic Pain. J Pain. 2013 doi: 10.1016/j.jpain.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XQ, Zhong XL, Li ZB, Wang HT, Zhang J, Li F, Zhang JY, Dai RP, Xin-Fu Z, Li CQ, Li ZY, Bi FF. Differential roles of hippocampal glutamatergic receptors in neuropathic anxiety-like behavior after partial sciatic nerve ligation in rats. BMC Neurosci. 2015;16:14. doi: 10.1186/s12868-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci. 2014;34(33):10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]