Abstract
Measurement of cortisol in hair provides a chronic index of hypothalamic-pituitary-adrenal (HPA) axis activity and has been applied to assessments of temperament (stable behavioral differences between individuals). However, the extent to which chronically high HPA axis activity relates to a correspondingly high degree of behavioral reactivity is as yet unknown. Therefore, the goal of the present experiment was to assess the relationship between hair cortisol and a reactive temperament. We administered the Human Intruder Test (HIT) twice to 145 (80 male) rhesus macaques (Macaca mulatta) in order to assess behavioral reactivity. The HIT presents monkeys with an unfamiliar experimenter and is composed of a Baseline phase (no intruder) followed by three experimental phases in which the orientation of the intruder changes (Profile, Stare, Back). Behavioral responses to the test were videotaped and behaviors thought to reflect a reactive response to the intruder were scored for duration. Hair samples collected within ±1 month of the first HIT session were analyzed for cortisol by enzyme immunoassay. Subjects were assigned to 3 groups based on hair cortisol concentration: high, intermediate, and low cortisol phenotypes. Monkeys with the high cortisol phenotype were more reactive to the presence of the intruder than those with the low cortisol phenotype: they were more aggressive, scratched more, and spent more time in the back half of the cage. Males yawned significantly more while females spent more time immobile and in the back of the cage. Overall, monkeys with higher hair cortisol demonstrated an exaggerated response to the presence of the human intruder, supporting a relationship between high levels of chronic HPA axis activity and a reactive temperament. These results indicate that high levels of HPA axis activity, which may result from either genetic variation or environmental stress, correspond with heightened behavioral responses to a stressful experience.
Key phrases: Hair cortisol, Hypothalamic-pituitary-adrenal axis, Human intruder test, temperament
Introduction
The hypothalamic-pituitary-adrenocortical (HPA) axis becomes activated in response to a threatening stimulus. This activation results in the release of the glucocorticoid hormone cortisol, which prepares the body to respond to the stimulus [Charmandari et al., 2005]. Stressful stimuli, including those that are perceived as threatening, also give rise to psychological and behavioral responses; however, the magnitude of these responses to a stressful event frequently differs from HPA reactivity to the same event. Schlotz and colleagues [2008] have termed this finding a “lack of psychoendocrine covariance.” Although those authors argue that a failure of psychoendocrine covariance may arise because of mis-timing of collection of blood or saliva samples for hormone measurement, another possibility is that better concordance of psychological/behavioral and hormonal measures would be obtained using trait (i.e., long-term) rather than state (i.e., short-term) assessments of these variables.
Until approximately 10 years ago, studies of cortisol responses to stress relied entirely on measuring the hormone in plasma, saliva, urine, or feces [Novak et al., 2013]. Despite the well-known utility of these sample matrices, they are limited because they index HPA axis activity only during a short window of time: on the scale of minutes for plasma and saliva, and up to 24 hours for urine and feces. The intrinsic variability of cortisol concentrations in these sample matrices limits their usefulness for determining chronic HPA activity. Fortunately, this limitation has been overcome by the development and validation of hair cortisol measurement by our laboratory and others [Davenport et al., 2006; Kirschbaum et al., 2009; Raul et al., 2004; Sauvé et al. 2007]. Hair cortisol concentrations provide a more stable, or trait-like, measurement of HPA axis activity that might be considered indicative of an organism's cortisol “phenotype” [Meyer & Novak, 2012; Russell et al., 2012]. Consistent with this view, variation in hair cortisol concentrations has been related to individual differences in temperament in nonhuman primates [Laudenslager et al., 2011] and also to human neuropsychiatric disease states [e.g., Staufenbiel et al., 2013].
Temperament refers to trait-like and stable behavioral differences between individuals. Although this concept is most widely used to describe human behavioral traits, it has also been applied to nonhuman primates [Coleman, 2012]. Recent evidence suggests that hair cortisol levels are heritable and might vary with temperamental characteristics. Fairbanks and coworkers [2011] demonstrated significant heritability of hair cortisol in female vervet monkeys exposed to both low and higher stress environments. In another study, vervet monkeys with high hair cortisol concentrations were less likely to approach a novel and possibly threatening object placed outside of their home environment [Laudenslager et al., 2011]. This resistance to novelty seeking behavior in monkeys with high levels of HPA axis activity was consistent across sessions and therefore likely represents a reactive temperament. A similar pattern was observed in dogs where reactivity to audio playback of thunderstorms and canine vocalizations was greater in dogs with high levels of hair cortisol [Siniscalchi et al., 2013]. Finally, high hair cortisol concentrations in infant rhesus monkeys have also been linked to poorer performance on object permanence tasks [Dettmer et al., 2009]. These authors proposed that poor performance on cognitive tasks may be related to a higher level of emotionality or a reactive temperament. Based on these findings, hair cortisol seems to provide a stable measure with which to examine the relationship between HPA activity and temperamental characteristics under a variety of conditions.
Despite growing evidence for a relationship between chronic HPA activity (assessed using hair cortisol) and temperament, there remains a gap in the literature with respect to this relationship in adult rhesus macaques. The present study was designed to fill this gap by examining the relationship between phenotypic HPA axis activity and the behavioral responses of adult rhesus macaques in a mildly anxiogenic social situation, the human intruder test (HIT). The HIT was first developed by Ned Kalin and Steve Shelton [1989] to assess behavioral temperament in infant macaques by presenting the animals with an unfamiliar human intruder. The response of the infants varied depending on the orientation of the intruder. When the intruder oriented his profile towards the infants, they responded by freezing, whereas when he stared at the infants, they responded with aggressive barking. The infants' responses were consistent across sessions, suggesting that the type and level of responsiveness to the HIT might reflect trait-like behavioral reactivity [Kalin & Shelton, 1989]. Kalin and Shelton also demonstrated that the amount of freezing positively correlated with plasma cortisol levels [Kalin et al., 1998b]. Similarly, monkeys determined to have an anxious temperament through behavioral observations displayed higher levels of barking and fear grimacing and also had higher plasma cortisol levels in response to the HIT [Capitanio et al., 2011]. However, as plasma cortisol levels are susceptible to sampling stress, these data suffer from the potential confound that elevated reactivity to the HIT reflected an enhanced response to the capture and venipuncture procedures. The current study avoided this problem by relating hair cortisol, a validated measure of chronic cortisol levels, to monkeys' responses to the HIT in order to assess the relationship between trait-like behavioral reactivity and HPA axis activity. We predicted that monkeys with a high cortisol phenotype would have a greater behavioral reaction (i.e., exaggerated levels of both aggressive and fearful behaviors) to the presence of an unfamiliar human intruder than monkeys with lower chronic cortisol levels.
Methods
Subjects
We tested 145 rhesus macaques (Macaca mulatta), 80 of which were male, housed at one of four national primate centers (Washington National Primate Research Center, Oregon National Primate Research Center, Southwest National Primate Center, and New England Primate Research Center). Subjects ranged from 3 to 30 years of age (mean of 9.9 years) and were housed individually (n=122), in pairs (n=11), or in protected grooming contact (n=12) in which the monkey is separated from their social partner but able to physically access them through widely spaced vertical bars. Housing conditions conformed to the rules outlined in the Guide and Use of Laboratory Animals [National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011]. The subjects' diet consisted of twice-daily feedings of formulated monkey biscuits (Lab Diet, PMI, St Louis, MO) and was supplemented with fruits, vegetables, and grains. Water was available ad libitum. All subjects were maintained under the specifications of the facilities' Institutional Animal Care and Use Committees and all four centers were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The study adhered to laws governing research with non-human primates and the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates.
Hair Cortisol Analysis
In order to determine subjects' hair cortisol concentrations, hair samples were collected from the nape of the neck during routine health exams between February and July 2012. As none of the subjects participated in any breeding activity, the potential influence of mating, pregnancy, or lactation on hair cortisol was avoided. The hair samples were stored at -20°C in labeled tin foil packets and then shipped to the University of Massachusetts Amherst for analysis (see Meyer et al., 2014 for detailed protocol). Briefly, hair samples were washed twice in isopropyl alcohol to remove external contaminants, dried, and then ground to a fine powder using a Retsch MM200 ball mill. Cortisol was extracted from the powder into methanol, which was then dried down in a vacuum evaporator and the cortisol was reconstituted in assay buffer. The reconstituted samples were analyzed in duplicate with an enzyme immunoassay (Salimetrics, State College, PA), following the procedure recommended by the manufacturer. The intra-assay coefficient of variation (CV) was 1.9% and was calculated as the average of the CVs generated from all 145 sample-duplicates multiplied by 100, where each individual CV was the mean of the 2 duplicate measurements divided by their standard deviation (SD). The inter-assay CV was 6.9% and was calculated using the mean and SD values for a quality control (QC) sample consisting of a pooled monkey hair extract that was included in each assay.
Human Intruder Test
The present study used a version of the HIT developed for adult monkeys (see Coleman et al., this issue). In studies following the original HIT procedure, subjects are removed from their colony room and placed alone in a separate testing room [Kalin & Shelton, 1989]. The version we presented, termed the cage side HIT, is administered to subjects in their home cage and is composed of 4 consecutive 2 minute phases. Before the test began, a video camera was placed within 2 m of the subject's home enclosure and, in the case of the 11 pair-housed monkeys, the subject was separated from its cage-mate. Ten minutes were allowed to pass for the subject to acclimate to the presence of the camera; the final 2 minutes of this phase were designated as the Baseline period. The human intruder then entered the colony room and positioned herself 60 cm from the subject, standing quietly with her profile oriented towards the monkey for 2 minutes (Profile Phase). The intruder then turned 90° to face the monkey, making direct eye contact for 2 minutes (Stare Phase). Finally, the intruder turned 180° to orient her back towards the subject for 2 minutes (Back Phase). At the end of the final phase, the intruder left the room and the test was completed. Each subject was administered the HIT twice, with an inter-trial interval of approximately 2-3 weeks. The first HIT session was administered within ±1 month of the health exam during which hair collection had occurred or was scheduled to occur.
Videos captured during both HIT sessions were sent to the University of Massachusetts Amherst for scoring. Eight behaviors of interest identified as reflecting a reactive response were scored for duration: (1) back of cage (subject positioning itself with at least 3 limbs in the back half of the cage), (2) lipsmack (rapidly opening and closing the lips) [Maestripieri, 2005], (3) fear grimace (a large grin-like facial expression showing the teeth) [Maestripieri, 2005], (4) aggress (shaking the cage or threatening [open-mouth stare, lunging or swiping at] the intruder), (5) pace (locomotion that exceeds 3 identical patterns), (6) scratch [Triosi et al., 1991], (7) yawn [Hadidian, 1980], and (8) freeze (remaining immobile for longer than 2 seconds) [Kalin & Shelton, 1989]. Videos were scored in a frame-by-frame analysis using MPEG Streamclip by seven trained observers with an inter-observer reliability of > 90%. Reliability was determined on each individual measure using test videos and was calculated as a percent agreement score: (number of scores in agreement)/(total number of scores) with agreement defined as scores within 30 frames (< 1 second). Start and stop times for each behavior were noted and duration of each behavioral bout was calculated as Stop Time - Start Time. For each behavior, individual bout durations were totaled within each phase to create a behavioral score for each of the 4 individual phases (Baseline, Profile, Stare, and Back). Finally, the durations from each phase were summed to create an overall total for each behavioral category.
Data Analysis
We reasoned that a possible association of hair cortisol concentration with a reactive temperament might be revealed by comparing groups of animals with relatively high and relatively low cortisol levels. Accordingly, we divided the subjects into tertiles based on their hair cortisol concentration, thereby yielding groups characterized by low, intermediate, and high hormone levels. To compare the two extreme cortisol phenotypes, we discarded the subjects in the intermediate group, which resulted in a final sample size of 97 subjects distributed across the four primate facilities. A chi-square analysis was implemented to assess the distribution of males and females across low cortisol and high cortisol phenotypes. Repeated measures Analyses of Variance were performed for each individual behavior (between subjects variables: hair cortisol phenotype and sex; within subject variables: behavioral durations for each of the four individual HIT phases). Post-hoc independent sample t-tests were performed to assess differences between monkeys with high and low cortisol phenotypes during specific HIT phases. Pearson correlations were applied to the total duration scores for each target behavior across the two rounds of testing to determine if response to the HIT was consistent across sessions. Two behavioral categories with a low frequency of occurrence (fear grimace and yawn) were not normally distributed (Shapiro-Wilk test, P<0.001) and, therefore, the data in these categories were log transformed. The log transformed values were used in all of the above analyses; however, for purposes of consistency we used the untransformed data for all graphical presentations.
Results
Hair Cortisol Phenotype
Overall, the macaques had an average hair cortisol concentration of 59.5 pg/mg ± 1.72 SE (range: 28.3-165.7). Monkeys with a low cortisol phenotype had a mean hair cortisol concentration of 41.5 pg/mg ± 0.7 SE (range: 28.3-49.9) and monkeys with a high cortisol phenotype had a mean hair cortisol concentration of 80.7 pg/mg ± 3.1 SE (range: 62.4-165.7). Both phenotypes had a similar distribution of males and females (X2=1.238, P=0.266); the low cortisol phenotype group was composed of 29 males and 20 females, whereas the high cortisol phenotype group was composed of 23 males and 25 females (see Table I).
Table 1. Distribution of Subjects across Sex, Facility, and Hair Cortisol Phenotype.
| Sex | Cortisol Phenotype | Facility | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| A | B | C | D | Totals | ||
| Males | High | 11 | 2 | 5 | 5 | 23 |
| Low | 8 | 5 | 9 | 7 | 29 | |
| Females | High | 9 | 8 | 7 | 1 | 25 |
| Low | 4 | 1 | 15 | 0 | 20 | |
|
| ||||||
| Totals | 32 | 16 | 36 | 13 | 97 | |
Human Intruder Test Response
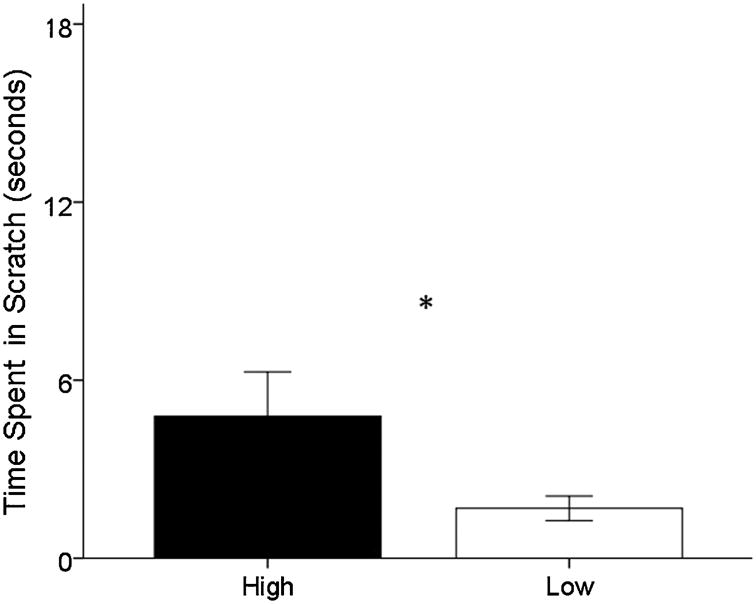
Repeated measures ANOVAs for the first HIT session (Round 1) identified a significant main effect of hair cortisol phenotype on the amount of time spent scratching, such that monkeys with the high hair cortisol phenotype scratched significantly more than monkeys with the low cortisol phenotype (F(1,91)=4.830, P=0.031; Fig. 1).
Fig. 1. The effect of hair cortisol phenotype on amount of time spent in scratch during the Round 1 HIT, * P-value <0.05; black: high cortisol phenotype, white: low cortisol phenotype.
Other behavioral responses of high and low cortisol phenotype monkeys were modified by test phase. First, there was a significant interaction between hair cortisol phenotype and HIT phase on the amount of time spent in the back of the cage (F(3,273)=2.824, P=0.039; Fig. 2a). During the Profile phase, monkeys with the high cortisol phenotype spent significantly more time in the back of the cage than monkeys with the low cortisol phenotype (t(93)=2.660, P=0.009). Additionally, the interaction between hair cortisol phenotype and HIT phase on time spent freezing trended towards significance (F(3,273)=2.420, P=0.066; Fig. 2b), but not in the predicted direction; monkeys with the low cortisol phenotype tended to spend more time in freeze during the Stare phase than those with the high cortisol phenotype (t(93)=-1.826, P=0.066). There was also an interaction between HIT phase and cortisol phenotype on amount of time spent aggressing (F(3,273)=3.610, P=0.014; Fig. 2c). Monkeys with the high cortisol phenotype aggressed the intruder significantly more during the Stare phase than counterparts with the low cortisol phenotype (t(93)=2.158, P=0.034) (Table II).
Fig. 2. The effect of HIT phase and hair cortisol phenotype on amount of time spent in (a) back of cage (BOC), (b) freeze, and (c) aggress during the Round 1 HIT; * P-value < 0.05, + P-value = 0.06; solid line: high cortisol phenotypes, dashed line: low cortisol phenotypes.
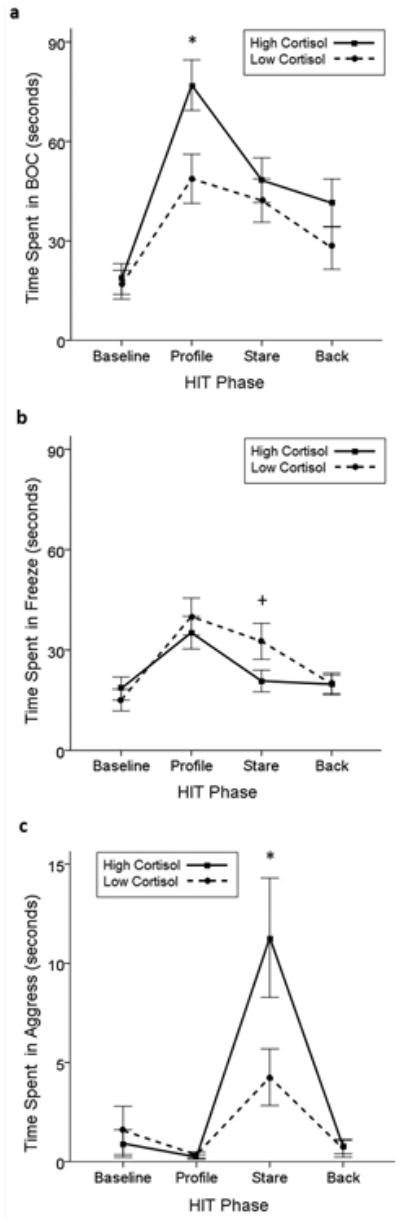
Table 2.
Results of Repeated Measures ANOVAs on Round 1 Behavioral Data Presented as P-values.
| BOC | LS | FG | Aggress | Pace | Scratch | Yawn | Freeze | |
|---|---|---|---|---|---|---|---|---|
| HC Phenotype | 0.180 | 0.141 | 0.664 | 0.086 | 0.947 | 0.031 | 0.457 | 0.124 |
| Phenotype × Phase | 0.039 | 0.087 | 0.854 | 0.014 | 0.548 | 0.248 | 0.211 | 0.066 |
| Sex | 0.002 | 0.512 | 0.992 | 0.598 | 0.595 | 0.124 | <0.001 | <0.001 |
| Sex × Phase | 0.007 | 0.792 | 0.668 | 0.195 | 0.173 | 0.180 | 0.160 | 0.158 |
| Phase × Sex × HC Phenotype | 0.154 | 0.141 | 0.134 | 0.695 | 0.394 | 0.644 | 0.953 | 0.007 |
Note: Bolded values (P<0.05), italicized values (P<0.07 and >0.05); HC: Hair cortisol, BOC: Back of cage, LS: Lipsmack, FG: Fear grimace)
Analysis of the second HIT session (Round 2) data revealed strong trends towards main effects of cortisol phenotype on time spent freezing (F(1,83)=6.964, P=0.050; low cortisol > high cortisol, Fig. 3a), time spent aggressing the intruder (F(1,83)=3.888, P=0.052, high cortisol > low cortisol, Fig. 3b), and time spent fear grimacing (F(1,83)=4.730, P=0.01, high cortisol > low cortisol, Fig. 3c). As before, the influence of cortisol phenotype was significantly modified by test phase (F(3,249)=3.662, P=0.014). This analysis revealed that the difference between high and low cortisol monkeys in aggressive behavior was manifested during the Stare phase (t(85)=2.118, P=0.037) but not at other times. In contrast, the two other behavioral categories with main effect trends did not show significant phase-related patterns (Table III).
Fig. 3. The main effect of hair cortisol phenotype on amount of time spent in (a) freeze, (b) aggress, and (c) fear grimace during the Round 2 HIT, * P-value < 0.05, + P=0.05; black: high cortisol phenotypes, white: low cortisol phenotypes.
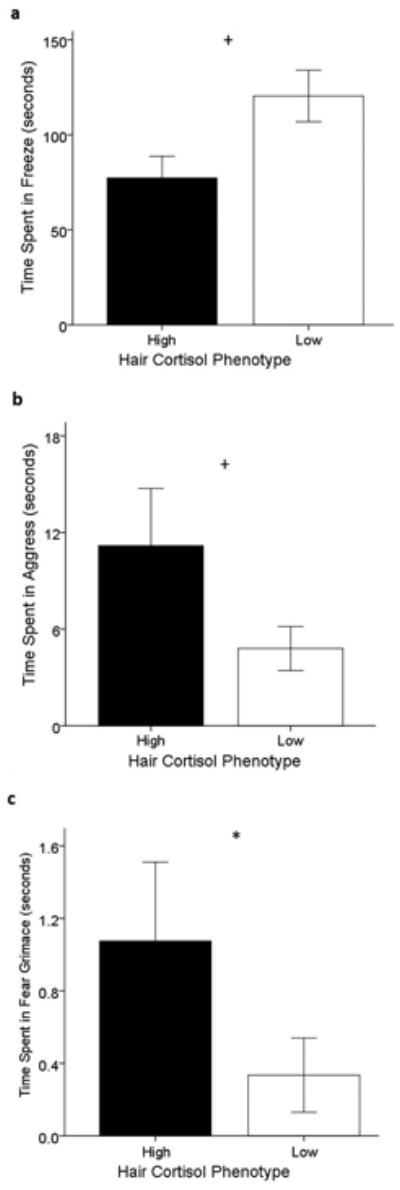
Table 3.
Results of Repeated Measures ANOVAs on Round 2 Behavioral Data Presented as P-values.
| BOC | LS | FG | Aggress | Pace | Scratch | Yawn | Freeze | |
|---|---|---|---|---|---|---|---|---|
| HC Phenotype | 0.121 | 0.897 | 0.010 | 0.052* | 0.223 | 0.516 | 0.086 | 0.050 |
| Phenotype × Phase | 0.936 | 0.918 | 0.750 | 0.014* | 0.537 | 0.503 | 0.304 | 0.163 |
| Sex | <0.001* | 0.830 | 0.138 | 0.292 | 0.223 | 0.067 | <0.001* | 0.015* |
| Sex × Phase | <0.001* | 1.00 | 0.811 | 0.273 | 0.310 | 0.731 | 0.405 | 0.002 |
| Phase × Sex × HC Phenotype | 0.456 | 0.107 | 0.646 | 0.432 | 0.340 | 0.664 | 0.385 | 0.420 |
Note: Bolded values are significant (P<0.05) and italicized values are a trend (P<0.07 and >0.05),
indicates a replication of Round 1 findings (BOC: Back of cage, LS: Lipsmack, FG: Fear grimace
Pearson correlations between Round 1 and Round 2 data for each behavioral category revealed that all behaviors, with the exception of fear grimace, were highly correlated across the two HIT sessions (Back of Cage: r=0.541, P<0.001; Lipsmack: r=0.520, P<0.001; Aggress: r=0.750, P<0.001; Pace: r=0.540, P<0.001; Scratch: r=0.734, P<0.001; Yawn: r=0.445, P<0.001; Freeze: r=0.596, P<0.001). Fear grimace occurred with such low frequency in the first HIT round that there was no significant correlation with Round 2 data (r<0.142, P=0.088).
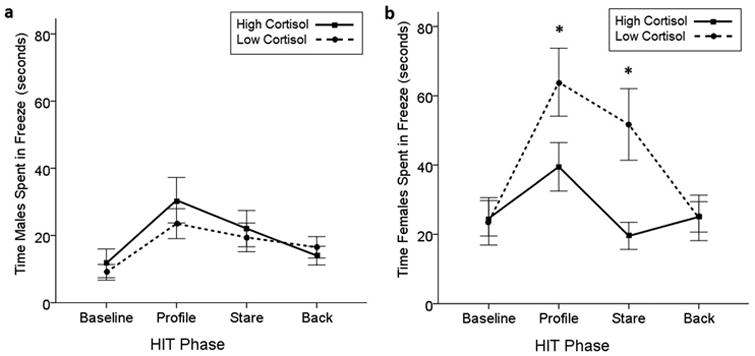
The sex of the monkeys also significantly influenced how they responded to the HIT, both overall and during the individual HIT phases. In the first session (Round 1), there was a significant main effect of sex (F(1,91)=9.954, P=0.002) and an interaction between HIT phase and sex (F(3,273)=4.162, P=0.007) on amount of time spent in the back of the cage. Females spent more time in the back of the cage, with the effects most prominent during the 3 intruder phases. Males yawned significantly more than females overall (F(1,91)=21.773, P<0.001) while females spent more time in freeze (F(1,91)=18.473, P<0.001). Finally, a significant three-way interaction between sex, hair cortisol phenotype, and HIT phase was identified (F(3,273)=4.100, P=0.007); females with the low cortisol phenotype spent significantly more time in freeze than male monkeys and females with the high cortisol phenotype, particularly during the Profile (t(42)=-2.074, P=0.044) and Stare (t(42)=-3.110, P=0.003) phases (Fig. 4 and Table II).
Fig. 4. The effect of hair cortisol phenotype on amount of time spent in freeze in (a) males and (b) females during the Round 1 HIT, * P-value <0.05; solid line: high cortisol phenotypes, dashed line: low cortisol phenotype.
Repeated measures ANOVAs of the Round 2 data replicated many of the main sex differences observed in the first round (Back of Cage: F(1,83)=20.264, P<0.001; Yawn: F(1,83)=13.078, P<0.001; Freeze: F(1,83)=6.166, P=0.015), as well as the interaction between sex and the HIT phases (Back of Cage: F(3,249)=6.378, P<0.001). Analyses of the Round 2 data also identified a trend towards a main effect of sex on amount of time spent scratching (F(1,83)=3.444, P=0.067) and a significant interaction between sex and HIT phase on time spent freezing (F(3,249)=4.905, P=0.002) (Table III). Females froze significantly more than males during the Profile (t(85)=3.060, P=0.002) and Stare (t(85)=1.866, P=0.001) phases of the test.
Discussion
These data support our prediction that monkeys with a high concentration of hair cortisol would demonstrate a more reactive pattern of responses to the HIT. Monkeys with the high cortisol phenotype responded to the presence of the unfamiliar experimenter with more aggression, scratching, and fear grimacing, and less time freezing. Furthermore, the monkeys' responses were dependent on the orientation of the experimenter. During the Profile phase, when the experimenter had first entered the colony room, monkeys with the high cortisol phenotype spent significantly more time in the back of the cage. During the Stare phase, monkeys with the high cortisol phenotype responded to the intruder with aggression, threatening and cage shaking, whereas monkeys with the low cortisol phenotype tended to spend more time freezing. Overall, the response of individual monkeys to the HIT was stable, as behaviors were highly correlated across the two sessions.Understanding the behavioral differences between high and low cortisol monkeys requires a consideration of the typical interpretation of each behavior in conjunction with the test phase during which the behavior was elicited. Independent of test phase, the high cortisol monkeys spent more time both scratching and fear grimacing than low cortisol monkeys. Although scratching behavior obviously can occur in response to a pruritic stimulus, in the absence of such a stimulus this behavior has been associated with increased anxiety [Troisi et al., 1991]. Grimacing is a communicative expression in macaques that is commonly elicited in a fear-inducing situation [Maestripieri, 2005]. Consequently, these phenotypic differences strongly suggest that overall, the HIT elicits a greater degree of fear/anxiety in high cortisol than in low cortisol monkeys.
Other differences between high and low cortisol monkeys occurred specifically in the Stare and Profile phases of the HIT. Because staring at a monkey's eyes is a threatening gesture, this phase is considered to be the most stressful component of the test. High cortisol monkeys responded to the Stare phase with aggressive behavior, whereas monkeys with a low cortisol phenotype instead responded with freezing behavior. Thus, both phenotypes reacted strongly to this threatening situation, but the pattern of reactivity was offensive (i.e., challenging the intruder back) in one case and defensive (i.e., remaining immobile) in the other. Kalin and colleagues [1998a] similarly reported a positive correlation between plasma cortisol concentrations and aggressive behavior (amount of barking and other hostile behaviors) during the Stare phase of the HIT in infant rhesus monkey subjects.
The Profile phase of the test is less threatening than the Stare phase, yet the high cortisol monkeys spent substantially more time in the back of the cage during the Profile phase than the low cortisol monkeys. This may be interpreted as a withdrawal response that increases the distance between the monkey and the intruder. If such withdrawal reflects fear of the intruder, then the back of cage results are in accordance with the scratching and fear grimacing data, suggesting that monkeys with the high cortisol phenotype are more defensive and fearful/anxious than low-cortisol monkeys during the HIT phase that represents an intermediate level of threat (i.e., more than Back, less than Stare). Yet, high-cortisol monkeys become aggressive during the Stare phase when they are challenged by the gaze of the intruder. These results indicate that overall, monkeys with a high cortisol phenotype demonstrate exaggerated responses to the intruder; however, the nature of these responses is highly contextual, manifesting as either fearful or aggressive depending on the intruder's orientation and the presumptive degree of threat posed by each orientation. While it may initially seem paradoxical that the same animals could show high levels of both fear and aggression, we note that an analysis of the Round 1 data across all subjects (both high and low cortisol) revealed a significant positive correlation between levels of fear grimacing and levels of threat during the HIT (data not shown). Thus, these two response patterns are not mutually exclusive within the present testing paradigm.
The response of individual subjects to the HIT was highly correlated from the first to the second round; monkeys that were highly reactive during the first phase continued to be highly reactive in the second phase. Kalin and Shelton [1989] demonstrated consistent responses in infant rhesus monkeys exposed to a variation of the HIT. For example, infants demonstrated similar levels of freezing behavior when they were tested again five months after an initial HIT exposure. This behavioral consistency supports the notion that response of monkeys to the HIT may be related to individual temperament. However, certain relationships between hair cortisol and behavior observed in Round 1 were not observed in Round 2, including time spent in the back of the cage and scratching. The loss of these differences may reflect habituation to the test leading to diminished reactivity during the second round. We also note that aggression was significantly related to hair cortisol phenotype in both Round 1 and Round 2, whereas levels of freezing behavior only trended towards significance in both rounds. Aggression is a common rhesus monkey species-typical response and, therefore, may be a particularly robust temperamental characteristic.
Sex also significantly influenced the response of the monkeys to the HIT. For example, females consistently spent more time in the back of the cage and more time freezing. This finding contrasts with a previous report that female monkeys spent less in time freezing than males (Rogers et al., 2008). However, the average age of the subjects in the Rogers et al. study was 1.5 years of age, whereas the subjects in the present study were adult monkeys with an average age of 9.9 years. Testosterone has been shown to be associated with decreased levels of anxiety [Aikey et al., 2002; Hermans et al., 2007], which could explain why adult male monkeys in the present study demonstrated both lower levels of freeze and less time spent in the back of the cage. Another sex difference observed in our data was that males consistently yawned more than females. This effect was expected, as males have very large canine teeth, whereas females do not. Yawning displays these large, and potentially dangerous, teeth and therefore, yawning would be predicted to occur more frequently in situations where the male feels threatened [Hadidian, 1980]. Overall, sex differences were extremely stable from the first to the second HIT session, more so than the relationships between behavior and hair cortisol phenotype.
Monkeys with high levels of hair cortisol presumably experienced increased activation of the HPA axis, compared to low cortisol monkeys, during the period of time the hair was growing. However, it is unclear at this time whether these increases in activity are due to a higher incidence of stressful experiences or to a highly reactive HPA axis (i.e., greater cortisol secretion to the same level of stress experienced by the low cortisol monkeys). Either hypothesis would be consistent with the heightened behavioral reactivity demonstrated by monkeys with a high hair cortisol phenotype. For example, a greater level of stress in high cortisol monkeys could have primed the animals to respond more strongly to the HIT. Alternatively, even if stress exposure was similar in the two groups, the two different behavioral and endocrine patterns of reactivity may have been previously programmed by various genetic and/or developmental factors. For example, freezing behavior and orienting to the intruder in young rhesus monkeys tested on the HIT showed significant heritability with respect to the macaque serotonin transporter promoter gene polymorphism [Rogers et al., 2008]. Suomi and colleagues found that approximately 20% of their rhesus macaque population are “high reactors”, which are monkeys that demonstrate heightened behavioral responses to stimuli other monkeys find benign, as well as increased and prolonged HPA axis activity compared to conspecifics [Suomi, 2000]. Importantly, patterns of behavioral and endocrine reactivity in these animals are thought to be programmed by specific gene × environment interactions (e.g, serotonin transporter gene allele and early rearing condition [Suomi, 2006]). The correspondence between the subjects identified as having a high cortisol phenotype in our dataset (about 30% of our subject pool) and Suomi's high reactors is striking, which suggests that these differing experimental methodologies may identify the same (or at least a very similar) subgroup of rhesus macaques.
Previous studies relating behavioral responsiveness on the HIT to the HPA axis in rhesus monkeys have been conducted on infant animals using plasma cortisol as an index of adrenocortical activity. Notably, Kalin and colleagues [1998b] reported a positive correlation between baseline plasma cortisol levels and freezing behavior (interpreted to be a fear response) at 10 months of age in mother-reared infants that were tested 2-3 months earlier on a version of the HIT involving exposure only to the intruder's profile. Another study from the same group [Kalin et al., 1998a] performed on 1-year-old rhesus monkeys found a positive correlation between plasma cortisol and aggressiveness (barking and “hostility”) when the intruder stared at the infant, but no significant relationship between either freezing or cooing and plasma cortisol during the profile phase of the test. A later study by Capitanio et al. [2011] found that mother-reared infants at 3-4 months of age rated as being high in nervous temperament had increased cortisol levels compared to low nervous infants under a range of experimental conditions. When tested on a version of the HIT containing both profile and stare phases, the high nervous monkeys showed a combination of heightened aggressiveness (bark and threat) and fearfulness (fear grimace), but lower levels of anxiety-related behaviors (scratch and self-groom). The latter finding seems surprising considering that a “nervous” temperament might be expected to manifest as increased anxiety in a threatening situation. Moreover, the Capitanio et al. [2011] paper did not separate the behavioral responses by phase of the HIT. In conclusion, we are unaware of any previous research demonstrating our primary finding that chronically high cortisol levels in adult rhesus macaques are associated with exaggerated reactivity on the HIT challenge, the characteristics of which are modulated by the level of threat posed by different phases of the test.
A few limitations of the present study should be noted. First, because of the constraints associated with colony husbandry activities at the four participating primate centers, hair collection during health exams was spread over a period from February to July. Consequently, although (as mentioned in the Methods section) none of the monkeys participated in any breeding activities, we cannot rule out the possibility of seasonal influences on hair cortisol concentrations or on behavioral responses in the HIT. Second, our particular interest in cortisol phenotypes at the high and low ends of the distribution led us to discard animals from the middle tertile of hair cortisol levels. While this analytical approach identified a number of interesting relationships between cortisol phenotype and behavior, it is worth noting that most of these statistically significant relationships were lost when separate analyses were performed using all of the animals. This result is consistent with the notion that adverse consequences may follow from cortisol levels that are either too high or too low [Staufenbiel et al., 2013]. Lastly, we acknowledge the limitations of the hair cortisol approach to assessing HPA activity [Meyer and Novak, 2012]. This approach is unable to discern changes in baseline circulating cortisol, changes in circadian rhythmicity, or the magnitude or rate of recovery of responses to acute stressors. In contrast to rodent and human studies that can readily make use of repeated plasma or saliva sampling respectively, such repeated sampling is much more challenging in primate research. On the other hand, a growing number of laboratory and field studies has shown the applicability of hair cortisol measurements to a variety of different questions in primate research (for example, see [Carlitz et al., 2014; Dettmer et al., 2009; 2012; Fairbanks et al., 2011; Fourie et al., 2015a; 2015b; Laudenslager et al., 2011]).
In conclusion, our data reveal a relationship between high HPA axis activity, as measured by hair cortisol, and behavioral reactivity to a mildly threatening and stressful challenge in rhesus macaques. The manifestation of this reactivity in high-cortisol monkeys seems to depend on the context, specifically the degree of threat present in each phase of the challenge. Our results further demonstrate that combining hair cortisol measurements with HIT reactivity is an effective method of assessing an animal's temperament, meaning trait-like behavioral and physiological responsiveness. This approach could be used in applications for which determination of temperament is important, such as selection of individually housed monkeys for social pairing (see, for example, [Capitanio et al., 2015]).
Acknowledgments
The authors would like to thank Olivia Forshtay, Elana Varner, Saif El-Mallah, Lauren Stanwicks, Bianca Corey, and Lily Augustini for videoscoring and Brittany Peterson and Nicola Robertson for administering the Human Intruder Test. This research was supported in part by grant numbers R24OD01180-15 to Melinda Novak, P51OD011133 to Texas Biomedical Research Institute (SNPRC), 8P51OD011092-53 to the Oregon National Primate Research Center (ONPRC), P51OD010425 to the Washington National Primate Research Center (WaNPRC), and P51OD011103 to the New England Primate Research Center (NEPRC). Finally, the authors would like to acknowledge that all research herein has been approved by an Institutional Animal Care and Use Committee (IACUC), conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and complied with the Animal Welfare Act of the United States.
References
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Hormones and Behavior. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan BJ. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. American Journal of Primatology, on-line before print. 2015 doi: 10.1002/ajp.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Cole SW. Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol's influence on trafficking. Brain, Behavior, and Immunity. 2011;25:151–159. doi: 10.1016/j.bbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlitz EH, Kirschbaum C, Stalder T, van Schaik CP. Hair a long-term retrospective cortisol calendar in orang-utans (Pongo spp.): New perspectives for stress monitoring in captive management and conservation. General and Comparative Endocrinology. 2014;195:151–156. doi: 10.1016/j.ygcen.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Coleman K. Individual differences in temperament and behavioral management practices for nonhuman primates. Applied Animal Behavior Science. 2012;137:106–113. doi: 10.1016/j.applanim.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MF, Novak MA, Meyer JS, Suomi SJ. Hair cortisol predicts object performance in infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2009;51:706–713. doi: 10.1002/dev.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology. 2011;36:1201–1208. doi: 10.1016/j.psyneuen.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie NH, Jolly CJ, Phillips-Conroy JE, Brown JL, Bernstein RM. Variation of hair cortisol concentrations among wild populations of two baboon species (Papio anubis, P. hamadryas) and a population of their natural hybrids. Primates. 2015a;56:259–272. doi: 10.1007/s10329-015-0469-z. [DOI] [PubMed] [Google Scholar]
- Fourie NH, Turner TR, Brown JL, Pampush JD, Lorenz JG, Bernstein RM. Variation in vervet (Chlorocebus aethiops) hair cortisol concentrations reflects ecological disturbance by humans. Primates. 2015;56:365–373. doi: 10.1007/s10329-015-0486-y. [DOI] [PubMed] [Google Scholar]
- Hadidian J. Yawning in an old world monkey, Macaca nigra (Primates: Cercopithecidae) Behavior. 1980;75:133–147. [Google Scholar]
- Hermans EJ, Putman P, Bass JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenutates the integrated central stress response in healthy young women. Psychoneuroendocinolgy. 2007;8-10:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behavioral Neuroscience. 1998a;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behavioral Neuroscience. 1998b;112:51–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production -- Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, Grzywa R, Fairbanks LA. A novelty seeking phenotype is related to chronic hypothalamic-pituitary-adrenal activity reflected by hair cortisol. Physiology & Behavior. 2011;104:291–295. doi: 10.1016/j.physbeh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Gestural communication in three species of macaques (Macaca mulatta, M. nemestrina, M. arcotoides): use of signals in relation to dominance and social context. Gesture. 2005;5:57–73. [Google Scholar]
- Meyer JS, Novak MA. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153:4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Hamel AF, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments. 2014;83:e50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. Stress, the HPA axis, and nonhuman primate well-being: A review. Applied Animal Behaviour Science. 2013;143:135–149. doi: 10.1016/j.applanim.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raul JS, Crimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry. 2004;37:1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes, Brain, and Behavior. 2008;7:463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and Investigative Medicine. 2007;30:E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wüst S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosomatic Medicine. 2008;70:787–796. doi: 10.1097/PSY.0b013e3181810658. [DOI] [PubMed] [Google Scholar]
- Siniscalchi M, McFarlane JR, Kauter KG, Quaranta A, Rogers LJ. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Research in Veterinary Science. 2013;93:49–54. doi: 10.1016/j.rvsc.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Pennix B, Spijker AT, Elzinga BM, van Rossum E. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin. 2000;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1994:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Troisi A, Schino G, D'Antoni M, et al. Scratching as a behavioral index of anxiety in macaque mothers. Behavioral and Neural Biology. 1991;56:307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]