Abstract
SHANK3 is a synaptic scaffolding protein localized in the postsynaptic density and has a crucial role in synaptogenesis and neural physiology. Deletions and point mutations in SHANK3 cause Phelan–McDermid Syndrome (PMS), and have also been implicated in autism spectrum disorder (ASD) and intellectual disabilities, leading to the hypothesis that reduced SHANK3 expression impairs basic brain functions that are important for social communication and cognition. Several mouse models of Shank3 deletions have been generated, varying in the specific domain deleted. Here we report impairments in cognitive function in mice heterozygous for exon 13–16 (coding for the PDZ domain) deletion. The touchscreen pairwise discrimination task was chosen by virtue of its: (a) conceptual and technical similarities to the Cambridge Neuropsychological Test Automated Battery (CANTAB) and NIH Toolbox Cognition Battery used for testing cognitive functions in humans, (b) minimal demand on motor abilities, and (c) capability to measure many aspects of learning and memory and complex cognitive functions, including cognitive flexibility. The similarity between our mouse tasks and human cognitive assays means a high translational validity in future intervention studies using preclinical models. Our study revealed that Shank3B heterozygous mice (+/–) were slower to reach criterion in the pairwise visual discrimination task, and exhibited trends toward making more errors (first trial errors) and more correction errors than wildtype mice (+/+). Open field activity was normal in +/–, ruling out hypo- or hyperactivity as potential confounds in the touchscreen test. Sociability in the three chamber test was also normal in both +/+ and +/–. These results indicate a deficit in discrimination learning in the Shank3B model of PMS and ASD, suggesting that this mouse model is a useful preclinical tool for studying neurobiological mechanisms behind cognitive impairments in PMS and ASD. The current findings are the starting point for our future research in which we will investigate multiple domains of cognition and explore pharmacological interventions.
Keywords: SHANK3, Phelan–McDermid Syndrome, autism, touchscreen, mouse models, associative learning
Introduction
SHANKs are scaffolding proteins enriched in the postsynaptic density. They are crucial for the formation and stabilization of synapses (Qualmann et al., 2004; Grabrucker et al., 2009). SHANK3 (also referred to as PROSAP2) encodes a structural component of excitatory synapses important for synaptic morphology and functions (Herbert, 2011; Harony-Nicolas et al., 2015). Consisting of five domains (ankyrin repeats, SH3, PDZ, proline-rich, and SAM) (Naisbitt et al., 1999; Sheng and Kim, 2000; Bourgeron, 2007; Buxbaum, 2009), SHANK3 can interact with multiple key synaptic components, including glutamate receptor complexes, anchoring proteins, and actin cytoskeleton (Bockers et al., 2001; Roussignol et al., 2005; Baron et al., 2006; Durand et al., 2008; Bertaso et al., 2010). Heterozygous deletions or point mutations of SHANK3 are thought to be the main cause of Phelan–McDermid Syndrome (PMS, also referred to as 22q13 Deletion Syndrome), a genetic disorder characterized by global developmental delays, delayed or absent speech, moderate to severe intellectual disability, autism, some dysmorphic features, neonatal hypotonia, and seizures (Bonaglia et al., 2001; Phelan, 2008; Phelan and McDermid, 2012; Harony-Nicolas et al., 2015). Haploinsufficiency of SHANK3 due to deletion or de novo mutations occurs in approximately 1% of autism spectrum disorder (ASD) cases, making SHANK3 abnormalities one of the most common genetic causes of autism (Durand et al., 2007; Moessner et al., 2007; Buxbaum, 2009; Betancur and Buxbaum, 2013; Boccuto et al., 2013).
In addition to impaired social communication and repetitive behaviors, a hallmark feature of autism is restricted interests, deficits in set shifting and behavioral inflexibility (Dawson et al., 2002; D'Cruz et al., 2013; de Vries et al., 2015; Miller et al., 2015). A crucial step toward understanding cognitive inflexibility in ASD is to characterize associative learning in this disorder.
Our present study aimed at evaluating associative learning in the Shank3B model of PMS and ASD. Four independent groups have generated mouse models of Shank3 deficiency or ablation (Bozdagi et al., 2010; Peca et al., 2011; Wang et al., 2011; Kouser et al., 2013), and impaired learning and memory have been reported in all models. The current study employed the PDZ domain deletion model originally generated in the Feng lab (Peca et al., 2011). This model has both construct validity (reduced expression of Shank3 mRNA and protein) and some face validity (Excessive/injurious repetitive self-grooming and altered sociability) (Peca et al., 2011). +/+ and +/– were used in the current study, because: (a) heterozygous deletion is translational and analogous to deletions found in clinical populations, (b) excessive/injurious self-grooming and low general locomotor activity in null mutants of this line could confound results of the touchscreen operant learning task. Since no previous studies have evaluated complex learning in any of the deletion models, we chose the automated touchscreen task for its conceptual and technical similarities to the Cambridge Neuropsychological Test Automated Battery (CANTAB), an automated computerized battery of cognitive assays commonly used to test cognitive function in humans. In order to rule out hypo- or hyperactivity as confounds in this cognitive assay, we conducted the open field test to measure general locomotor activity. In a previous study on the Shank3B model, altered sociability was found in null mutants, but no data were reported in heterozygous mutants (Peca et al., 2011). Given the relevance of heterozygous deletions to the human disease condition, we therefore also evaluated sociability in the three chamber test in the current study.
Experimental Procedures
Subjects
All procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of California Davis, and followed the NIH Guide for the Care and Use of Laboratory Animals. The Shank3B line characterized by a mutation within the PDZ domain was originally generated by the Feng lab (Peca et al., 2011). A neo cassette replaced exons 13–16 of the Shank3 gene, resulting in a deficiency of isoforms Shank3α and Shank3β, and a reduction in expression of the Shank3γ isoform. Breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, Maine, stock #01768). Genotype was determined by standard PCR, with the following primers: primer F1b (GAGCTCTACTCCCTTAGGACTT) and R1b (TCCCCCTTTCACTGGACACCC) for the wild-type allele (316 base pairs), and F1b and R2 (TCAGGGT-TATTGTCTCATGAGC; in the neo cassette) for the mutant allele (360 base pairs). The neo cassette was not removed. Heterozygous (+/–) males and females were bred to generate subject mice used in the present study. Juveniles were weaned between 21 and 24 days of age and housed by sex in cages of 2–4 littermates per cage. Group-housed male subjects were tested between 3 and 5 months of age. Cohort 1 was used for the touchscreen experiment, Cohort 2 for the open field assay, and Cohort 3 for the social approach assay and repetitive self-grooming. Standard rodent chow and tap water were available ad libitum prior to the start of the touchscreen experiment. In addition to standard bedding, a Nestlet square and a cardboard tube (Jonesville Paper Tube Corp., Michigan) were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle with lights on at 7:00 AM, and at approximately 20 °C and 55% humidity. Behavioral testing was conducted between 9:00 AM and 5:00 PM.
Touchscreen pairwise discrimination
Pairwise visual discrimination was tested in the automated Bussey-Saksida touchscreen apparatus for mice (Campden Instruments Ltd/Lafayette Instruments, Lafayette, IL, USA), using a procedure modified based on original methods described previously (Bussey et al., 2000; Brigman and Rothblat, 2008; Bussey et al., 2012; Brigman et al., 2013; DePoy et al., 2013; Oomen et al., 2013; Silverman et al., 2015b). The reinforcer was 20 μl of a palatable liquid nutritional supplement (Strawberry Ensure Plus, Abbott, IL, USA) diluted to 50% with water. Each session was conducted under overhead lighting (∼60 lux). A standard tone cue was used to signal the delivery of the reinforcer during pre-training and acquisition. Prior to pre-training, subject mice were weighed, and placed on a restricted diet of 2–4 g of rodent chow per mouse per day, to induce a 15% weight loss. Body weight was carefully monitored throughout the experiment, to ensure that a minimum of 85% of free feeding body weight was maintained for each mouse.
An efficient pre-training regimen was validated with pilot animals (personal communication with Dr. Stacey Rizzo, Jackson Laboratory) and utilized based on previously published work (McTighe et al., 2013). The pre-training consisted of four stages. Stage 1 consisted of two days of habituation (20 min on day 1, and 40 min on day 2) to the chamber and the liquid diet with no images on the screen under overhead lighting (∼60 lux). Stage 2 was a single 45-min session in which entering and exiting the food magazine initiates the next trial and triggers additional reward under overhead lighting. During Stage 3, subjects were trained in daily 45-min sessions during which an image (a random picture from a selection of 40 images) was presented in one of the two windows, and remained on the screen until it was touched. Mice must complete 30 trials/day for two consecutive days in order to advance to the next stage. In Stage 4, subjects were trained in 45-min daily sessions in which touching the blank side of the screen was discouraged with a 5-s time-out during which the overhead lighting turned off. Completion of at least 30 trials, at an average accuracy of 80%, on two consecutive days, is required for advancement. Images used in Stages 3 and 4 were not used in the subsequent discrimination task. Only mice that completed all stages of pre-training were advanced to the pairwise visual discrimination task. Subjects were trained to discriminate between two novel images, a spider and an airplane, presented in a spatially pseudo-randomized manner in the two windows of the touchscreen. Each 45-min session consisted of unlimited number of trials separated by 15-s inter-trial intervals (ITI). Designation of the correct and incorrect images was counterbalanced across mice within each genotype. Correct responses were rewarded. Each incorrect response was followed by a correction trial in which the images were presented in an identical manner to the previous trial, until a correct response was made. Criterion was completing at least 30 trials, at an accuracy of 80% or higher, on two consecutive days. Days to reach criterion, percentage of mice reaching criterion on each day, number of errors, correction errors, and total trials were compared between genotypes. Our more time-consuming, five-stage pre-training procedure was described previously (Silverman et al., 2015a,b; Yang et al., 2015).
Open field activity
Open field exploratory activity was evaluated as previously described (Yang et al., 2009; Silverman et al., 2010a–c; Yang et al., 2012; Silverman et al., 2015a,b). Briefly, each animal was tested in a VersaMax Animal Activity Monitoring System (Accuscan, Columbus, OH, USA) for a 30-min session. Total distance traversed, horizontal activity, vertical activity, and time spent in the center were automatically measured.
Automated three-chambered social approach task
Social approach was assayed using methods modified based from our previous studies (Yang et al., 2009; Silverman et al., 2010a–c; Silverman et al., 2011; Yang et al., 2011, 2012; Silverman et al., 2015a,b). The current methods were recently described in (Silverman et al., 2015a,b). Each rectangular three-chambered apparatus (40 cm × 60 cm × 23 cm) was made of non-reflective matte white finished acrylic (P95 White, Tap Plastics, Sacramento, CA, USA). Opaque retractable doors (12 cm × 33 cm with 5 cm × 10 cm doorways) separated the compartments and allowed entries across chambers. Time spent in each chamber was detected using the EthoVision XT videotracking software (Version 9.0, Nol-dus Information Technologies, Leesburg, VA, USA). Sniffing was defined as head facing the cup enclosure (inverted wire cup, Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com) with the nose point within 2 cm from the enclosure. Two infrared sensitive cameras (Ike-gami ICD-49, B&H Photo, New York, NY, USA) mounted directly above four three-chambered units recorded the test sessions. Infrared lighting (Nightvisionexperts.com) provided uniform dim illumination. Time spent in each chamber and time spent sniffing each cup were automatically measured using the Ethovision software (Noldus Information Tech Inc., Leesburg, VA, USA).
Repetitive self-grooming
The self-grooming test was conducted in empty clean mouse cages. Each animal was habituated to the cage (with the plastic lid on and the food hopper off) for 10 min and recorded for self-grooming behavior for the following 10 min. Recorded videos were scored by two investigators blinded of genotype information. Interrater reliability was >95%.
Statistical analysis
Touchscreen parameters (days to reach criterion, trials to criterion, errors to criterion, and correction errors to criterion) were analyzed with paired t-test. Log-rank Mantel-Cox test was used to analyze the percentage of animals that reached criteria in the survival/completion analysis for the touchscreen test. Open field parameters (total distance traveled, horizontal activity, vertical activity, and center time) were analyzed with Repeated Measures ANOVA, with genotype as the between-group factor and time as the within-group factor. Repeated Measures ANOVA (∼ paired t-test) was used to analyze social approach data. Comparisons between time spent in the chamber with the novel mouse and time spent in the chamber with the novel object were compared within each genotype. Similarly, time sniffing the novel stimulus mouse versus time sniffing the novel object were compared within each genotype, as previously described (Silverman et al., 2010; Yang et al., 2011; Silverman et al., 2015a,b). Self-grooming data were analyzed using paired t-test.
Results
Poor performance of −/− during pre-training stages
Eight null mutants (−/−) were initially included in the study. Genotype differences were not statistically significant for numbers of trials completed on habituation day 1 (F2,26 = 1.40, NS) or habituation day 2 (F2,26 = 1.82, NS), although trends were observed for −/− to complete fewer trials than +/+ on both days. As shown in Table 1, significant genotype effects were found in a number of trials completed in Stage 2 (F2,26 = 3.63, p < .05) and days to reach criterion in Stage 3 (F2,26 = p < 5.29, p < .05). Tukey's post hoc analysis indicated that −/− completed significantly fewer trials in Stage 2 and required more days to reach criterion in Stage 3, as compared to +/+ (p < .05 for each comparison).
Table 1.
Pre-training performance in Shank3B mice. Shank3 homozygous mutants (−/−) exhibited trends toward completing fewer trials in Stage 1, completed significantly fewer trials in Stage 2, and required significantly more days to reach criterion in Stage 3.
| Pre-training stage | +/+ (N = 7) | +/– (N = 14) | −/− (N = 8) | ANOVA p value |
|---|---|---|---|---|
| Stage 1: Habituation Day 1 # of trials | 15.9 ± 3.8 | 15.2 ± 2.9 | 8.8 ± 1.7 | 0.26 |
| Stage 1: Habituation Day 2 # of trials | 78.6 ± 11.2 | 74.2 ± 14.1 | 41.1 ± 14.1 | 0.08 |
| Stage 2: # of trials | 38.1 ± 10.6 | 25.4 ± 3.8 | 13.1 ± 4.4* | 0.04 |
| Stage 3: Days to reach criterion | 2.4 ± 0.4 | 4.0 ± 0.76 | 6.63 ± 1.0* | 0.01 |
p < .05 or less vs. +/+
Touchscreen pairwise discrimination deficits
As illustrated in Fig. 1, +/– mice required significantly more training days to learn to discriminate two images displayed on the touchscreen (Fig. 1A, t = 2.36, p < .05). Analysis of survival curves, i.e. percentage of mice that reached the 80% accuracy criterion on each training day indicated that the percentage of mice that reached criterion was significantly lower in +/– than in +/+ (Fig. 1B, Log-rank Mantel-Cox test, χ2 = 19.39, p < .001).
Fig. 1.
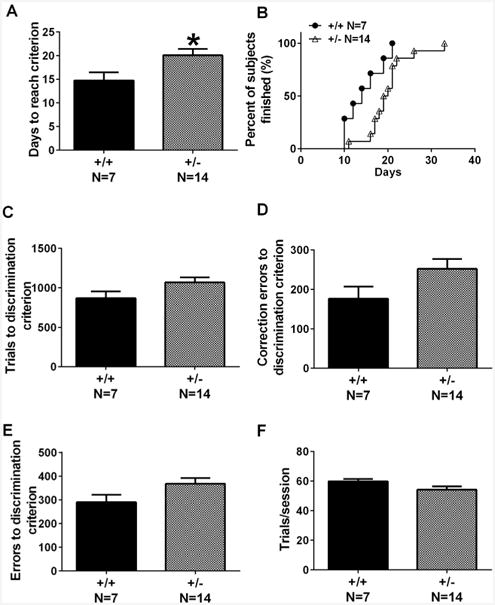
Touchscreen pairwise discrimination deficits in Shank3B mice. (A) +/– took significantly more training days to reach the criterion of 80% correct responses on the pairwise visual discrimination during the initial acquisition. (B) The percentage of mice that reached criterion across the training days was significantly lower in +/– than in +/+. (C–E) During discrimination training, +/– exhibited trends toward making more trials to reach criterion, more correction errors, and more errors. (F) No genotype differences were found in trials per session. Data are presented as mean ± standard error of the mean in all figures (except (B)). *p < .05 vs. +/+.
Analysis of additional parameters indicated that +/– mice exhibited a trend toward requiring more trials to reach criterion, compared to +/+ controls (Fig. 1C, t = 1.87, p = 0.078), suggesting slower learning. Trends were also detected for +/– to make more errors (first trial error) (Fig. 1E, t = 1.93, p = .069) and more correction errors (Fig. 1D, t = 1.86, p = 0.085) compared to +/+. Importantly, the two genotypes did not differ in average trials per session (Fig. 1F, t = 1.62, p = 0.12), indicating that both genotypes were actively engaged in the learning task. These data corroboratively indicated a deficit in visual discrimination learning in +/–.
Pre-training performance could reveal motor or motivational deficits, as well as general deficits in acquiring touchscreen tasks. During pre-training stages, we analyzed trials/session across three genotypes for Stages 1 and 2, and between +/+ and +/– for Stages 3 and 4. As shown in Table 1, trials/session did not differ between +/+ and +/– in Stages 1 and 2. No significant genotype differences were found in trials/ session in Stage 3 (t = −1.267, NS) or Stage 4 (t = 1.747, p = 0.097). In Stages 3 and 4, the animals either touch the window with an image in it, or touch the blank window. We termed the response “image touch” and “blank touch”. In Stage 3 (in which the animals were trained to touch the window with an image instead of the blank window), no genotype differences were found in days to reach criterion (Fig. 2A, t = −2.7, NS), total trials to criterion (Fig. 2B, t = −1.27, NS), and % blank touches expressed as blank touches/total touches × 100 (Fig. 2C, t = 1.45, NS). In Stage 4 (in which the animals were given a brief timeout for each incorrect response), no significant genotype differences were found for days to reach criterion (Fig. 2D, t = −0.14, NS), total trials to criterion (Fig. 2E, t = 1.747, NS), and % blank touches (Fig. 2F, t = 1.196, NS), suggesting that +/– did not have deficits in acquiring or participating in the touchscreen assay. −/− were not advanced to pairwise visual discrimination, due to their poor performance in pre-training. To detect genotype differences in task-participation at different pre-training stages, we analyzed trials/session across three genotypes for Stages 1 and 2, and between +/+ and +/– for Stages 3 and 4. As shown in Table 1, trials/session did not differ between +/+ and +/– in Stages 1 and 2. No significant genotype differences were found in trials/session in Stage 3 (t = −1.27, NS) or Stage 4 (t = 1.75, p = 0.097). No genotype differences were found in blank touches/session in Stage 3 (t = −0.30, NS) or Stage 4 (t = 1.19, NS). Data not shown.
Fig. 2.
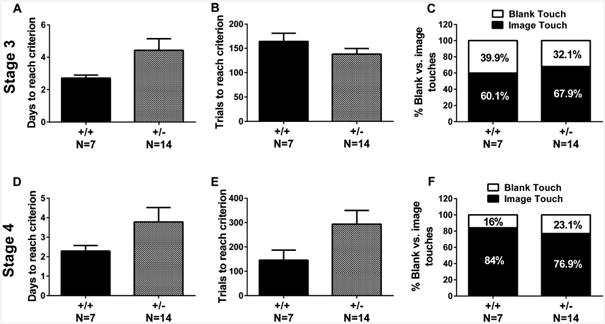
Normal pre-training performance in Shank3B +/– mice. Pre-training performance could reveal motor or motivational deficits in +/–. (A, D) +/+ and +/– did not differ in days to reach criterion in Stages 3 or 4. (B, E) Trials to reach criterion was not different between genotypes in Stages 3 or 4. (C, F) % blank touches did not differ between +/+ and +/– in Stages 3 or 4.
Open field
Fig. 3 illustrates normal open field activity in +/– and reduced activity in –/–. Significant genotype differences were found in total distance traveled (F2,41 = 3.35, p < .05), horizontal activity (F2,41 = 4.5, p < .01), vertical activity (F2,41 = 7.8, p < .01), and center time (F2,41 = 5.5, p < .01). Post-hoc analysis revealed that −/− were significantly reduced on all four parameters (p < .01 for each comparison).
Fig. 3.
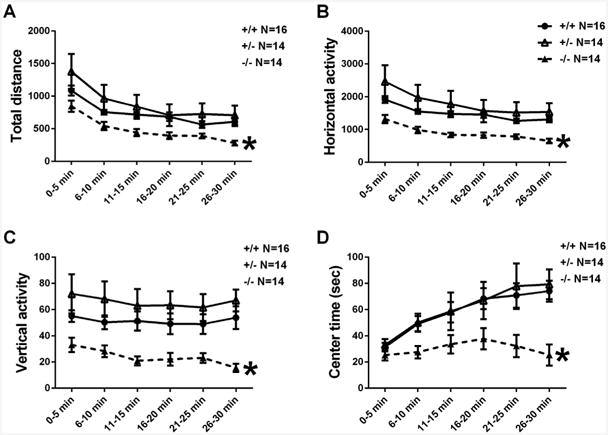
Normal open field activity in Shank3B +/– mice. (A–D) Significant genotype differences were found on total distance traveled, horizontal activity, vertical activity, and center time. Post hoc analysis revealed that −/− were significantly reduced on all four parameters. We excluded −/− from the touchscreen experiment, because of their low locomotor activity could greatly confound touchscreen performance.
Social approach in the three-chambered apparatus
As Fig. 4 illustrates, normal sociability in the three-chambered task was detected in both +/+ and +/– genotypes. Both genotypes spent more time in the chamber with the novel stimulus mouse than in the chamber with the novel object (+/+: F1,13 = 14.7, p < .01; +/–: F1,13 = 6.3, p < .05). Similarly, both genotypes spent more time sniffing the novel mouse than the novel object (+/+: F1,13 = 4.3, p < .05; +/–: F1,13 = 10.0, p < .01). Number of transitions across chambers was not different between genotypes during the 10-min habituation phase (Fig 4D, F1,26 = 0.54, NS) or in the sociability phase (Fig 4C, F1,26 = 1.62, NS), ruling out hypo- or hyperactivity as influencing factors and providing corroborating measures to the open field data.
Fig. 4.
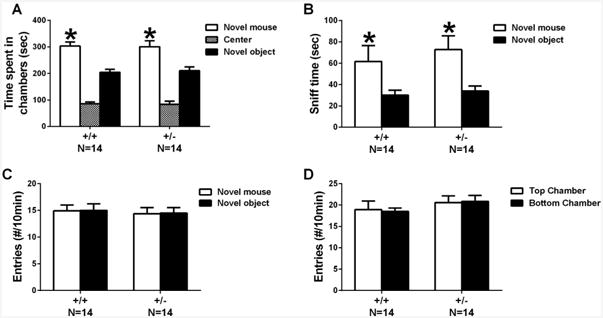
Normal social approach in Shank3B +/– mice. (A) Both genotypes spent more time in the chamber with the novel stimulus mouse than in the chamber with the novel object. (B) Both genotypes spent more time sniffing the novel mouse than the novel object. (C, D) Number of transitions across chambers was not different between genotypes during the 10-min habituation phase or in the sociability phase. *p < .05 novel mouse vs. novel object.
Repetitive self-grooming
As shown in Fig. 5, a trend was observed for +/– to exhibit increased self-grooming as compared to +/+ (t = 1.80, .05 < p < .10, NS). No skin lesions were observed in +/–.
Fig. 5.
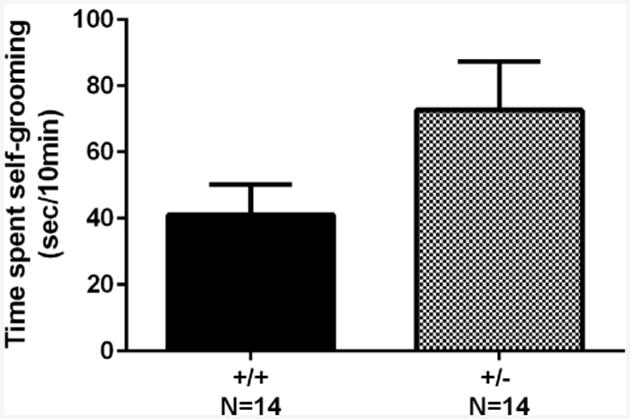
Modestly increased self-grooming in Shank3B +/– mice. +/– mice exhibited modestly increased self-grooming. No skin lesions were observed in +/–.
Discussion
Mouse models are indispensable tools for studying neurobiological mechanisms behind cognitive impairments caused by genetic abnormalities. Cognitive functions have been studied in a number of Shank3 deletion models, using simple assays (Bozdagi et al., 2010; Peca et al., 2011; Wang et al., 2011; Kouser et al., 2013). One line of mice homozygous for the exon 4–9 (coding for ankryin domain) deletion exhibited impaired novel object recognition but normal fear conditioning and spatial learning (Yang et al., 2012). Null mutants of a second line of the exon 4–9 deletion exhibited impaired reversal learning in the Morris water maze test (Kouser et al., 2013). Mice homozygous for the exon 13–16 (coding for the PDZ domain) deletion exhibited normal spatial learning (Peca et al., 2011). Mice homozygous for exon 21 (coding for the Homer binding domain) deletion exhibited impaired reversal learning in the Morris water maze (Kouser et al., 2013). +/– mice of the exon 21 model exhibited impaired eye-blink conditioning, a cerebellar-dependent learning task (Kloth et al., 2015). The present study evaluated complex cognitive function in the Shank3B model using a touchscreen pairwise visual discrimination task – a computerized cognitive task with high translational value and a potential to reveal preclinical phenotypes that are directly relevant to clinical research. Results indicated that +/– Shank3B mice exhibited impaired pairwise visual discrimination learning in the automated touchscreen task. As compared to +/+ controls, +/– mice were slower to reach criterion in the pairwise visual discrimination task, and exhibited trends of making more errors (first trial errors) and more correction errors. This is the first study to evaluate complex learning in a Shank3B deletion model, and thus opens a whole new pathway to explore the role of Shank3 in complex learning, attention-shifting, behavioral flexibility, and inference learning, all of which can be evaluated in our automated, optimized touchscreen system. While it remains possible that +/– have a mild deficit in Stage 4 of the pre-training, the lack of genotype differences in % blank touches in pre-training Stages 3 or 4 does not support this interpretation.
Deficits in operant learning tasks could be confounded by several factors. Two primary factors, motor activity and performance motivation, were examined in detail in this study. We found no genotype differences in trials completed per session during the visual discrimination task. In the last stage of pre-training, +/– more trials per session than +/+. In addition, our preliminary analysis revealed no genotype differences in latencies to make correct responses, incorrect responses, or to retrieve reward (data not shown due to inadequate power). These results suggest that the learning deficit observed in +/– is unlikely attributable to impaired motor activity or poor motivation. Further, we did not detect significant genotype differences in days to complete Stages 3 and 4 of pre-training, indicating that the learning deficit observed in +/– was not due to a general deficit in acquiring touchscreen tasks, but indicates an impairment specific to the pairwise discrimination.
A crucial finding in the current study is that −/− were incapable of completing pre-training tasks, a profound deficit most likely attributable to high level of repetitive self-grooming and low locomotor activity, which adds to the model's face validity. Eight −/− subjects were included in pre-training Stages 1–3, but were excluded for rest of the experiment. We found that the −/− completed approximately 50% fewer trials in the habituation Stage 1 (Days 1 and 2), markedly fewer trials in Stage 2, and required three times longer to reach criterion in Stage 3. Since poor performance in pre-training will significantly confound results in the discrimination learning task, −/− were not advanced to Stage 4 or beyond. Data from behavioral assays presented in conjunction with our touchscreen results suggest that high levels of self-grooming and low general activity are two confounds in the touchscreen task, and perhaps all operant learning tasks, revealing challenges in testing additional complex cognitive functions in other mouse models that exhibit these phenotypes (Peca et al., 2011; Zhou et al., 2016). We will pursue and develop adapted protocols that dissociate motor skills, motivation, and learning in our future experiments in the null mutants. Notably, impaired motor activity, the mouse phenotype that incurred methodological difficulties in our experiments, has important translational value. PMS, the genetic disorder caused by terminal deletions in 22q13.3 (a region that encompasses the SHANK3 gene), is associated with a number of motor problems, including generalized developmental delay, neonatal hypotonia, low energy and muscle/motor weakness – symptoms not commonly found in ASD cases unrelated to SHANK3 mutations (Phelan and McDermid, 2012; Harony-Nicolas et al., 2015; Mieses et al., 2016.). Other studies of rare intellectual disability disorders have also shown associations between lower IQ scores and the severity of motor delays (Bishop et al., 2016). The Shank3B mouse model thus recapitulates motor symptoms and cognitive deficits in PMS.
Our finding that −/− struggled in our current pre-training regimen raised an important question on pre-training procedures which vary considerably across laboratories. Pre-training (also called auto-shaping) protocols vary in: weekend break (yes or no), days of habituation to the chamber, initiate trials by a nose-poke in the empty well (yes or no), inter-trial interval duration, size and complexity of the visual stimuli, and “passing” criteria (Bussey et al., 2008; Horner et al., 2013; McTighe et al., 2013; Dickson et al., 2010, 2014; Brigman et al., 2012; Yang et al., 2015; Silverman et al., 2015a,b). Some animals may possess sophisticated cognitive functions but, for a number of reasons, fail to pass all stages of pre-training. It is possible that our present fast-paced, efficient pre-training regimen adapted from McTighe et al., 2013 was simply too challenging for −/−. Our previous protocol (Silverman et al., 2015a,b; Yang et al., 2015), while conceptually and procedurally similar to the current protocol, takes more time to complete: It includes five stages of pre-training, with more time for habituation (60 min/day for up to 5 days), longer sessions in Stages 2, 3, 4 (60 min/day as compared to 45 min/day in the current protocol), and an additional Stage 5 in which the animals were given a “timeout” for each incorrect response (30 min/day, up to 5 days). It is conceivable that the current new protocol, while efficient and sufficient for many lines of mice, is too difficult for Shank3B −/−. Particularly, longer, and/or more frequent habituation sessions could be beneficial for Shank3B −/− mutants which have global motor deficits. To our knowledge, a systematic comparison of pre-training variables has not been conducted in mice. A study on variables in the pairwise choice discrimination has been performed, but only in rats (Bussey et al., 2008). It is also of interest to note that environmental novelty is known to increase anxiety-like behaviors and repetitive behaviors (Thomas et al., 2009; Kalueff and Tuohimaa, 2005). It is possible that novelty-induced anxiety and/or repetitive behaviors were still high in −/− after two quick habituation sessions (20 min on day 1, and 40 min on day 2), and that the mice did not attend to environmental cues in the chamber as much as +/+ and +/− did. The trend for −/− to perform fewer trials in Stage 1 manifested further in Stages 2 and 3, and resulted in our decision to exclude −/− starting from Stage 4. Future experiments will explore the possibility that Shank3B −/− could learn the pre-train tasks in an alternative pre-training regimen.
Operant visual discrimination depends on normal functions of interconnected cortical and subcortical regions. The ability to initiate, select, and shift action involves the ventromedial and orbitofrontal regions of the prefrontal cortex, and the dorsal striatum (Jones and Mishkin, 1972; Bussey et al., 1997a,b; Schoenbaum et al., 1999; Brigman and Rothblat, 2008; Bissonette et al., 2014). The consensus in the cognitive behavioral literature suggests that it is inappropriate to draw conclusions on the reversal data if the animals are significantly impaired in the initial discrimination choice task. The data will also be difficult to interpret on the circuitry level, given that studies in multiple species have established a role of the interconnected corticostriatal and orbitofrontal circuits in both acquisition discrimination and reversal across sensory modals (Jones and Mishkin, 1972; Bussey et al., 1997a,b; Schoenbaum et al., 1999; Brigman et al., 2013). One strategy in approaching this issue is to examine progression of reversal learning using sub-stage analysis: Early stage performance reflects retention of previously acquired response; at the middle stage, the animals usually respond at chance level. Performance in the late stage is usually above chance level, reflecting the acquisition of the reversal task. Sub-stage analysis will reveal the nature of reversal deficits, differentiating deficits in choice learning (acquisition and late reversal) from the flexibility component (early reversal). Uniquely, only the late stage of reversal is impaired in ASD cases, reflecting deficits in maintaining newly acquired response (Miller et al., 2015). We are in the process of establishing the extensive baseline data required for these further analyses. We plan to delineate the main patterns of choice learning and relearning, and implement rigorous analytic measurements in our future studies. In addition, analyses of covariance could be performed, to account for variances in reversal explained by discrimination performance (Gastambide et al., 2013; Bissonette et al., 2015).
These findings are consistent with the role of Shank3 in synaptic functions. Anatomical changes in mice homozygous for the exon 21 deletion include increases in white matter structures, specifically the corpus callosum and fimbria, and the cortex (Ellegood et al., 2015). Ultrastructural analysis revealed more perforated synapses in mice heterozygous for the exon 4–9 deletion at a juvenile age, an alteration that diminished in adulthood (Uppal et al., 2015). Electrophysiological experiments revealed reduced cortical-striatal transmission in homozygous Shank3B mice (Peca et al., 2011), but no studies have reported anatomical or electrophysiological changes in +/– mice of the Shank3B model, making it difficult to conjure mechanistic explanations for our current results. Other deletion models may offer some insights: Impaired hippocampal LTP was detected in mutants of three ankyrin deletion models (Bozdagi et al., 2010; Wang et al., 2011; Yang et al., 2012; Jaramillo et al., 2015). Impaired hippocampal transmission and NMDAR functions were found in the exon 21 deletion model (Kouser et al., 2013; Duffney et al., 2015). Our plans for future research include studying physiology and plasticity in cortical and subcortical areas that are important for learning and memory.
Sociability in the three-chamber task was tested in all existing Shank3 models. Sociability was normal in mutants of one exon 4–9 deletion model (Yang et al., 2012; Drapeau et al., 2014), and subtly impaired in another exon 4–9 deletion model (Wang et al., 2011). Deletion of exon 21 had minimal effects on sociability and self-grooming, with +/– exhibiting reduced sociability and older −/− exhibiting increased self-grooming (Kouser et al., 2013; Duffney et al., 2015). Homozygous Shank3B mice exhibited impaired sociability and excessive/injurious self-grooming (Peca et al., 2011). Our current finding of normal sociability in +/– Shank3B mice adds an important piece to the existing literature on the role of Shank3 in sociability. Overall, sociability is only impaired in animals with injurious repetitive self-grooming (Peca et al., 2011).
Normal open field activity in Shank3B +/– mice provided strong evidence that impaired touchscreen learning was not attributable to hypo- or hyperactivity. These results are also in accord with previous studies that reported normal open field activity in mutants of the exon 4-9 deletion models (Wang et al., 2011; Yang et al., 2012; Drapeau et al., 2014; Jaramillo et al., 2015) and the exon 21 deletion model (Kouser et al., 2013).
Conclusion
We report for the first time that pairwise discrimination associative learning is disrupted in +/– Shank3B mice, opening a new pathway to study neurobiological mechanisms behind intellectual disabilities caused by deletions/mutations in SHANK3. The touchscreen task requires habit forming, rule following, and attending to specific sensory stimuli (auditory, visual, and olfactory). Resistance to change or cognitive inflexibility could manifest as deficits in reversal learning. Over or under-responsiveness to sensory stimuli could also impair several aspects of a wide variety of executive functions. Our future research will explore attention-shifting, cognitive inflexibility, and inference learning in genetic models of autism.
Acknowledgments
This work was supported by the Joe P. Tupin Research Award in Psychiatry and the MIND Institute's Intellectual and Developmental Disabilities Research Center (NICHD-IDDRC) HD079125-01 (JLS). The authors are grateful to Drs. Jonathan Brigman (University of New Mexico) and Stacey J. Sukoff Rizzo for their knowledgeable suggestions on our protocol designs.
Abbreviations
- ASD
autism spectrum disorder
- PMS
Phelan–McDermid Syndrome
References
- Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU. An architectural framework that may lie at the core of the postsynaptic density. Science. 2006;311:531–535. doi: 10.1126/science.1118995. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Roussignol G, Worley P, Bockaert J, Fagni L, Ango F. Homer1a-dependent crosstalk between NMDA and metabotropic glutamate receptors in mouse neurons. PLoS One. 2010;5:e9755. doi: 10.1371/journal.pone.0009755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Buxbaum JD. SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism. 2013;4:17. doi: 10.1186/2040-2392-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Thurm A, Farmer C, Lord C. Autism spectrum disorder, intellectual disability, and delayed walking. Pediatrics. 2016;137:1–8. doi: 10.1542/peds.2015-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav Brain Res. 2014;259:143–151. doi: 10.1016/j.bbr.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Schoenbaum G, Roesch MR, Powell EM. Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biol Psychiatry. 2015;77(5):454–464. doi: 10.1016/j.biopsych.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Lauri M, Sarasua SM, Skinner CD, Buccella D, Dwivedi A, Orteschi D, Collins JS, Zollino M, Visconti P, Dupont B, Tiziano D, Schroer RJ, Neri G, Stevenson RE, Gurrieri F, Schwartz CE. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet. 2013;21:310–316. doi: 10.1038/ejhg.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ. Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin. J Biol Chem. 2001;276:40104–40112. doi: 10.1074/jbc.M102454200. [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O. Disruption of the ProSAP2 gene in a t(12;22) (q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001;69:261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol. 2007;72:645–654. doi: 10.1101/sqb.2007.72.020. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 2008;187:405–410. doi: 10.1016/j.bbr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Powell EM, Mittleman G, Young JW. Examining the genetic and neural components of cognitive flexibility using mice. Physiol Behav. 2012;107:666–669. doi: 10.1016/j.physbeh.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997a;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997b;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- D'Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27:152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M, Prins PJ, Schmand BA, Geurts HM. Working memory and cognitive flexibility-training for children with an autism spectrum disorder: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56:566–576. doi: 10.1111/jcpp.12324. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Del Mar N, Martin LA, Heck D, Blaha CD, Goldowitz D, Mittleman G. Behavioral flexibility in a mouse model of developmental cerebellar Purkinje cell loss. Neurobiol Learn Mem. 2010;94:220–228. doi: 10.1016/j.nlm.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Calton MA, Mittleman G. Performance of C57BL/ 6J and DBA/2J mice on a touchscreen-based attentional set-shifting task. Behav Brain Res. 2014;261:158–170. doi: 10.1016/j.bbr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Dorr NP, Elder GA, Buxbaum JD. Absence of strong strain effects in behavioral analyses of Shank3-deficient mice. Dis Model Mech. 2014;7:667–681. doi: 10.1242/dmm.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, Ma K, Dietz DM, Kajiwara Y, Buxbaum JD, Yan Z. Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Rep. 2015;11:1400–1413. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Chaste P, Fauchereau F, Betancur C, Leboyer M, Bourgeron T. Alterations in synapsis formation and function in autism disorders. Med Sci (Paris) 2008;24:25–28. doi: 10.1051/medsci/200824125. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau BA, Crawley JN, Lin L, Genestine M, DiCicco-Bloom E, Lai JK, Foster JA, Penagarikano O, Geschwind DH, Pacey LK, Hampson DR, Laliberte CL, Mills AA, Tam E, Osborne LR, Kouser M, Espinosa-Becerra F, Xuan Z, Powell CM, Raznahan A, Robins DM, Nakai N, Nakatani J, Takumi T, van Eede MC, Kerr TM, Muller C, Blakely RD, Veenstra-VanderWeele J, Henkelman RM, Lerch JP. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry. 2015;20:118–125. doi: 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Gilmour G, Robbins TW, Tricklebank MD. The mGlu positive allosteric modulator LSN2463359 differentially modulates motor, instrumental and cognitive effects of NMDA receptor antagonists in the rat. Neuropharmacology. 2013;4:240–247. doi: 10.1016/j.neuropharm.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Grabrucker AM, Vaida B, Bockmann J, Boeckers TM. Efficient targeting of proteins to post-synaptic densities of excitatory synapses using a novel pSDTarget vector system. J Neurosci Methods. 2009;181:227–234. doi: 10.1016/j.jneumeth.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Harony-Nicolas H, De Rubeis S, Kolevzon A, Buxbaum JD. Phelan McDermid syndrome: from genetic discoveries to animal models and treatment. J Child Neurol. 2015;30:1861–1870. doi: 10.1177/0883073815600872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR. SHANK3, the synapse, and autism. N Engl J Med. 2011;365:173–175. doi: 10.1056/NEJMcibr1104261. [DOI] [PubMed] [Google Scholar]
- Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, Alsio J, Oomen CA, Holmes A, Saksida LM, Bussey TJ. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–1984. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Liu S, Powell CM. Altered striatal synaptic function and abnormal behaviour in Shank3 Exon4-9 deletion mouse model of autism. Autism Res. 2015 doi: 10.1002/aur.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur J Pharmacol. 2005;508(1–3):147–153. doi: 10.1016/j.ejphar.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Kloth AD, Badura A, Li A, Cherskov A, Connolly SG, Giovannucci A, Bangash MA, Grasselli G, Penagarikano O, Piochon C, Tsai PT, Geschwind DH, Hansel C, Sahin M, Takumi T, Worley PF, Wang SS. Cerebellar associative sensory learning defects in five mouse autism models. Elife. 2015;4:e06085. doi: 10.7554/eLife.06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, Liu S, Jaramillo TC, Bangash M, Xiao B, Worley PF, Powell CM. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci. 2013;33:18448–18468. doi: 10.1523/JNEUROSCI.3017-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieses AM, Tavassoli T, Li E, Soorya L, Lurie S, Wang AT, Siper PM, Kolevzon A. Brief report: sensory reactivity in children with Phelan–McDermid Syndrome. J Autism Dev Disord. 2016 doi: 10.1007/s10803-016-2754-0. [DOI] [PubMed] [Google Scholar]
- Miller HL, Ragozzino ME, Cook EH, Sweeney JA, Mosconi MW. Cognitive set shifting deficits and their relationship to repetitive behaviors in autism spectrum disorder. J Autism Dev Disord. 2015;45:805–815. doi: 10.1007/s10803-014-2244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt DJ, Hough SJ, Gill HJ, Pirmohamed M, Kitteringham NR, Park BK. Cellular disposition of sulphamethoxazole and its metabolites: implications for hypersensitivity. Br J Pharmacol. 1999;126:1393–1407. doi: 10.1038/sj.bjp.0702453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8:2006–2021. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC. Deletion 22q13.3 syndrome. Orphanet J Rare Dis. 2008;3:14. doi: 10.1186/1750-1172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, McDermid HE. The 22q13.3 deletion syndrome (Phelan–McDermid Syndrome) Mol Syndromol. 2012;2:186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24:2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113(Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010c;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN. GABAB receptor agonist R-Baclofen reverses social deficits and reduces repetitive behavior in two mouse models of Autism. Neuropsychopharmacology. 2015a;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb Cortex. 2015b:1133–1142. doi: 10.1093/cercor/bht293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal N, Puri R, Yuk F, Janssen WG, Bozdagi-Gunal O, Harony-Nicolas H, Dickstein DL, Buxbaum JD, Hof PR. Ultrastructural analyses in the hippocampus CA1 field in Shank3-deficient mice. Mol Autism. 2015;6:41. doi: 10.1186/s13229-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(2):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley J. Automated three-chambered social approach task for mice. Curr Protoc Neurosci Unit. 2011;8:26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Lewis FC, Foley G, Sarvi M, Crawley JN. 16p11.2 Deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn Mem. 2015;22(12):622–632. doi: 10.1101/lm.039602.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kaiser T, Monteiro P, Zhang X, Van der Goes MS, Wang D, Barak B, Zeng M, Li C, Lu C, Wells M, Amaya A, Nguyen S, Lewis M, Sanjana N, Zhou Y, Zhang M, Zhang F, Fu Z, Feng G. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron. 2016;89(1):147–162. doi: 10.1016/j.neuron.2015.11.023. http://dx.doi.org/10.1016/j.neuron.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


