Abstract
Background & Aims
Patients with chronic ulcerative colitis are at increased risk for colorectal neoplasia (CRN). Surveillance by white-light endoscopy (WLE) or chromoendoscopy may reduce risk of CRN, but these strategies are underused. Analysis of DNA from stool samples (sDNA) can detect CRN with high levels of sensitivity, but it is not clear if this approach is cost effective. We simulated these strategies for CRN detection to determine which approach is most cost effective.
Methods
We adapted a previously published Markov model to simulate the clinical course of chronic ulcerative colitis, the incidence of cancer or dysplasia, and costs and benefits of care with 4 surveillance strategies. These strategies were: analysis of sDNA and diagnostic chromoendoscopy for patients with positive results, analysis of sDNA with diagnostic WLE for patients with positive results, chromoendoscopy with targeted collection of biopsies, or WLE with random collection of biopsies. Costs were based on 2014 Medicare reimbursement. The primary outcome was the incremental cost effectiveness ratio (incremental cost/incremental difference in quality-adjusted life years) compared with no surveillance and a willingness to pay threshold of $50,000.
Results
All strategies fell below the willingness to pay threshold at 2 year intervals. Incremental cost effectiveness ratios were $16,362 per quality-adjusted life year for sDNA analysis with diagnostic chromoendoscopy; $18,643 per quality-adjusted life year for sDNA analysis with diagnostic WLE; $23,830 per quality-adjusted life year for chromoendoscopy alone; and $27,907 per quality-adjusted life year for WLE alone. In sensitivity analyses, sDNA analysis with diagnostic chromoendoscopy was more cost effective than chromoendoscopy alone, up to a cost of $1135 per sDNA test. sDNA analysis remained cost effective at all rates of compliance; when combined with diagnostic chromoendoscopy, this approach was preferred over chromoendoscopy alone, when the specificity of the sDNA test for CRN was above 65%.
Conclusion
Based on a Markov model, surveillance for CRN is cost effective for patients with chronic ulcerative colitis. Analysis of sDNA with chromoendoscopies for patients with positive results was more cost effective than chromoendoscopy or WLE alone.
Keywords: ICER, QALY, cost benefit analysis, inflammatory bowel diseases
Introduction
Population-level data show an increased incidence of colorectal cancer (CRC) in inflammatory bowel disease (IBD), specifically among patients with extensive, long-standing ulcerative colitis (UC)1 and Crohn's disease (CD) of the colon.2 Recent data show that CRC incidence and mortality in patients with UC is reduced with surveillance colonoscopy by white light endoscopy (WLE).3 Even prior to this evidence, surveillance colonoscopy became standard of care, based on several smaller scale case-control and cohort studies, which in aggregate, suggested a benefit from earlier-stage CRC diagnosis.4 Over the last decade, a growing body of literature suggests that dye-spray-enhanced surveillance with chromoendoscopy may be superior for the detection of dysplastic precursors to CRC. While multiple specialty society guidelines endorse surveillance colonoscopy with WLE,5 the recent SCENIC consensus statement, jointly issued by the American Gastroenterology Association and the American Society for Gastrointestinal Endoscopy, now recommends chromoendoscopy over standard definition WLE for CRC surveillance in UC patients.6
Since its introduction over a decade ago7 uptake of chromoendoscopy has been slow, due to the requirement for additional training, perceived increased procedure time, and higher costs.8 A recent study showed that chromoendoscopy might be more cost-effective than WLE but both modalities appeared to be above societal willingness to pay thresholds when used at currently recommended intervals.9 Complicating matters, patient compliance with CRC surveillance is unacceptably low, even among commercially-insured UC patients.10 Non-invasive testing by stool DNA (sDNA) is a new potential strategy, hypothesized to reduce costs and improve surveillance adherence.11-13 Multi-target sDNA has recently been approved by the United States Food and Drug Administration for average-risk CRC screening following a pivotal study in which sensitivity and specificity of sDNA for CRC were similar to gold-standard WLE.14
The approved multi-target sDNA test, commercialized as Cologuard™ (Exact Sciences, Madison WI), assays mutant KRAS, methylated BMP3, methylated NDRG4 and a fecal immunochemical test (FIT). FIT cannot logically be applied in CD and UC as test specificity may be compromised by hemorrhagic inflammatory activity. However, recent case-control data show that stool assay of aberrant DNA methylation markers, achieved >90% sensitivity at 90% specificity for CRC in patients with IBD.15, 16
Several important questions remain that must be addressed before sDNA can be used for CRC surveillance in patients with UC and CD. What sensitivity and specificity thresholds should be required of an sDNA test in this setting? How frequently should the test be applied? Will sDNA be cost-effective, and if so, at what price? We approached these knowledge gaps by modeling the cost-effectiveness of sDNA in comparison to WLE, chromoendoscopy or no surveillance in a hypothetical cohort of UC patients. Further, we sought to inform test design by 1-way sensitivity analyses of test performance, interval and cost. Lastly, probabilistic sensitivity analyses were performed to assess the reliability of model outputs.
Methods
This study was exempt from Institutional Review Board review.
Model Design
We adapted a previously published Markov model with Monte Carlo simulations9 and imputed a clinical course of UC, the incidence of colorectal neoplasia (CRN, cancer + dysplasia), and costs & benefits of care with patients committed to one of four surveillance strategies: sDNA with diagnostic chromoendoscopy for sDNA test positives, sDNA with diagnostic WLE for sDNA positives, chromoendoscopy with targeted biopsies only, or WLE with random biopsies. The model was originally created with TreeAge Pro 2009 (TreeAge, Williamstown, MA) and updated with TreeAge 2015. The Markov cycle length was 1 year. The simulation sampled hypothetical patients, 8 years after population-based age distribution of UC diagnosis, the recommended duration to begin surveillance for dysplasia.17 Termination criteria were death or a patient age greater than 90 years with 10 years of surveillance. Pancolitis and left-sided disease were treated equally according to current guidelines for surveillance.5 Health states included chronic UC, chronic UC with dysplasia, status post-colectomy, CRC, and death due to either baseline mortality or mortality related to colonoscopy, surgery, or CRC (Figure 1). Base case values and ranges for transition probabilities and health state utilities (Supplemental Table 1) were obtained from a systematic literature search as previously described.9 Several key adaptations were included to address the specific aims of this study (Table 1).
Figure 1.
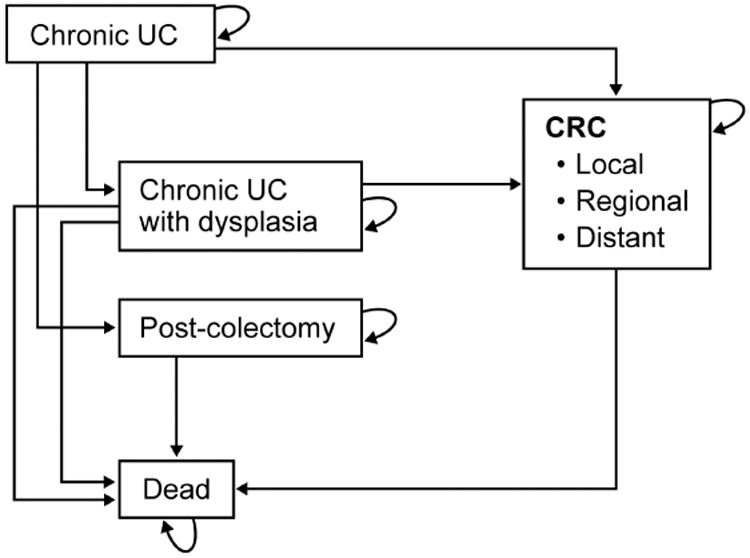
Model health state transition diagram from chronic ulcerative colitis (UC) to dysplasia, colorectal cancer (CRC), surgery or death
Adapted from Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014 Mar;79(3):455-65 and used with permission.
Table 1. Transition probabilities and healthcare costs.
| Variable | Base case | Standard Deviation (SD)* | Monte Carlo distribution type* | 2.5th/97.5th Percentile* | References |
|---|---|---|---|---|---|
| Stool DNA | |||||
| Sensitivity LGD | 0.41 | SD 0.018 | Beta | 0.38 / 0.45 | 14 |
| Sensitivity HGD | 0.69 | SD 0.072 | Beta | 0.56 / 0.85 | 14 |
| Sensitivity CRC | 0.92 | SD 0.35 | Beta | 0.84 / 0.98 | 14 |
| Specificity | 0.87 | SD 0.0036 | Beta | 0.86 / 0.87 | 14 |
| Ulcerative colitis | |||||
| % Adhering to surveillance | 0.5 | SD 0.11 | Beta | 0.28 / 0.71 | 10 |
| Age of UC diagnosis | 17 | ||||
| 10 years | 0.075 | - | - | - | 17 |
| 30 years | 0.354 | - | - | - | 17 |
| 50 years | 0.279 | - | - | - | 17 |
| 70 years | 0.238 | - | - | - | 17 |
| 90 years | 0.054 | - | - | - | 17 |
| Annual rate of CRC incidence by UC duration | |||||
| At 8th year | 0.0107 | - | Beta | 0.00045 – 0.044 | 18 |
| 9-15 years | 0.00135 | - | Beta | 0.000032 - 0.0046 | 18 |
| 16-20 years | 0.00205 | - | Beta | 0.000044 – 0.0089 | 18 |
| ≥21years | 0.00207 | Beta | 0.000067 – 0.0075 | 18 | |
| Probability CRC stage at diagnosis | |||||
| Surveillance | |||||
| Dukes A/B | 0.86 | 0.13 | Beta | 0.50 / 1.00 | 19 |
| Dukes C | 0.10 | 0.012 | Beta | 0.08 / 0.13 | 19 |
| Dukes D | 0.04 | 0.0065 | Beta | 0.03 / 0.06 | 19 |
| No Surveillance | |||||
| Dukes A/B | 0.47 | 0.071 | Beta | 0.33 / 0.60 | 19, 31 |
| Dukes C | 0.38 | 0.044 | Beta | 0.29 / 0.49 | 19, 31 |
| Dukes D | 0.15 | 0.024 | Beta | 0.11 / 0.20 | 19, 31 |
| Costs | |||||
| Annual care UC | $8,485 | $1,061 | Gamma | $6,326 / $11,048 | 20 |
| Stool DNA test | $599 | $75 | Gamma | $460 / $744 | CPT G0464 |
| CE with biopsies | $1,221 | $153 | Gamma | $932 / $1,589 | CPT 88305 CPT 45380 |
| WLE with biopsies | $1,405 | $177 | Gamma | $978 / $1714 | CPT 88305 CPT 45380 |
| Adverse event from colonoscopy | $7,075 | $2,653 | Gamma | $2,636 / $15,386 | 27 |
| Colectomy cost + 6 month postoperative care | $62,453 | $35,858 | Gamma | $17,004 / $153,379 | 32, 33 |
| Routine care with IPAA (annual) | $6,058 | $758 | Gamma | $4,540 / $7,238 | 20 |
| Cancer related expenses, initial | |||||
| Local | $48,945 | $6,115 | Gamma | $37,994 / $64,953 | 21, 22 |
| Regional | $64,746 | $8,091 | Gamma | $52,209 / $85,461 | 21, 22 |
| Distant | $64,384 | $8,046 | Gamma | $49,446 / $82,898 | 21, 22 |
| Cancer related expenses, continuing | |||||
| Local | $3,796 | $475 | Gamma | $3,126 / $4,799 | 21, 22 |
| Regional | $7,887 | $986 | Gamma | $6,078 / $9,916 | 21, 22 |
| Distant | $26,570 | $3,321 | Gamma | $19,531 / $33,248 | 21, 22 |
| Cancer related expenses, terminal | |||||
| Local | $20,453 | $2,556 | Gamma | $16,126 / $25,261 | 21, 22 |
| Regional | $36,509 | $4,562 | Gamma | $27,348 / $47,145 | 21, 22 |
| Distant | $49,792 | $6,223 | Gamma | $39,566 / $62,789 | 21, 22 |
Apply to probabilistic sensitivity analysis only
CE, chromoendoscopy; CRC, colorectal cancer; HGD, high-grade dysplasia; IPAA, ileal pouch anal anastomosis; LGD, low-grade dysplasia; SD, standard deviation; UC, ulcerative colitis; WLE, white light endoscopy
Please see Supplemental Table 1 for additional model inputs and distributions
Risk of colorectal cancer and benefit of surveillance
Patients in the simulation cohort entered the model at an age consistent with population-level measurements of natural history.17 The probability of developing CRC, given no prior dysplasia, was modeled on population-level estimates; patients entered the simulation at 8 years after diagnosis with a 1.07% incidence of CRC to match cumulative incidence estimates of 2% at 15 years, 3% at 20 years and 4% at 25 years.18 Surveillance colonoscopy has been recently shown to reduce colorectal cancer incidence and all-cause mortality.3 To reflect this benefit, the model was designed to confer improved survival from CRC if detected by surveillance in accordance with previously published estimates.19
Stool DNA test performance in ulcerative colitis
Base-case sDNA performance estimates were derived from average-risk sDNA test performance,14 which are more conservative than those available from the limited literature in IBD patient populations.15, 16 Also, each surveillance strategy assumed base case compliance of 0.5, consistent with US surveillance adherence estimates.10
Costs and utilities
Care and procedure expenditures were based on 2014 Medicare reimbursement for specific procedures (Table 1) or updated to 2014 United States dollars from literature estimates (Supplemental Table 1) 20-22 using published medical Consumer Price Index inflation factors. Because sDNA cost for use in UC has not been determined, and because Medicare has not issued a national coverage determination for sDNA testing in IBD, the base cost was set at $599, the current price billed to commercial insurance for the Cologuard multi-target sDNA test for average-risk CRC screening (Exact Sciences, Madison WI). All costs were estimated from the payer perspective and did not include indirect patient expenses (e.g., travel, lost work time).
Transient utilities included UC flare requiring colectomy, immediate postoperative state, and adverse events from either surgery or colonoscopy; these were modeled with 1 month duration. In patients simulated to have those events, yearly utilities were adjusted by a weighted average of the transient utility and the underlying utility attributed to chronic UC, post-IPAA state or cancer (Dukes stage A/B vs C vs D).
All costs and utilities were discounted at a rate of 3% per year.
Analyses
Base-case analysis was conducted by Markov modeling with Monte Carlo microsimulation which sampled all costs, utilities and probabilities to create 500,000 hypothetical patients and disease courses. The primary outcome was the incremental cost effectiveness ratio (ICER, incremental cost/incremental difference in quality adjusted life years [QALY]) for each surveillance strategy at two year intervals compared to no surveillance. A willingness to pay (WTP) threshold of $50,000 per QALY was used to estimate cost-effectiveness.23
One-way sensitivity analyses evaluated the impact of surveillance compliance, sDNA test cost, and sDNA sensitivity & specificity. A second set of sensitivity analyses studied cost thresholds for a 1-year sDNA surveillance program, compared to 2-year intervals for chromoendoscopy and WLE.
To estimate the degree of uncertainty around the model assumptions, probabilistic sensitivity analysis (PSA) was then performed by simulating 100 separate cohorts of 100,000 patients. Cost, utility and probability variable inputs were sampled by the simulation software according to the distribution type and margin of error around the base-case point estimates (Table 1). Model uncertainty was also examined using cost-effectiveness acceptability curves which utilize a net monetary benefit calculation (NMB) that combines cost, effectiveness and WTP into a single measurement (NMB = effectiveness × WTP − cost). The strategy with the highest NMB is the most cost-effective given the fixed WTP parameter. The sampling variation in the PSA was used to estimate the probability that a surveillance strategy is cost-effective for a given WTP (i.e. the decision maker's threshold ICER). Where the probabilistic sensitivity analysis disagreed with the results of the base case analysis, additional acceptability curves were generated to identify input variables which might potentially disrupt to the expected outcome.
Results
Base Case Analysis
Relative to no surveillance, the primary cost-effectiveness analysis demonstrated that ICERs for annual, biennial and every 3 year sDNA, chromoendoscopy and WLE fell below the WTP threshold of $50,000 (Figure 2). While all options were cost-effective, the ICERs for sDNA-based surveillance were lower than and not dominated by endoscopic-based options. Consistent with prior results,9 chromoendoscopy modalities were more cost-effective that WLE.
Figure 2.
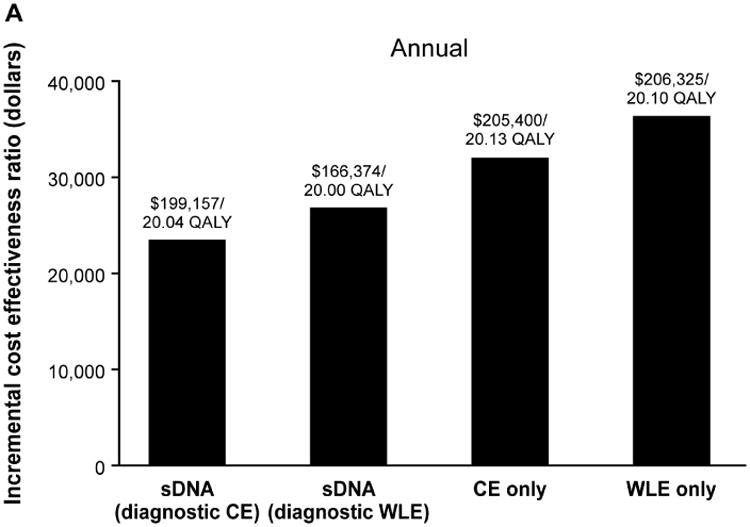
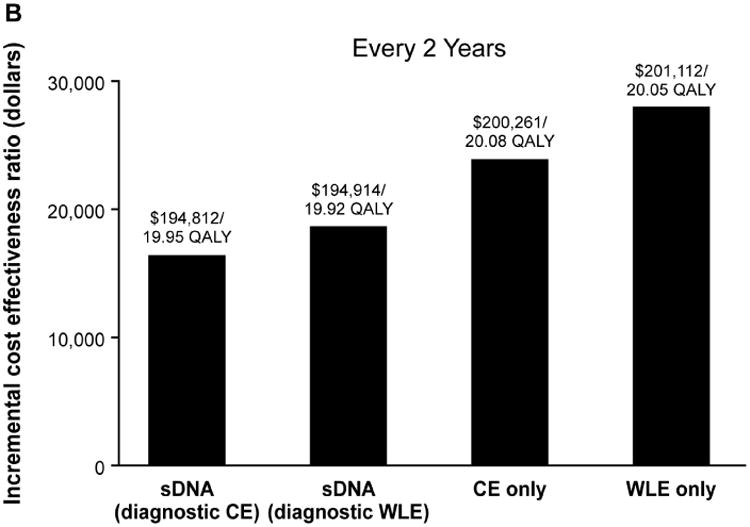
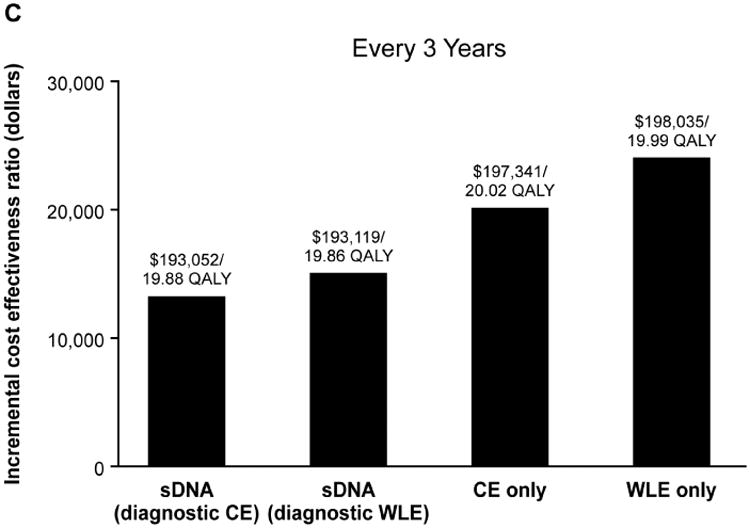
Incremental cost-effectiveness ratios for each surveillance strategy at (A) annual, (B) biennial and (C) every 3-years surveillance intervals in comparison to no surveillance, which cost $189,960 for 19.65 quality-adjusted life years (QALY). CE, chromoendoscopy; sDNA, stool DNA; WLE, white light endoscopy
In the no surveillance strategy on 500,000 hypothetical patients, the model generated 95,987 CRC cases, resulting in 81,502 CRC-related deaths over the full duration of the simulation. Biennial WLE reduced CRC cases by 53,800 and associated fatalities by 42,274. To prevent one additional CRC case, 9 patients would have to participate in programmatic WLE surveillance. Biennial chromoendoscopy prevented 52,539 cases and 42,104 hypothetical deaths with 10 surveillance program participants needed to prevent one additional CRC. Though less costly, sDNA with diagnostic chromoendoscopy, and sDNA with diagnostic WLE, prevented 17,681 and 16,230 hypothetical CRC fatalities, respectively. To prevent one additional CRC case, 18 and 19 patients would have to participate in programmatic surveillance with sDNA with diagnostic chromoendoscopy or diagnostic WLE, respectively. Assuming 50% base-case compliance with colonoscopy-based surveillance, and that non-compliant patients used sDNA, 58,504 to 59,785 CRC fatalities would be prevented. For this combination approach, only 5-8 patients would need to participate in programmatic surveillance with to prevent 1 additional CRC fatality.
Sensitivity Analyses on Individual Variables
All biennial surveillance modalities became more expensive as patient compliance increased, but at all levels of compliance at sDNA remained cost-effective. Unless the specificity of sDNA fell below 0.65, sDNA with diagnostic chromoendoscopy was preferred over chromoendoscopy alone. Diagnostic sensitivity of sDNA did not impact cost-effectiveness across a wide range of performance (0.25-0.99) for any target lesion, which included low-grade dysplasia (LGD), high-grade dysplasia (HGD) or CRC. Test cost for sDNA was prohibitive above $1,135 at which point chromoendoscopy became more cost-effective (Table 2). Similarly, in comparison of sDNA with diagnostic WLE against WLE alone, sDNA remained preferred unless the specificity of sDNA fell below 0.66 or cost of sDNA exceeded $1,109.
Table 2. One way sensitivity analyses assuming biennial testing intervals.
| Comparison strategy | Variable | Base case value | N simulated individual patients | Range of sensitivity analysis | Threshold value |
|---|---|---|---|---|---|
| Chromoendoscopy | Percent adhering to sDNA Screening | 0.50 | 500,000 | 25% to 100% | n/a |
| Cost of sDNA Test | $599 | 100,000 | $600 to $1500 | $1,135 | |
| Specificity of sDNA | 0.866 | 100,000 | 0.25 - 0.99 | 0.65 | |
| Sensitivity sDNA to CRC | 0.923 | 100,000 | 0.25 - 0.99 | n/a | |
| Sensitivity sDNA to HGD | 0.692 | 100,000 | 0.25 - 0.99 | n/a | |
| Sensitivity of sDNA to LGD | 0.409 | 100,000 | 0.25 - 0.99 | n/a | |
| White light endoscopy | Percent adhering to sDNA Screening | 0.50 | 500,000 | 25% to 100% | n/a |
| Cost of sDNA Test | $599 | 100,000 | $600 to $1800 | $1,109 | |
| Specificity of sDNA | 0.866 | 100,000 | 0.25 - 0.99 | 0.66 | |
| Sensitivity sDNA to CRC | 0.923 | 100,000 | 0.25 - 0.99 | n/a | |
| Sensitivity sDNA to HGD | 0.692 | 100,000 | 0.25 - 0.99 | n/a | |
| Sensitivity of sDNA to LGD | 0.409 | 100,000 | 0.25 - 0.99 | n/a |
CRC, colorectal cancer; HGD, high-grade dysplasia; LGD, low-grade dysplasia; sDNA, stool DNA
If sDNA testing were to be performed annually, the threshold cost for sDNA would decrease to $601 and $675 in order to remain cost-effective when compared to biennial interval chromoendoscopy or WLE, respectively.
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analyses supported the findings of the base-case analysis (Figure 3, Supplemental Figure 1). Compared to no surveillance, sDNA with diagnostic chromoendoscopy was below the WTP threshold of $50,000 per QALY or dominant in 71 of 100 hypothetical patient cohorts. Stool DNA with diagnostic WLE or chromoendoscopy alone were below the WTP of $50,000 per QALY or dominant in 66 of the cohorts; WLE alone was below the WTP threshold or dominant 55% of the time.
Figure 3.
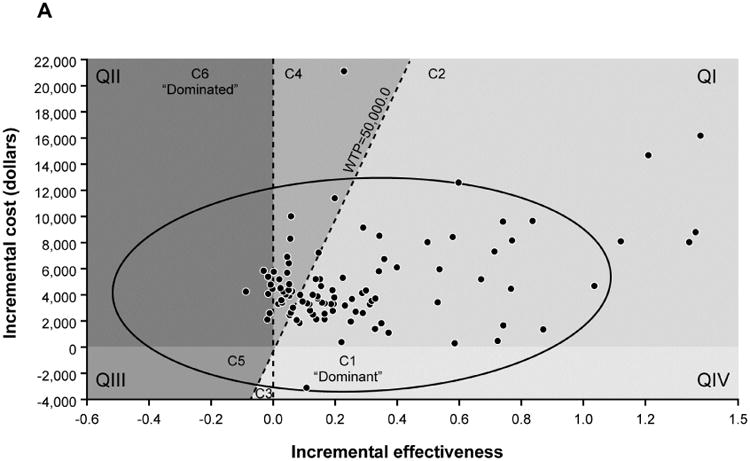
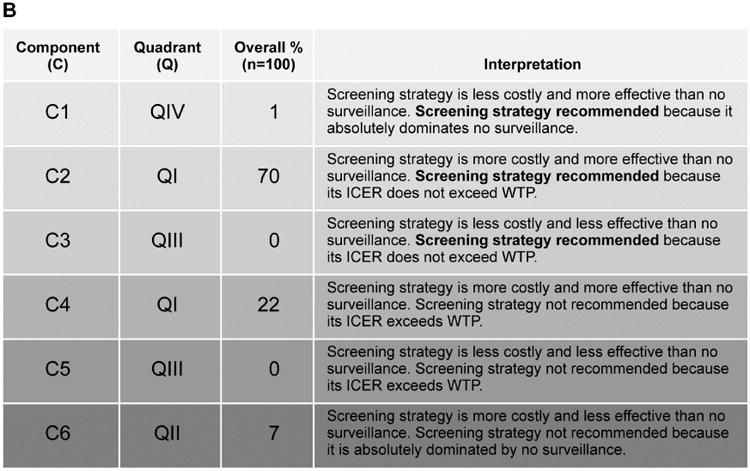
(A) Incremental cost-effectiveness ratio (ICER) scatter plot and 95% confidence interval ellipse in relationship to $50,000 willingness to pay threshold (WTP) for stool DNA with diagnostic chromoendoscopy in reference to no surveillance. Quadrants (QI-IV) are defined by cost and effectiveness axes and components (C1-6) define where stool DNA is recommended or not in relationship to WTP. (B) Interpretation key shows that stool DNA with diagnostic chromoendoscopy was the recommended surveillance strategy in the majority (71%) of cohorts in which the full range of model parameters was sampled.
Base case analyses anticipated that sDNA-based options would show maximal NMB if payers were willing to pay $18,000-$45,000 per QALY (Supplemental Table 2); however, acceptability curves for the full set of input variables provided contradictory results to the base case. To determine why this PSA was discordant with base case expectations, the PSA repeated with stratified sampling by each of the combined utility, cost and probability variables to identify those that might be disruptive when sampled across the entire Monte Carlo distribution. With this approach, the output of all utility and cost variables remained consistent with base-case expectations, while results based on probability variables appeared contradictory (Figure 4, A-C). Sampling by individual probability variables generated curves which were each consistent with base case, therefore interaction among probability variables was suspected. To further investigate this, all probability inputs were sampled in clusters of 2-4 variables at a time in combination with the CRC incidence variable; however, each of these acceptability curves matched base-case expectations (Figure 4 D, Supplemental Figure 2).
Figure 4.
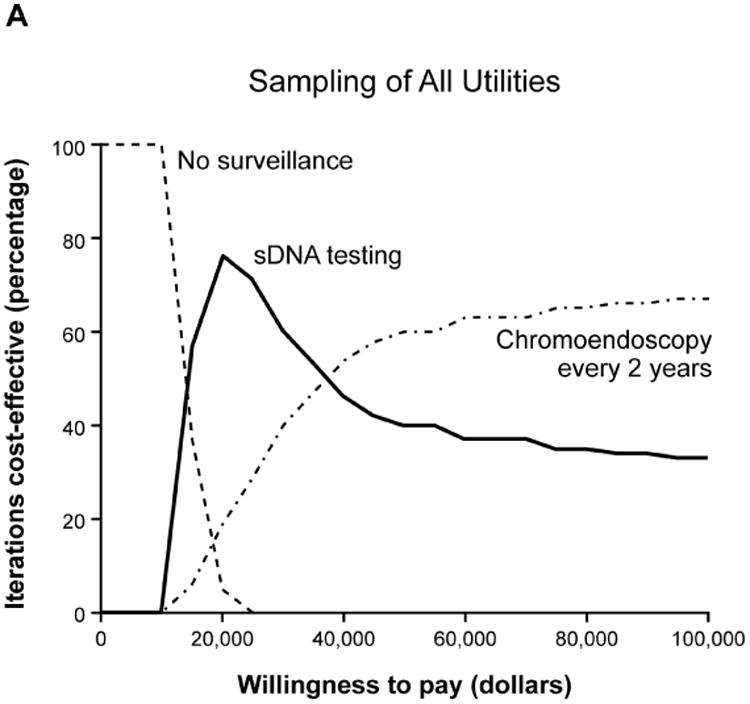
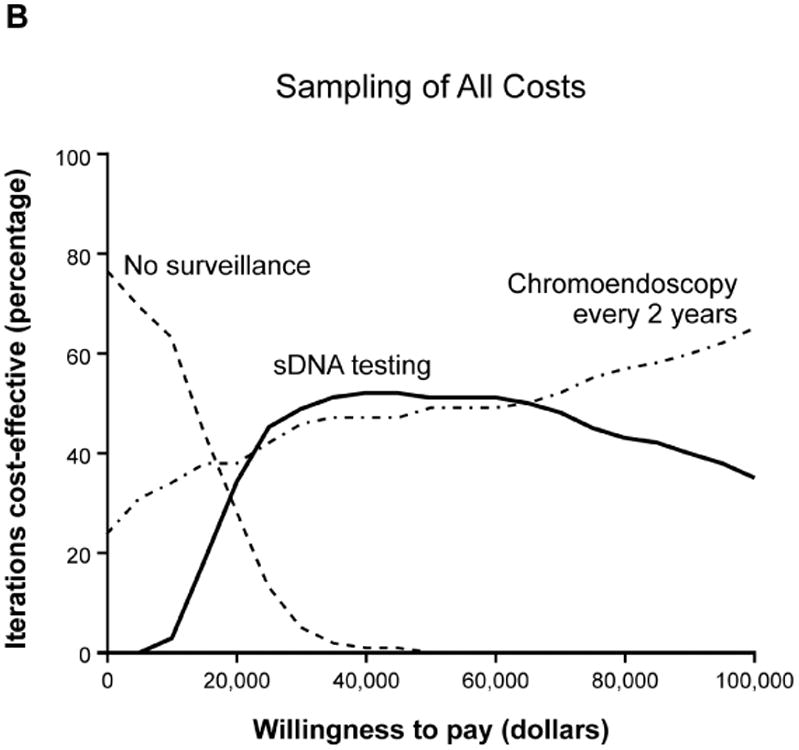
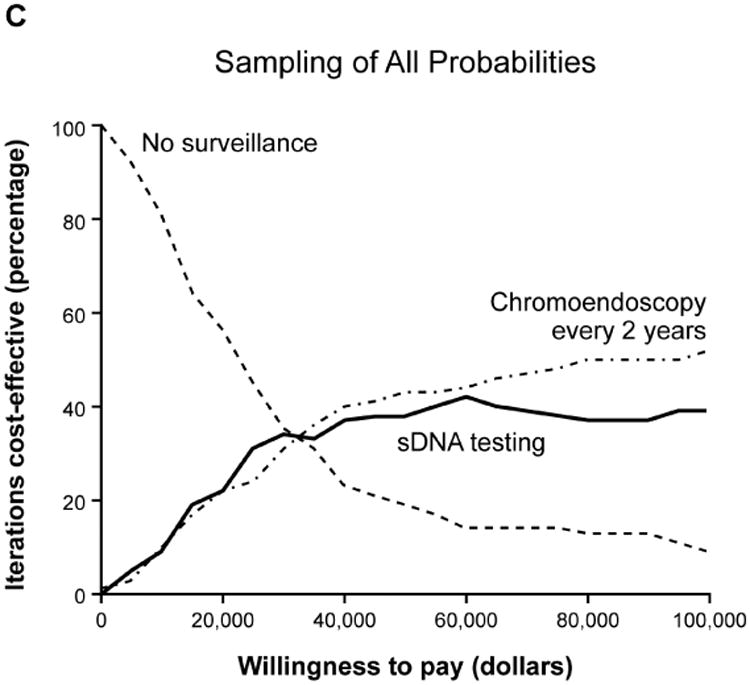
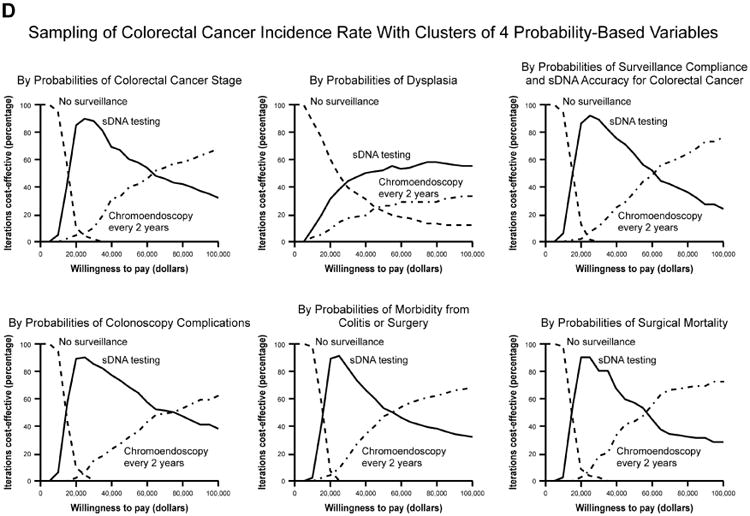
Acceptability curves for the each of the major input variable categories (A) utilities, (B) costs and (C) probabilities. Probabilities were further examined in smaller clusters of variables at a time (D) which all met base-case expectations.
Discussion
In this analysis of CRC surveillance strategies, sDNA appears to be more cost-effective when used with either diagnostic chromoendoscopy or diagnostic WLE than chromoendoscopy alone or WLE alone. Though chromoendoscopy has recently been endorsed by the SCENIC consensus statement,6 this modality is not yet widely utilized, leaving many patients and providers to rely on WLE. Endoscopy-based surveillance could become more cost-effective if sDNA specificity falls below 0.65 in comparison to chromoendoscopy surveillance alone or below 0.66 in comparison to WLE. These specificity thresholds are well below currently published estimates of sDNA performance in either IBD patients15, 16 or in the average risk population.14 The relative cost-effectiveness of sDNA was maintained across a wide spectrum of patient compliance, test sensitivity, and test costs. Even annual sDNA testing was cost-effective in comparison to either annual or biennial endoscopy-based options.
Regardless of test strategy, surveillance for dysplasia and CRC in UC patients appears cost-effective. These findings shed new light on a long-standing clinical uncertainty as previous economic analyses of endoscopic surveillance for CRC in UC patients have yielded mixed results. Provanzale, and colleagues performed a cost-effectiveness analysis of WLE colonoscopy in UC at 1-5 year intervals in comparison to no surveillance.24 While surveillance at intervals shorter than 5 years appeared to exceed WTP, the ICERs for all intervals were comparable to other commonly performed screening tests, such as cervical cancer screening.24 Subsequent threshold analyses and critical reviews on surveillance cost-effectiveness have highlighted that test cost, CRC incidence, mortality reduction from surveillance and quality of life after proctocolectomy are critical factors, whereas diagnostic accuracy of colonoscopy did not greatly impact results.9, 25-27 Consistent with these model outputs, our present analysis did not reveal significant threshold effects when varying the sensitivity of sDNA, though thresholds for specificity and cost were observed.
The present results also update a recent economic analysis of chromoendoscopy and WLE strategies which found dominance by chromoendoscopy but at costs exceeding a WTP of $100,000.9 The substantial relative QALY gains we observed were most likely due to the incorporation of recent data showing that WLE lowers CRC incidence and improves mortality.3 Surveillance of UC patients with WLE has only been shown to reduce CRC incidence in one observational study; two others showed earlier stage CRC diagnosis with corresponding mortality benefit.19, 28 There has been further concern that chromoendoscopy may overestimate risk of subsequent advanced neoplasia in patients with LGD by finding more lesions with uncertain natural history.13, 29 A recent update of the St. Mark's Hospital surveillance cohort in the United Kingdom showed that the rate of progression from LGD to CRC was similar across each of 4 decades suggesting that the more recent introduction of chromoendoscopy has not changed the apparent risk attributable to LGD.28 The present model also relied on encouraging but preliminary available data on sDNA performance in IBD.15, 16 Because small sample size and single-center experience should prompt a cautious approach, we opted to use more conservative sDNA performance inputs from a large clinical trial of average-risk, asymptomatic patients in the general population,14 and utilized 1-way and probabilistic sensitivity analyses to evaluate sDNA cost-effectiveness across wide hypothesized ranges of test performance. Results from a large multi-center case-control study on sDNA performance in IBD patients are eagerly anticipated (ClinicalTrials.gov identifier NCT01819766).
There are several potential limitations to this study. The acceptability curve generated from the full set of variables appeared contrary to the base-case results. Stratifying by inputs of utilities, costs and probabilities appeared to isolate probability variables; however, despite sampling probability variables individually and in groups of 2-4 at a time, we were unable to identify any potentially disruptive inputs. Therefore, we remain confident in the base-case findings. There is also the potential to under estimate costs. Inputs for costs of endoscopic procedures, complications, surgery and cancer care were modeled from the Medicare perspective, and may be lower than reimbursement rates from commercial insurance carriers. Also, the model did not include any indirect costs. While there is precedent for their exclusion due the difficulty of accurate measurement,24, 25, 27 this may under-estimate the total burden to society.
We are encouraged that surveillance for CRC in UC patients appears cost-effective, as the ICER estimates of all options fell below a WTP of $50,000. Based on our modeling, sDNA was most cost-effective relative to no surveillance. The user-friendly features of sDNA may improve patient compliance,30 and its addition as an option in the surveillance arsenal may enhance overall effectiveness.
Supplementary Material
Supplemental Figure 1: A. Probabilistic Sensitivity Analysis Summary: Biennial Stool DNA Testing with Diagnostic White Light Colonoscopy
Supplemental Figure 1: B. Probabilistic Sensitivity Analysis Summary: Biennial Chromoendoscopy
Supplemental Figure 1: C. Probabilistic Sensitivity Analysis Summary: Biennial White Light Colonoscopy
Supplemental Figure 2: Sampling of CRC incidence rate with additional clusters of two or four probability-based variables
Supplemental Table 1: Additional transition probabilities, utilities and model variables
Acknowledgments
This was made possible by grants (to J.B.K.) from the Jack and Maxine Zarrow Family Foundation of Tulsa Oklahoma and the Paul Calabresi Program in Clinical-Translational Research (NCI CA90628). Additional support was provided by a National Institutes of Health KL2 grant (to G.G.K) (NCATS TR001112). Additional partial support was provided by the Carol M. Gatton endowment for Digestive Diseases Research. Additional funding was provided by Exact Sciences (Madison WI).
Abbreviations
- CRC
Colorectal cancer
- CRN
Colorectal neoplasia
- HGD
High-grade dysplasia
- ICER
Incremental cost-effectiveness ratio
- IPAA
Ileal pouch anal anastomosis
- LGD
Low-grade dysplasia
- QALY
Quality-adjusted life-year
- sDNA
Stool DNA
- WLE
White light endoscopy
- WTP
Willingness to pay threshold
Footnotes
Disclosures: Mayo Clinic has entered into an intellectual property agreement with Exact Sciences (Madison WI) whereby Drs. Kisiel and Ahlquist may receive royalties in accordance with Mayo Clinic policy. Drs. Konijeti, Chandra, Goss, Farraye & Ananthakrishnan and Mr. Piscitello have no relevant disclosures.
Presented in part at Digestive Disease Week, May 2015, Washington DC
Author Contributions: John B. Kisiel: study concept and design, acquisition of data, interpretation of data, drafting the manuscript, obtained funding, study supervision
Gauree G. Konijeti: study design, acquisition of data, interpretation of data, critical revision of the manuscript
Andrew J. Piscitello: analysis and interpretation of data, statistical analysis, critical revision of manuscript, technical support
Tarun Chandra: analysis and interpretation of data, statistical analysis, critical revision of manuscript, technical support
Thomas F. Goss: study design, interpretation of data, critical revision of manuscript
David A. Ahlquist: study design, interpretation of data, critical revision of manuscript
Francis A. Farraye: study design, interpretation of data, critical revision of manuscript
Ashwin N. Ananthakrishnan: study design, analysis and interpretation of data, critical revision of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin Gastroenterol Hepatol. 2012 Jun;10(6):639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN, Cagan A, Cai T, et al. Colonoscopy Is Associated With a Reduced Risk for Colon Cancer and Mortality in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2015 Feb;13(2):322–329. doi: 10.1016/j.cgh.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins PD, Mpofu C, Watson AJ, et al. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006:CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74. 774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501 e26. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Rutter MD, Saunders BP, Schofield G, et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–60. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullman TA. Should chromoendoscopy be the standard of care in ulcerative colitis dysplasia surveillance? Gastroenterol Hepatol. 2010;6:616–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Konijeti GG, Shrime MG, Ananthakrishnan AN, et al. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455–65. doi: 10.1016/j.gie.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velayos FS, Liu L, Lewis JD, et al. Prevalence of colorectal cancer surveillance for ulcerative colitis in an integrated health care delivery system. Gastroenterology. 2010;139:1511–8. doi: 10.1053/j.gastro.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Kwah J, Farraye FA. Current and Future Status for Evaluation of Dysplasia and Carcinoma in IBD. Curr Treat Options Gastroenterol. 2014;12:90–102. doi: 10.1007/s11938-013-0006-3. [DOI] [PubMed] [Google Scholar]
- 12.Rutter MD, Riddell RH. Colorectal dysplasia in inflammatory bowel disease: a clinicopathologic perspective. Clin Gastroenterol Hepatol. 2014;12:359–67. doi: 10.1016/j.cgh.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Marion JF, Sands BE. The SCENIC consensus statement on surveillance and management of dysplasia in inflammatory bowel disease: praise and words of caution. Gastroenterology. 2015;148:462–7. doi: 10.1053/j.gastro.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N Engl J Med. 2014;370:1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 15.Kisiel JB, Yab TC, Nazer Hussain FT, et al. Stool DNA testing for the detection of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:546–54. doi: 10.1111/apt.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisiel JB, Taylor WR, Allawi H, et al. Detection of Colorectal Cancer and Polyps in Patients With Inflammatory Bowel Disease by Novel Methylated Stool DNA Markers. Gastroenterology. 2014;146:S-440–S-441. [Google Scholar]
- 17.Jess T, Simonsen J, Jorgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–381. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Stewenius J, Adnerhill I, Anderson H, et al. Incidence of colorectal cancer and all cause mortality in non-selected patients with ulcerative colitis and indeterminate colitis in Malmo, Sweden. Int J Colorectal Dis. 1995;10:117–22. doi: 10.1007/BF00341210. [DOI] [PubMed] [Google Scholar]
- 19.Choi PM, Nugent FW, Schoetz DJ, Jr, et al. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–24. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 20.Gunnarsson C, Chen J, Rizzo JA, et al. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–91. doi: 10.1007/s10620-012-2289-y. [DOI] [PubMed] [Google Scholar]
- 21.Lang K, Lines LM, Lee DW, et al. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clin Gastroenterol Hepatol. 2009;7:198–204. doi: 10.1016/j.cgh.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 24.Provenzale D, Wong JB, Onken JE, et al. Performing a cost-effectiveness analysis: surveillance of patients with ulcerative colitis. Am J Gastroenterol. 1998;93:872–80. doi: 10.1111/j.1572-0241.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 25.Delco F, Sonnenberg A. A decision analysis of surveillance for colorectal cancer in ulcerative colitis. Gut. 2000;46:500–6. doi: 10.1136/gut.46.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inadomi JM. Cost-effectiveness of colorectal cancer surveillance in ulcerative colitis. Scand J Gastroenterol Suppl. 2003:17–21. doi: 10.1080/00855910310001430. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein JH, Waljee AK, Jeter JM, et al. Cost effectiveness of ulcerative colitis surveillance in the setting of 5-aminosalicylates. Am J Gastroenterol. 2009;104:2222–32. doi: 10.1038/ajg.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi CR, Rutter MD, Askari A, et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol. 2015;110:1022–1034. doi: 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins PD. Editorial: Miles to Go on the SCENIC Route: Should Chromoendoscopy Become the Standard of Care in IBD Surveillance? Am J Gastroenterol. 2015;110:1035–1037. doi: 10.1038/ajg.2015.179. [DOI] [PubMed] [Google Scholar]
- 30.Schroy PC, 3rd, Lal S, Glick JT, et al. Patient preferences for colorectal cancer screening: how does stool DNA testing fare? Am J Manag Care. 2007;13:393–400. [PubMed] [Google Scholar]
- 31.Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Holubar SD, Long KH, Loftus EV, Jr, et al. Long-term direct costs before and after proctocolectomy for ulcerative colitis: a population-based study in Olmsted County, Minnesota. Dis Colon Rectum. 2009;52:1815–23. doi: 10.1007/DCR.0b013e3181b327a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Silva S, Ma C, Proulx MC, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:972–80. doi: 10.1016/j.cgh.2011.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: A. Probabilistic Sensitivity Analysis Summary: Biennial Stool DNA Testing with Diagnostic White Light Colonoscopy
Supplemental Figure 1: B. Probabilistic Sensitivity Analysis Summary: Biennial Chromoendoscopy
Supplemental Figure 1: C. Probabilistic Sensitivity Analysis Summary: Biennial White Light Colonoscopy
Supplemental Figure 2: Sampling of CRC incidence rate with additional clusters of two or four probability-based variables
Supplemental Table 1: Additional transition probabilities, utilities and model variables


