Abstract
Redundancy is an important feature of the motor system, as abundant degrees of freedom are prominent at every level of organization across the central and peripheral nervous systems, and musculoskeletal system. This basic feature results in a system that is both flexible and robust, and which can be sustainably adapted through plasticity mechanisms in response to intrinsic organismal changes and dynamic environments. While much early work of motor system organization has focused on synaptic-based plasticity processes that are driven via experience, recent investigations of neuron–glia interactions, epigenetic mechanisms and large-scale network dynamics have revealed a plethora of plasticity mechanisms that support motor system organization across multiple, overlapping spatial and temporal scales. Furthermore, an important role of these mechanisms is the regulation of intrinsic variability. Here, we review several of these mechanisms and discuss their potential role in neurorehabilitation.
Keywords: plasticity, sensorimotor, dynamical systems, mechanisms, neurorehabilitation
Introduction
Redundancy permeates every level of motor system organization, as a given action can be equivalently accomplished through different combinations of neural network and musculoskeletal pathways (Bernstein 1967; Lashley 1933). While a redundant system requires greater allocation and maintenance of computational resources (i.e., greater energy investment), and more complex solutions for controlling actions (i.e., “the degrees of freedom problem”) (Bernstein 1967; Contreras-Vidal and others 1997), it also affords two crucial ecological and evolutionary benefits (Shadmehr and Wise 2005). Redundancy endows the motor system with extraordinary flexibility and robustness, allowing motor skills to be acquired within highly uncertain and non-stationary environments (Bullock and others 1993; Cisek and others 1998) and enables the rehabilitation of these skills following injury or disease through alternative system pathways (Lashley 1933; Yehuda and others 2015). Thus, optimizing the trade-off between redundancy-based costs (i.e., energy expenditures and control complexity) and benefits (i.e., robustness and system adaptability) is a primary and continuous challenge for motor system organization over time (Ajemian and others 2013; Bassett and others 2011; Dias and Ressler 2014; Graziano 2016).
Several computational models motivated by Donald Hebb’s foundational theory of experience-based plasticity (Heijmans and others 2008) have described intrinsic (i.e., internal representations of actions within the brain) and extrinsic (i.e., sensory feedback) influences underlying motor learning, adaptation, and control (Bullock and others 1993; Bullock and others 1998; Bullock and Grossberg 1988; Cisek and others 1998; Contreras-Vidal and others 1997). More recently, dynamical systems theory has provided a way to understand how the motor system optimizes the redundancy-based cost–benefit trade-off (Ajemian and others 2013; Bassett and others 2013; Bassett and others 2015; Churchland and others 2010; Churchland and others 2012; Shenoy and others 2011; Shenoy and others 2013). Within this framework, the heterogeneity of synaptic weights across a network, which cumulatively encode knowledge about past interactions between an organism and the environment, result in the formation of network attractors (Bullock and Grossberg 1988; Dias and Ressler 2014; Shadmehr and Wise 2005) (Box 1; Figure 1). Attractors are stable network state representations of motor skills and represent an appropriate basis for understanding motor system organization (Box 2) (Churchland and others 2012; Flanders 2005; Graziano 2016). Attractor-based dynamics at the systems level reduce control complexity, while still maintaining the flexibility required to perform a skill in uncertain biological and environmental contexts (Ajemian and others 2013; Churchland and others 2012). Furthermore, some plasticity mechanisms appear to provide an important source of intrinsic noise, which result in chaotic attractor structure that supports flexibility. Chaotic attractors allow small variations in neural network activity patterns whenever a motor skill is performed, which promote the constant exploration and comparison of alternative pathways for its execution (Ajemian and others 2013; Bullock and others 1998). Thus, the plasticity-related development of chaotic attractors over time provides a strong foundation for utilizing redundancy in the motor system.
Box 1. Dynamical System Attractors.
At a given instant, the state of a dynamical system is the result of: (1) the initial intrinsic system state, (2) intrinsic noise, and (3) extrinsic inputs. Eventually, the system will settle on a stable set of states, which is a subset of all possible network state configurations. This is referred to as an attractor, and the boundary of a given attractor set is referred to as the attractor manifold. The system dynamics will remain within the manifold until a future extrinsic input is sufficient to drive the network toward a different attractor basin. Attractors are a function of asymmetry within the network. In turn, asymmetry is defined by the structural connectivity between neurons at different spatial scales, distribution of synaptic weight strengths, and action potential transmission speeds and delays. Plasticity mechanisms controlling these factors shape the creation and extinction of individual attractors, the location of the existing attractors within the network state-space, or even changes to their dimensional structure over time.
Network attractor structure can be described according to its dimensionality. Point attractors represent fixed, stationary network states, while line attractors represent stable behavior that still varies to some degree over one dimension. Additional attractors types include planes or rings, or even limit cycles (typically used to describe central pattern generators observed in the spinal cord, or periodic motor behaviors such as gait), which involve a set of states that is repeatedly traversed. Chaotic (or strange) attractors are yet another type, which are typically described by three-dimensional manifolds such as a sphere, torus, or more complex surfaces. Chaotic attractors have several interesting properties or relevance to biological neural networks. First, the set of states adopted within the manifold subspace is nonstationary and may vary stochastically over time. Second, the manifolds typically exhibit fractal structure. These features of chaotic attractors appear to be important for maintaining adaptive networks adept at operating within uncertain and non-stationary environments.
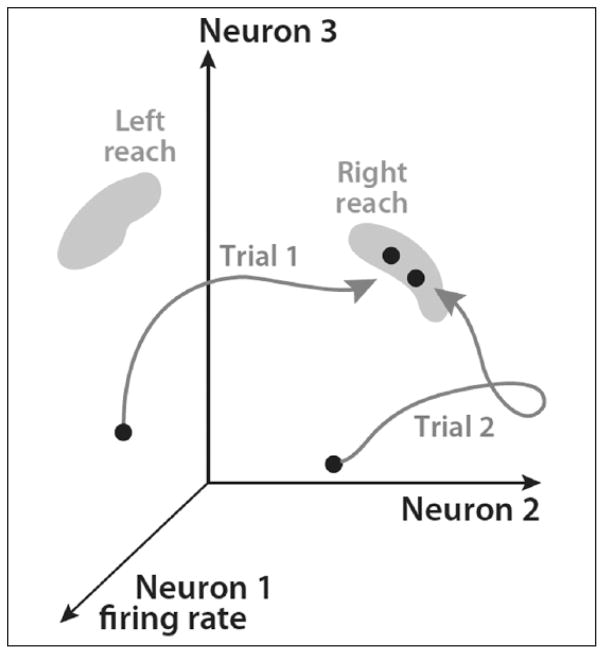
Figure 1.
Dynamical system attractors and motor skills. A schematic representation of a network state-space made up of three individual neurons. A leftward and rightward goal-directed reach is defined by two distinct attractor manifolds within the network state-space. The system must adopt states within these subspaces to execute either reach behavior. In this example, the evolution of delay-period preparatory activity is represented as a trajectory for two different instructed rightward reaches (Trial 1 vs. Trial 2). Individual trial differences between the state-space trajectory during preparation, as well as differences between where the system settles within the attractor subspace can be related to behavioral features for a given instance of the action (i.e., reaction time, movement time, reach error, etc). Figure modified from Shenoy and others (2013).
Box 2. Attractor-Based Organization of the Motor System.
Despite a long-standing debate over the topographic organization of the primary motor cortex spanning several decades, an emerging consensus is that the organization can be well understood from an attractor-based perspective. Stimulation-based mapping of the primary motor cortex (M1) neurons to peripheral muscle activation patterns does not appear to correspond to individual muscles or even groups of muscles that cross a single joint as originally thought (Flanders 2005). Instead, these maps appear to reflect representations of attractor manifolds present in the motor system, as stimulation of local neuron populations causes coherent patterns of activation in groups of muscles that contribute to actions which, given prior experience, are likely to be functionally relevant and regularly repeated (Bullock and others 1993; Flanders 2005; Graziano 2016; Graziano and others 2005; Shadmehr and Wise 2005). This is also consistent with the motor primitive (Mussa-Ivaldi and others 1994) or synergy (Jaric and Latash 1998) theoretical frameworks, which decompose the motor system into the basic units from which an organism’s entire action repertoire can be synthesized. Under this view, more elaborate motor skills (i.e., a tennis serve) emerge from network dynamics that move between different attractor subspaces, flexibly combining these basic units into a single coherent action (Davison and others 2015). For example, unlike summary statistics of M1 population activity (such as directional tuning, etc.), state-space trajectories encoded across the population network during movement planning is highly predictive of subsequent reaction times and movement execution parameters on a single-trial basis (Churchland and others 2006; Churchland and others 2012).
The dynamical systems framework has also been successfully applied to describe behavioral features of motor skill learning and control (Gera and others 2010; Latash and others 2007; Scholz and Schoner 1999; Schoner 1990). For example, skill gains for a given motor task are coincident with selective, context-dependent modifications of the variance observed between instances of a given action (inter-trial variability) (Scholz and Schoner 1999; Wu and others 2014). Variability at the behavioral level is controlled in at least two ways. First, as motor skill increases for a given action there is a specific reduction in variability at effector joints that results in more robust repeatability over time (Latash and others 2007). For example, elbow rotations along one dimension (i.e., flexion–extension) may be more strongly related with target-directed reaching accuracy than rotations along another dimension (pronation–supination). In this scenario, an increase in skill will be concurrent with a reduction in variability of elbow flexion–extension between instances of a reach, while no significant change pronation–supination variability will be observed. One formulation of this theoretical concept is the uncontrolled manifold hypothesis (Scholz and Schoner 1999; Schoner 1990). Second, evidence suggests that strategies for acquiring contextual information about the task environment evolve in a similar way. For example, during the novice stage, strategies are biased toward exploring task environments in a uniform way across the limits of the task space. Through experience, knowledge about dependencies between multiple context variables can be used to constrain the space and simplify the basis for selecting the most appropriate actions in order to achieve the intended goal (Cully and others 2015; Friston 2013). This has been described in the literature as dividing the contextual information into task-relevant and task-irrelevant subspaces (Wu and others 2014). A secondary consequence of this filtering process is that it allows for greater allocation of time and attentional resources to task-relevant information. Indeed, when contextual information is categorized in this way, experts display greater exploration of task-relevant information compared with novices (Scholz and Schoner 1999).
Experimental investigation of synaptic plasticity have provided a rich description of mechanisms affecting neuronal information processing over time (Feldman 2012). More recently, investigations of neuron-glia interactions, epigenetic modification of DNA, and systems-level network properties suggest a diverse and redundant landscape of plasticity mechanisms operating over different spatial and temporal scales during motor learning (Figure 2) (Bassett and others 2011; Bassett and others 2013; Bassett and others 2015; Fields and others 2015). Some processes operate over longer temporal scales (i.e., across an individual’s life span) and impart stability to the system through the maintenance of networks representing knowledge gained through prior experience (Kwok and others 2011). Alternatively, other processes operate over shorter temporal scales (i.e., across seconds to hours) and integrate new information into the motor network architecture in real time, supporting rapid learning of new skills or adaptation of existing ones as needs change. The interaction between these processes, described as the stability-plasticity dilemma (Ajemian and others 2013; Dias and Ressler 2014), changes in response to needs over the lifespan. In the healthy adult, there is a bias toward preserving existing knowledge and skills, while the incorporation of new information is enhanced during critical developmental periods, the learning of new skills, and in the period immediately following the onset of brain injury or disease where existing motor memories may no longer be relevant.
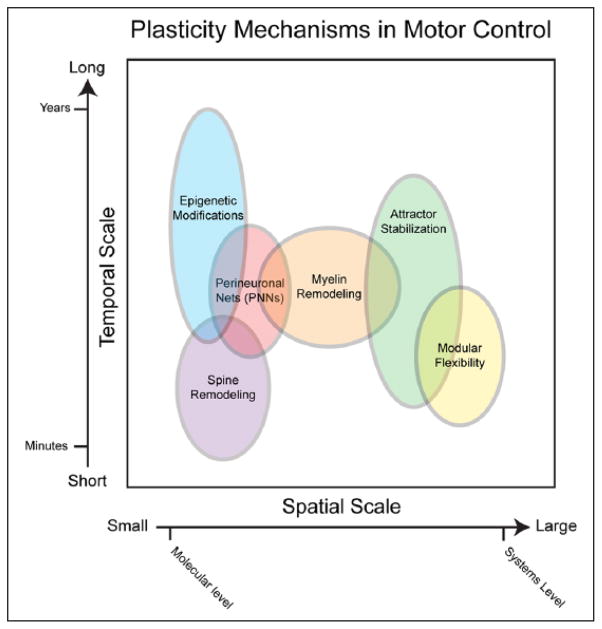
Figure 2.
Schematic of plasticity mechanisms in motor control along spatiotemporal scales. This figure depicts the spatiotemporal properties of various plasticity mechanisms along a continuum with small spatial scales and short temporal scales at the left/bottom and larger spatial scales and longer temporal scales on the right/top. Overlapping cellular-level (in purple, red and orange), epigenetic (blue), and systems-level (green and yellow) mechanisms are distributed throughout these scales.
Here, we discuss the roles different plasticity mechanisms play in relation to the stability-plasticity dilemma (Ajemian and others 2013; Churchland and others 2012; Kelso 1997; Pajevic and others 2014; Woldemichael and others 2014). We also propose that inter-individual differences in stability–plasticity interactions influence spontaneous recovery of motor function following brain injury, and response to neuromodulation (i.e., non-invasive brain stimulation).
Cellular-Level Mechanisms
Plasticity mechanisms use information gained through experience (i.e., learning) and intrinsic noise to continuously optimize network organization over time (Ajemian and others 2013; Pajevic and others 2014). In the past decade, imaging of dendritic spine remodeling (i.e., the addition and elimination of individual spines from the dendritic field) have revealed dynamics that appear to play an important role in skill learning (Ganguly and Poo 2013; Holtmaat and Svoboda 2009; Jung and Herms 2014; Roberts and others 2010). Spine remodeling occurs over relatively rapid temporal scales (on the manner of hours), suggesting it is an important mechanism for maintaining adaptability in motor systems. In vitro imaging of this process in rodent models has demonstrated two distinct classes of spines: (1) those that become established and remain stable over long periods and (2) a group that is continuously formed and eliminated in a stochastic manner over shorter time periods, and thus, may be a mechanistic source of intrinsic noise that appears to promote exploratory learning (Holtmaat and Svoboda 2009).
Interestingly, the rate of dendritic spine remodeling is directly related to the rate of skill acquisition and task environment complexity (Roberts and others 2010). For example, in juvenile birds with high levels of spontaneous spine turnover, hearing a tutor song leads to the rapid (24 hours) stabilization, accumulation, and enlargement of dendritic spines in specific functionally related brain areas. Moreover, in vivo intracellular recordings made immediately before and after the first day of tutoring revealed robust enhancement of synaptic activity in these same brain region. Thus, learning occurs when instruction results in stabilization and strengthening of synapses within task-relevant networks. Additionally, total spine density and number, and the turnover rate of transient exploratory spines depends on the complexity of the environment, and appears to be a system response to the acquisition and consolidation of skills within richer task contexts (Jung and Herms 2014). Notably, the local spatial nature of these changes suggests that similar remodeling effects must accumulate across populations of neurons before having an influence on system stability (Ajemian and others 2013).
A related cellular-level plasticity mechanism contributing to the stability–plasticity trade-off in motor systems is the formation and destabilization of perineuronal nets (PNs). PNs are lattice-like structures made up of extracellular matrix proteins that envelop the cell body of proximal neurites of individual neurons, and impose a physical restriction on new synapse formation. The development of PNs is tightly synchronized with the close of critical developmental periods, and thus appear to play a primary role in stabilizing motor networks within the healthy mature brain (Karetko and Skangiel-Kramska 2009). Furthermore, work in rodent models of ischemic stroke suggest that the destabilization of PNs occurs in a distributed fashion across motor regions in the first few days following injury, and are important predictors of motor recovery (Bidmon and others 1997; Hobohm and others 2005).
The regulation of axon myelination is another important component of information processing within the motor system, and is a process that remains much more dynamic throughout the lifespan than originally thought (Fields and others 2015). Myelination is the primary mechanism through which transmission speed is manipulated in individual pathways, and can influence how information is distributed and processed across the network. Recent data suggest that oligodendrocytes use activity-related feedback mechanisms to continuously remodel myelin sheath thickness and the spacing and structure of nodes of Ranvier across the length of the axon (Fields 2014; Fields and others 2014; Fields and others 2015). Although, the precise purpose for this remodeling remains unknown, it is likely that it is responsible for fine-tuning the synchronization of widespread oscillatory neuronal network activity (Fields and others 2015; Pajevic and others 2014). A recent computational model of dynamic regulation of myelination in cortical networks suggests that introduction of delays smaller than 1 ms, which are well within the range of latency changes that can be induced by myelin remodeling, can change oscillatory phase by up to 30%, and have significant effects on network output (Pajevic and others 2014).
Furthermore, the impairment of activity-dependent myelin regulation and inability to continuously adapt time delays to optimize network information transfer may contribute to disorders where hyper- and hypo-synchrony of neuronal firing leads to motor system dysfunction (Fields and others 2015).
While these are only a few examples of the many cellular mechanisms that participate in the stability–plasticity tradeoff, they exemplify issues that have substantial clinical implications. In rodent models of brain injury and stroke, spine remodeling rates continually increase in peri-infarct regions (but not in distant brain regions) over the first 2 weeks following the event before gradually declining (Brown and others 2007; Brown and others 2008; Brown and others 2010; Brown and Murphy 2008). This suggests that increased transient spine turnover rates may be a way that the system increases exploratory intrinsic noise in an attempt to re-stabilize the damaged network following injury, and that there is a critical period where the network can converge on new system attractors to replace or restore diminished behaviors. Another intriguing area of research is the targeted destabilization of PNs in animal models (Bidmon and others 1997; Hobohm and others 2005). Such work is being pursued as an approach for removing physical barriers to synaptogenesis, and to promote expansion of the therapeutic window following brain injury or disease. Future research might focus on how these two mechanisms—increasing spine turnover or destabilizing PNs—relate to spontaneous reduction of motor deficits following brain injury or disease. Prolonging these processes could provide a greater chance of recovery, especially in those who do not demonstrate spontaneous recovery early after injury. Furthermore, a better understanding of the dynamics of these processes could help predict patients who are more or less likely to experience spontaneous recover early after injury, and aid in the development of non-invasive techniques for manipulating these factors in humans to provide powerful therapeutic tools.
Epigenetic Mechanisms
Epigenetic modification of gene targets can result in alterations in dendritic spines and postsynaptic membrane receptor density (Bronfman and others 2014). Similar to the previously discussed cellular mechanisms, epigenetic mechanisms can also fit within the lens of dynamical systems theory as promoters of either long-term stability or flexible adaptability across various temporal and spatial scales.
One way that epigenetic mechanisms promote long-lasting stability is through hereditability via parental gametes so that information about organism–environment interactions are maintained from one generation to the next (Keverne 2014; Shadmehr and Wise 2005). Although some controversy remains regarding the specific mechanistic source, several recent studies have shown that epigenetic modifications affecting one generation can be passed down to their offspring. For example, a recent study in mice showed transgenerational fear conditioning which appears to have occurred through DNA hypomethylation of a specific odor-related receptor gene (Dias and Ressler 2014). Here, a group of mice were conditioned to associate fear with a particular scent through pairing with electric shocks. Interestingly, their subsequent offspring also showed a conditioned fear response to the same scent and the same hypomethylation of the odor-related gene, despite not undergoing behavioral conditioning themselves. This effect was evident through two generations of offspring and found to be passed down via the parental gametes (Dias and Ressler 2014). This suggests that environmental-based cueing of specific motor skill behaviors important to survival may also be passed between generations through similar mechanisms. Epigenetic mechanisms also contribute to learning and memory stability within one’s own life span (Grossberg 1980a, 1980b; Martinowich and others 2003; Woldemichael and others 2014). During long-term potentiation (LTP) and long-term depression (LTD), a slew of short-term molecular changes occur such as intracellular signaling cascades and increased protein synthesis. Epigenetic modifications enable longer lasting changes by either up-regulating or down-regulating promoter gene transcription that control protein synthesis required for motor memory consolidation (for reviews, see Day and Sweatt 2011; Guan and others 2015; Woldemichael and others 2014).
In parallel, several epigenetic mechanisms act to maintain CNS malleability supporting adaptive behavior that is customized to an individual’s specific interactions with the environment. Epigenetic changes have been shown to explain variability in adult monozygotic twins, suggesting adaptive expression of the same underlying DNA occurs in response to different environments and experiences (Feil and Fraga 2011; Fraga and others 2005). In line with this, there are numerous epigenetic mechanisms affecting synaptic plasticity that are activity-dependent and require the presence of specific behavioral training or environmental triggers (Martinowich and others 2003; Woldemichael and others 2014). For example, epigenetics may play a role in “gating” protein synthesis and synaptic remodeling cascades during learning that can lead to rapid changes in behavior (Dayan and Cohen 2011; Guan and others 2015; Martinowich and others 2003).
Fast plasticity mechanisms operate on many genes targets that underlie motor learning and memory. One of the most widely studied is brain-derived neurotrophic factor (BDNF), important for neurogenesis and LTP (Hall and others 2000; Jones and others 1994). Humans with the Val66Met BDNF polymorphism, which reduces activity-dependent BDNF secretion by 18% to 30% compared with individuals with the Val/Val polymorphism (Chen and others 2006), or mice with a BDNF-specific gene deletion, show a reduction in practice-dependent motor learning, motor-evoked potentials indicating cortical excitability, and responsiveness to noninvasive brain stimulation such as transcranial direct current stimulation (Fritsch and others 2010; Kleim and others 2006; McHughen and others 2010; Reis and others 2009). Epigenetic alterations of BDNF can occur in response to environmental challenges, stress, light exposure, drug abuse, exercise, and training.
The adaptive response of stability–plasticity interactions in individuals at progressive epochs following brain injury or disease is the direct result of interactions between measurable biological and environmental factors (Elder and others 2013). There have been some attempts to experimentally manipulate these factors with the aim of promoting either spontaneous or therapeutically driven impairment reduction following brain injury or disease. One example of an approach applied in animal models is ischemic tolerance, in which sublethal ischemia is induced in order to promote activation of neuroprotective agents (Gidday 2006; Sandu and others 2009). While the efficacy of this method is still under investigation, studies have found that ischemic tolerance leads to neuroprotective effects through the epigenetic down-regulation of genes involved in cell death and inflammation, and enhanced transcription of genes involved in promoting blood flow, repair mechanisms, and plasticity processes (Schweizer and others 2013; Simon and others 2012). In particular, epigenetic silencers such as polycomb group proteins (PcG) and modified histones become active after sublethal ischemia to regulate chromatin and transcription suppression and promote neuroprotective mechanisms (Elder and others 2013; Simon and others 2012). Other emerging therapies are exploring ways to modulate specific aspects of gene expression, such as inhibiting DNA methyltransferase and histone deacetylase enzymes in order to promote functional reorganization and neural repair (Qureshi and Mehler 2010; Schweizer and others 2013), and promoting polycomb proteins, which are thought to directly regulate the level of repair-based gene expression (Elder and others 2013). While much of this research is preliminary, it presents a promising pathway for harnessing neuroplasticity-related epigenetic mechanisms that become highly active following brain lesions like stroke to promote recovery. In addition, epigenetic variation could be explored as a potential source of the wide inter-individual variability found in spontaneous neurological recovery. Understanding epigenetic mechanisms of recovery and how these may be influenced by different environmental factors (i.e., stress, nutrition) may help in the design of appropriate hospital-based environments aimed to maximize plasticity and learning in the acute stage following brain injury.
Systems-Level Mechanisms
Simultaneous activity of many interconnected neurons results in widespread oscillations across neural networks that encode information unavailable at smaller scales, called emergent network properties. Similar to information processing at the single neuron level, emergent network properties can (1) support the transition between highly stable and highly adaptive states when needed, (2) adaptively regulate functional connectivity patterns, and (3) represent distinct plasticity mechanisms in the motor system.
A novel computational model developed by Ajemian and others (2013) offers a quantitative framework within which emergent systems-level plasticity mechanisms may be explored, as well as some interesting insight into the role they play in motor skill learning. They constructed a biologically constrained “hyperplastic” neural network model consisting of a highly redundant network of nodes possessing properties of both high learning rates and high levels of intrinsic noise consistent with known dendritic spine dynamics. The high learning rates for each node support rapid convergence on favorable, contextually based action–outcome pairings, while the intrinsic noise mechanisms allow for continuous exploration of alternative network solutions, even in the absence of overt errors. When sufficiently balanced, the interactions between these network features result in a systems-level structure adept at rapid transition from highly stable to highly adaptive states.
During the early stage of motor skill learning, rapid gains in performance typically occur at an exponential rate. At a later stage, performance gains slow considerably, and are best described by low-slope linear functions. The “hyperplastic network” model makes two specific predictions about systems level mechanisms underlying these two behaviorally defined stages of motor learning. The first prediction is that in the early stage, high synaptic learning rates result in rapid system convergence on a chaotic attractor space that represents a set of valid functional network solutions necessary for goal attainment when performing a particular skill. As stated earlier, the creation of the attractor constrains the dimensionality of the system. Many experimental paradigms focus on the acquisition of a single motor skill in laboratory settings. In real life, however, we are constantly developing a wide range of skills, and constantly switching back and forth between them. Thus, motor networks must create and maintain a vast number of attractors, which simultaneously coexist.
Since we accumulate many skills over our life spans, motor networks must be able to represent enormous numbers of attractors simultaneously. Furthermore, as new skills are acquired, the degradation or disruption of existing skills must be minimized. The hyperplastic network model proposed by Ajemian and others (2013) accommodates this by converging rapidly on subspaces where several attractor manifolds representing different motor skills overlap or intersect. The second prediction is that during this later stage, behavioral transfer may be observed between two motor skills sharing similar underlying neurophysiological and biomechanical effector features. One could think of this process as clustering attractors supporting similar skills within similar subspaces of the network. When the system settles on a subspace where multiple attractor manifolds intersect, performance gains become asymptotic and interference between similar motor skills may begin to emerge. Intrinsic noise mechanisms promote the chaotic nature of the attractor and maintain a fluid weight space that leads to eventual minimization of network resource overlap between attractors representing similar motor skills. Once attractors become maximally independent, interference between different motor skills is minimized. This process may provide a network-level description of motor memory consolidation (Brashers-Krug and others 1996), where a skill becomes resistant to interference from competing skills, or reconsolidation (Censor and others 2014; Walker and others 2003), which is where a previously consolidated memory transitions back from a stabile to labile stage.
Bassett and others (2011) used BOLD fMRI (blood oxygen level–dependent functional magnetic resonance imaging) to explore how network attractor structure varied over multiple timescales during learning of a novel motor task, and if this variability was predictive of how fast individuals learned. They used network analyses to cluster the nodes into attractor modules, which were empirically based on functional connectivity patterns. They then calculated a measure termed flexibility, which quantified the tendency for nodal membership of different modules to change over different timescales (within and between the three daily practice sessions). At the group-level, they found that the modular decomposition of large-scale brain networks display a tendency to become more flexible during early stages of learning, compared with both baseline and late learning stages (Figure 3). They also observed that at the individual subject level, the degree of flexibility observed in each participant was predictive of the learning rate observed for the same session, and actually predicted performance on subsequent sessions (Session 2 vs. Session 1; Session 3 vs. Session 2). This suggests that destabilization of existing network attractors may promote more rapid formation of new ones as new motor skills are acquired.
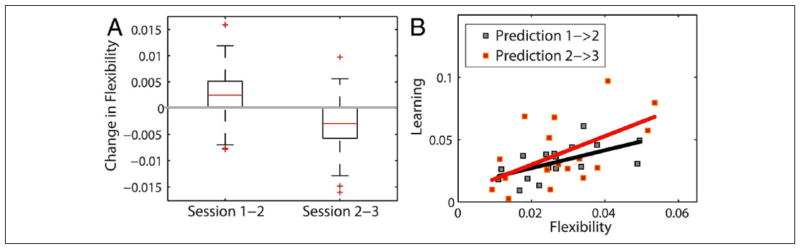
Figure 3.
Modular flexibility and learning. (A) Modular flexibility increases significantly during early learning of a novel motor skill (Sessions 1 to 2), and the decreases as performance gains plateau (Sessions 2 to 3). (B) The change in flexibility occurring between Sessions 1 and 2, and Sessions 2 and 3 is highly predictive of the learning exhibited in the latter session. This suggests that greater modular flexibility is indicative of high learning potential in the system. Figure modified from Bassett and others (2011).
At the behavioral level, a similar finding relating the structure of within-subject performance variability to skill learning or expertise has been observed at the level of both discrete (reaching, pointing and grasping) and rhythmic (gait) motor behaviors (Schoner 1990). Wu and others (2014) investigated the relationship between inter-subject variability and trial-by-trial learning rate in a reward-based motor learning task. They found that subjects who displayed greater variability early in learning showed faster learning rates (Figure 4). These subjects were also able to constrain this variability over time to task-specific domains. That is, they actively explored the task environment in a way that was constructive to achieving the goal, while simultaneously dampening movement variability that was independent of the task space. Several other studies have found similar relationships between variability and motor learning for different motor skills (Gera and others 2010; Scholz and Schoner 1999). Thus, increased task-relevant variability appears to result in faster formation of stronger network attractors.
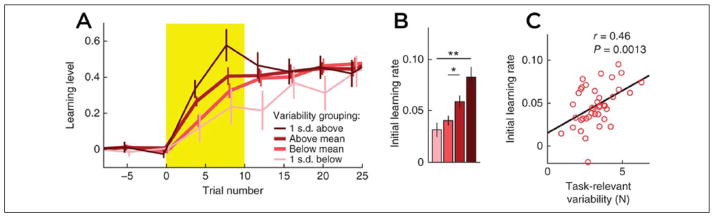
Figure 4.
(A, B) Learning curve comparisons for subgroups determined by task-relevant variability observed during baseline trials of a force-field adaptation task. There is a consistent and direct relationship between baseline task-relevant variability and initial learning rate across all subgroups. (C) This relationship holds at the single-subject level as well, where task-relevant variability observed during the baseline period is predictive of the initial learning rate observed during the first ten trials of the training period for the force-field adaptation task (error bars indicate ± standard error of the mean; **P > 0.005, *P > 0.05). Figure modified from Wu and others (2014).
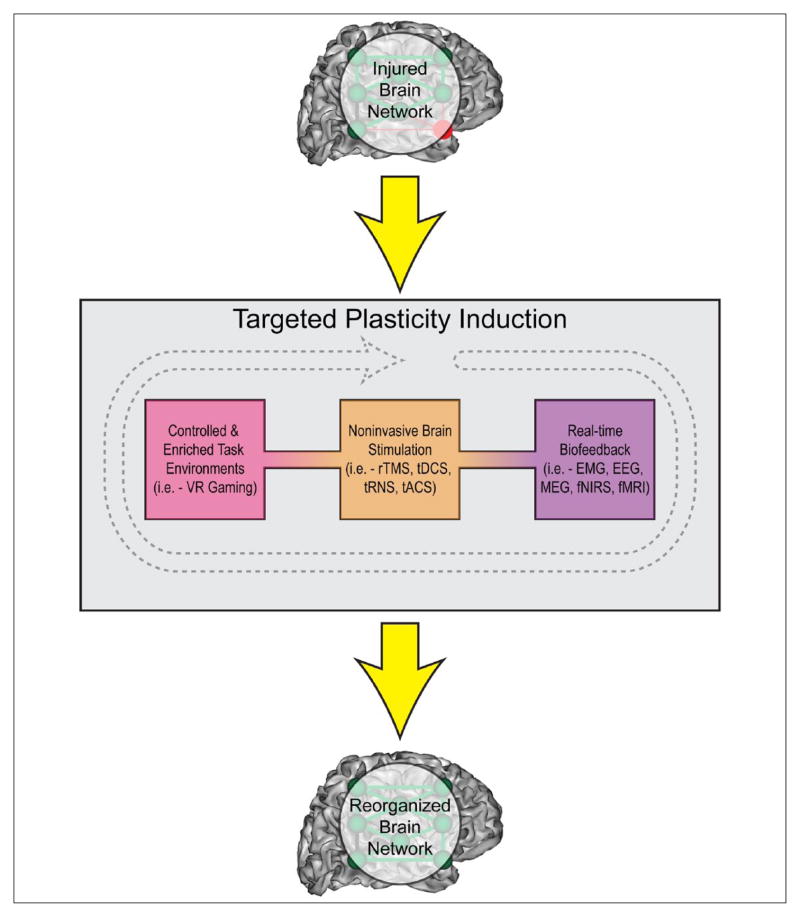
Regulation of neural network flexibility at the systems level may have clinical relevance, and play a large role in the design of interventional approaches for rehabilitation. Two major themes addressed throughout this paper that contribute to network flexibility are redundancy and variability. Following neural trauma, system redundancy provides a safeguard so that behaviors may be maintained, despite disruption of some information transmission pathways. In contrast, system variability or noise promotes the probability that new pathways may be discovered, with beneficial ones eventually reinforced. Thus, redundancy and variability work in tandem to provide the means for spontaneous recovery of function by re-routing information flow within the nervous system. These two characteristics of the nervous system also serve as potential targets for rehabilitative therapeutic advancement. While many attempts have been made at promoting impairment reduction or functional recovery through traditional physical therapy and practice, more recent attempts at modulating plasticity in the human brain have investigated the effects of real-time biofeedback, emersive virtual environments and non-invasive brain stimulation techniques (Figure 5) (Dayan and others 2013).
Figure 5.
Targeted clinical methods may induce plasticity and restore damaged neural networks. Plasticity may be induced in the injured brain via controlled and/or enriched task training environments (e.g., through virtual reality (VR), or gaming), noninvasive brain stimulation to modulate brain activity (e.g., repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS), and transcranial alternating current stimulation (tACS)), or real-time biofeedback, which allows individuals to learn to control their own neural network activity (e.g., using neuroimaging signals from electromyography (EMG), electroencephalography (EEG), magnetoencephalography (MEG), functional near-infrared spectroscopy (fNIRS), or functional magnetic resonance imaging (fMRI)). Greater understanding of how each of these techniques interact with one or more plasticity mechanisms will allow for a directed, individualized approach.
Non-invasive brain stimulation is a particularly promising neuromodulatory agent that involves the relatively safe application of transient electromagnetic pulses or electrical currents to the brain with the aim of exciting or inhibiting underlying neural tissue, resulting in both local and larger scale network changes (Dayan and others 2013; Liew and others 2014; Sandrini and Cohen 2013). In humans, these techniques influence LTP-like mechanisms, enhancing synaptic strength, and interacting with genes and neurotransmitters that are related to plasticity processes (e.g., BDNF, NMDA). They also appear to produce wide-scale network changes across the whole brain, not limited to the local stimulated region. In addition, pharmacological treatments, alone or in addition to non-invasive brain stimulation, further strengthen learning and plasticity (Khoutorsky and others 2013). It is unclear if this plasticity is occurring due to an increased strengthening of redundant, already-present connections, or due to augmentation of noise processes within the brain, leading to greater potential for new, adaptive changes. This area of research poses many new questions, such as whether patients with a greater capacity for increasing exploratory behavior following injury are more likely to display spontaneous recovery following stroke. However, before addressing this, there are also many basic questions remaining about the mechanisms by which noninvasive brain stimulation induces changes in the brain, especially in clinical populations, and at which level(s) of plasticity mechanisms brain stimulation works (Rothwell 2012). While practical matters such as the wide inter-individual variability in responsiveness to brain stimulation must be addressed (Lopez-Alonso and others 2015), we believe that exploring how noninvasive brain stimulation affects network dynamics may also shed light into both mechanisms of its use and potential as a clinical intervention. In particular, future research will investigate the use of non-invasive brain stimulation or pharmacologic agents to bias intrinsic system noise or learning rates in a manner that aids convergence on novel brain network attractors resulting in rehabilitated motor behaviors, or in prolongation of critical periods. Furthermore, the incorporation of multiple plasticity levels into future dynamical systems-based network models may provide important information about the role different mechanisms play in the stability–plasticity dilemma, and suggest target mechanisms for inducing appropriate rebalancing of these interactions to improve skill learning in healthy adults or reduce motor deficits in patients on an individual basis (Bestmann 2015).
Conclusion
The current review presents evidence that neuroplasticity in motor systems is multidimensional. Different mechanisms act in parallel across a spectrum of spatiotemporal scales. Fast-acting, local plasticity changes at the cellular level operate between predominantly stable and highly adaptive modes. More diffuse epigenetic and network changes that may relate to longer-term adaptive plasticity. Importantly, much of the observed plasticity is built upon tightly regulated intrinsic variability at each level of organization. Interactions between mechanisms on different levels allow for convergence upon, and stabilization of new attractors when necessary.
Future work will build upon our knowledge that it is possible to influence the stability-plasticity dilemma to augment plasticity in motor control networks using real-time biofeedback, emersive virtual environments, noninvasive brain stimulation and pharmacological agents, and examine the array of mechanisms through which these interventions operate. Computational models that integrate plasticity mechanisms over several levels of organization may help us understand how these adaptive changes permeate the motor system in both healthy and diseased states. Current research is exploring the use of computational models to understand basic mechanisms of brain stimulation (for excellent discussions, see Progress in Brain Research on Computational Brain Stimulation, and Bestmann 2015). Future neurorehabilitation approaches that utilize this understanding of motor system organization and dynamics may prove more effective for enhancing motor rehabilitation in a way that can be targeted to each individual.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Ajemian R, D’Ausilio A, Moorman H, Bizzi E. A theory for how sensorimotor skills are learned and retained in noisy and nonstationary neural circuits. Proc Natl Acad Sci U S A. 2013;110(52):E5078–87. doi: 10.1073/pnas.1320116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108(18):7641–6. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST. Task-based core-periphery organization of human brain dynamics. PLoS Comput Biol. 2013;9(9):e1003171. doi: 10.1371/journal.pcbi.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18(5):744–51. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA. The co-ordination and regulation of movements. Oxford, England: Pergamon Press; 1967. [Google Scholar]
- Bestmann S. Computational neurostimulation in basic and translational research. Prog Brain Res. 2015;222:xv–xx. doi: 10.1016/S0079-6123(15)00159-4. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Oermann E, Schleicher A, Kato K, Kinscherf R, Buchkremer-Ratzmann I, et al. Copper-zinc superoxide dismutase and isolectin B4 binding are markers for associative and transhemispheric diaschisis induced by focal ischemia in rat cortex. Neurosci Lett. 1997;228(3):163–6. doi: 10.1016/s0304-3940(97)00389-3. [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382(6588):252–5. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Bronfman ZZ, Ginsburg S, Jablonka E. Shaping the learning curve: epigenetic dynamics in neural plasticity. Front Integr Neurosci. 2014;8:55. doi: 10.3389/fnint.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Boyd JD, Murphy TH. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J Cereb Blood Flow Metab. 2010;30(4):783–91. doi: 10.1038/jcbfm.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27(15):4101–9. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Murphy TH. Livin’ on the edge: imaging dendritic spine turnover in the peri-infarct zone during ischemic stroke and recovery. Neuroscientist. 2008;14(2):139–46. doi: 10.1177/1073858407309854. [DOI] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39(4):1286–91. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Bullock D, Cisek P, Grossberg S. Cortical networks for control of voluntary arm movements under variable force conditions. Cereb Cortex. 1998;8(1):48–62. doi: 10.1093/cercor/8.1.48. [DOI] [PubMed] [Google Scholar]
- Bullock D, Grossberg S. Neural dynamics of planned arm movements: emergent invariants and speed-accuracy properties during trajectory formation. Psychol Rev. 1988;95(1):49–90. doi: 10.1037/0033-295x.95.1.49. [DOI] [PubMed] [Google Scholar]
- Bullock D, Grossberg S, Guenther FH. A self-organizing neural model of motor equivalent reaching and tool use by a multijoint arm. J Cogn Neurosci. 1993;5(4):408–35. doi: 10.1162/jocn.1993.5.4.408. [DOI] [PubMed] [Google Scholar]
- Censor N, Horovitz SG, Cohen LG. Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron. 2014;81(1):69–76. doi: 10.1016/j.neuron.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, et al. Neural population dynamics during reaching. Nature. 2012;487(7405):51–6. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68(3):387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006;26(14):3697–712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Grossberg S, Bullock D. A cortico-spinal model of reaching and proprioception under multiple task constraints. J Cogn Neurosci. 1998;10(4):425–44. doi: 10.1162/089892998562852. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Grossberg S, Bullock D. A neural model of cerebellar learning for arm movement control: cortico-spino-cerebellar dynamics. Learn Mem. 1997;3(6):475–502. doi: 10.1101/lm.3.6.475. [DOI] [PubMed] [Google Scholar]
- Cully A, Clune J, Tarapore D, Mouret JB. Robots that can adapt like animals. Nature. 2015;521(7553):503–7. doi: 10.1038/nature14422. [DOI] [PubMed] [Google Scholar]
- Davison EN, Schlesinger KJ, Bassett DS, Lynall ME, Miller MB, Grafton ST, et al. Brain network adaptability across task states. PLoS Comput Biol. 2015;11(1):e1004029. doi: 10.1371/journal.pcbi.1004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70(5):813–29. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16(7):838–44. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72(3):443–54. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J, Cortes M, Rykman A, Hill J, Karuppagounder S, Edwards D, et al. The epigenetics of stroke recovery and rehabilitation: from polycomb to histone deacetylases. Neurotherapeutics. 2013;10(4):808–816. doi: 10.1007/s13311-013-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75(4):556–71. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Myelin formation and remodeling. Cell. 2014;156(1–2):15–7. doi: 10.1016/j.cell.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Araque A, Johansen-Berg H, Lim SS, Lynch G, Nave KA, et al. Glial biology in learning and cognition. Neuroscientist. 2014;20(5):426–31. doi: 10.1177/1073858413504465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Woo DH, Basser PJ. Glial regulation of the neuronal connectome through local and long-distant communication. Neuron. 2015;86(2):374–86. doi: 10.1016/j.neuron.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M. Functional somatotopy in sensorimotor cortex. Neuroreport. 2005;16(4):313–6. doi: 10.1097/00001756-200503150-00001. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Active inference and free energy. Behav Brain Sci. 2013;36(3):212–3. doi: 10.1017/S0140525X12002142. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80(3):729–41. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Gera G, Freitas S, Latash M, Monahan K, Schoner G, Scholz J. Motor abundance contributes to resolving multiple kinematic task constraints. Motor Control. 2010;14(1):83–115. doi: 10.1123/mcj.14.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Graziano MS. Ethological action maps: a paradigm shift for the motor cortex. Trends Cogn Sci. 2016;20(2):121–32. doi: 10.1016/j.tics.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Aflalo TN, Cooke DF. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J Neurophysiol. 2005;94(6):4209–23. doi: 10.1152/jn.01303.2004. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Biological competition: decision rules, pattern formation, and oscillations. Proc Natl Acad Sci U S A. 1980a;77(4):2338–42. doi: 10.1073/pnas.77.4.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. Intracellular mechanisms of adaptation and self-regulation in self-organizing networks: the role of chemical transducers. Bull Math Biol. 1980b;42(3):365–96. doi: 10.1007/BF02460792. [DOI] [PubMed] [Google Scholar]
- Guan JS, Xie H, Ding X. The role of epigenetic regulation in learning and memory. Exp Neurol. 2015;268:30–6. doi: 10.1016/j.expneurol.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3(6):533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobohm C, Gunther A, Grosche J, Rossner S, Schneider D, Bruckner G. Decomposition and long-lasting down-regulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J Neurosci Res. 2005;80(4):539–48. doi: 10.1002/jnr.20459. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–58. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Jaric S, Latash ML. Learning a motor task involving obstacles by a multi-joint, redundant limb: two synergies within one movement. J Electromyogr Kinesiol. 1998;8(3):169–76. doi: 10.1016/s1050-6411(97)00017-5. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–99. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CK, Herms J. Structural dynamics of dendritic spines are influenced by an environmental enrichment: an in vivo imaging study. Cereb Cortex. 2014;24(2):377–84. doi: 10.1093/cercor/bhs317. [DOI] [PubMed] [Google Scholar]
- Karetko M, Skangiel-Kramska J. Diverse functions of perineuronal nets. Acta Neurobiol Exp (Wars) 2009;69(4):564–77. doi: 10.55782/ane-2009-1766. [DOI] [PubMed] [Google Scholar]
- Kelso JS. Dynamic patterns: the self-organization of brain and behavior. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Keverne EB. Significance of epigenetics for understanding brain development, brain evolution and behaviour. Neuroscience. 2014;264:207–17. doi: 10.1016/j.neuroscience.2012.11.030. [DOI] [PubMed] [Google Scholar]
- Khoutorsky A, Yanagiya A, Gkogkas CG, Fabian MR, Prager-Khoutorsky M, Cao R, et al. Control of synaptic plasticity and memory via suppression of poly(A)-binding protein. Neuron. 2013;78(2):298–311. doi: 10.1016/j.neuron.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71(11):1073–89. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Integrative functions of the cerebral cortex. Physiol Rev. 1933;13(1):1–42. [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Toward a new theory of motor synergies. Motor Control. 2007;11(3):276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- Liew S-L, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alonso V, Fernandez-Del-Olmo M, Costantini A, Gonzalez-Henriquez JJ, Cheeran B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin Neurophysiol. 2015;126(12):2342–7. doi: 10.1016/j.clinph.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–3. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20(5):1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Giszter SF, Bizzi E. Linear combinations of primitives in vertebrate motor control. Proc Natl Acad Sci U S A. 1994;91(16):7534–8. doi: 10.1073/pnas.91.16.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–47. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010;67(11):1316–22. doi: 10.1001/archneurol.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci. 2009;106(5):1590–5. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463(7283):948–52. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Clinical applications of noninvasive electrical stimulation: problems and potential. Clin EEG Neurosci. 2012;43(3):209–14. doi: 10.1177/1550059412444973. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Cohen LG. Noninvasive brain stimulation in neurorehabilitation. Handb Clin Neurol. 2013;116:499–524. doi: 10.1016/B978-0-444-53497-2.00040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu N, Cornelius J, Filis A, Arasho B, Perez-Pinzon M, Schaller B. Ischemic tolerance in stroke treatment. Expert Rev Cardiovasc Ther. 2009;7(10):1255–61. doi: 10.1586/erc.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126(3):289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Schoner G. A dynamic theory of coordination of discrete movement. Biol Cybern. 1990;63(4):257–70. doi: 10.1007/BF00203449. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Meisel A, Märschenz S. Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab. 2013;33(9):1335–46. doi: 10.1038/jcbfm.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Wise SP. The computational neurobiology of reaching and pointing: a foundation for motor learning. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Shenoy KV, Kaufman MT, Sahani M, Churchland MM. A dynamical systems view of motor preparation: implications for neural prosthetic system design. Prog Brain Res. 2011;192:33–58. doi: 10.1016/B978-0-444-53355-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, Churchland MM. Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci. 2013;36:337–59. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- Simon RP, Meller R, Zhou A, Henshall D. Can genes modify stroke outcome and by what mechanisms? Stroke. 2012;43(1):286–91. doi: 10.1161/STROKEAHA.111.622225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425(6958):616–20. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Woldemichael BT, Bohacek J, Gapp K, Mansuy IM. Epigenetics of memory and plasticity. Prog Mol Biol Transl Sci. 2014;122:305–40. doi: 10.1016/B978-0-12-420170-5.00011-8. [DOI] [PubMed] [Google Scholar]
- Wu HG, Miyamoto YR, Castro LN, Ölveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci. 2014;17(2):312–21. doi: 10.1038/nn.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.005. Epub Aug 12. [DOI] [PubMed] [Google Scholar]