Abstract
With increased access to high-speed Internet and smartphone devices, patients have started to use mobile applications (apps) for various health needs. These mobile apps are now increasingly used in integration with telemedicine and wearables to support fitness, health education, symptom tracking, and collaborative disease management and care coordination. More recently, evidence (especially around remote patient monitoring) has started to build in some chronic diseases, and some of the digital health technologies have received approval from the Food and Drug Administration. With the changing healthcare landscape and push for value-based care, adoption of these digital health initiatives among providers is bound to increase. Although so far there is a dearth of published evidence about effectiveness of these apps in gastroenterology care, there are ongoing trials to determine whether remote patient monitoring can lead to improvement in process metrics or outcome metrics for patients with chronic gastrointestinal diseases.
Keywords: Mobile Applications, Apps, Chronic Disease Management, Telemedicine, Wearables, Gastroenterology, Inflammatory Bowel Diseases
Despite annual expenditures exceeding $2.5 trillion (17% of gross domestic product), the quality of U.S. healthcare remains far from optimal.1 Soaring healthcare costs, significant variations in management, poor quality outcomes, and increasing fragmentation of care have become the major drivers of healthcare reforms, including pay-for-performance, meaningful use, and the Affordable Care Act of 2010.2 The Department of Health and Human Services recently declared that up to 50% of provider payments would be tied to healthcare quality (“performance”) by 2018.3 One of the major drivers of costs and push toward value-based care is the increasing prevalence of chronic diseases that affect almost 1 out of every 2 Americans and account for 75% of the U.S. healthcare burden.4,5 These changes affect the management of chronic digestive disorders.6–11
Digital health is the convergence of the digital and genetics revolutions with health, healthcare, living, and society, and the term is often interchangeably used with mHealth or mobile health because of the central role played by mobile devices.12 Digital health can be an effective tool in chronic disease management because of its widespread reach and efficiency it can bring to healthcare delivery. Remote patient monitoring (RPM) is a subset of digital health technologies that enable monitoring of patients outside of conventional clinical settings (eg, in the home), which may increase access to care and decrease healthcare delivery costs.13 The essential elements of RPM include smartphone and mobile apps, hardware sensors (including implantable, wearable, and Internet of Things) and software sensing technologies, health information technology including electronic health records, and genomics.
We will provide an overview of RPM strategies comprising mobile apps, telemedicine, and remote sensors and assess the potential opportunities of bringing these innovations for improving outcomes and experience of patients with chronic digestive disorders.
Remote Patient Monitoring
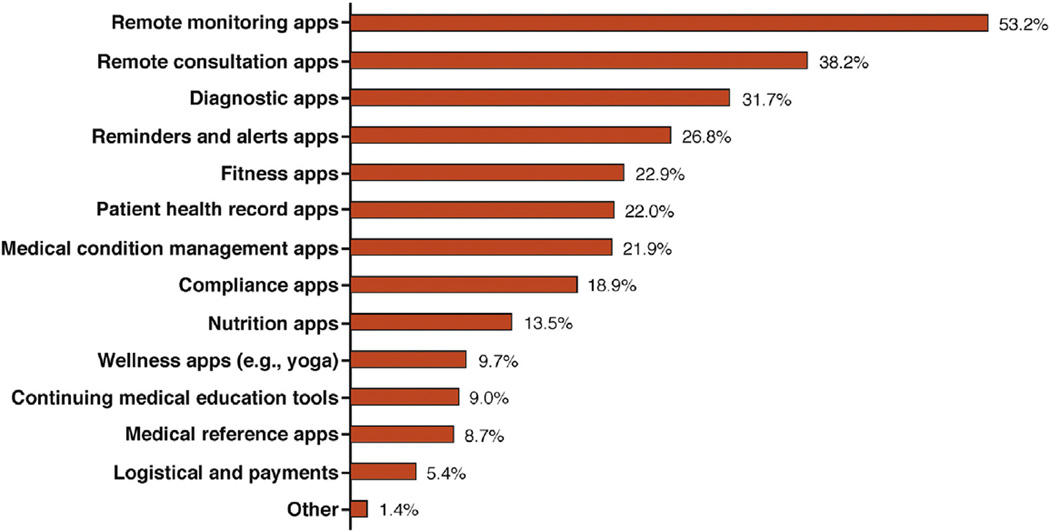
RPM can be helpful by managing patients while they remain in their homes, thus reducing unnecessary and even routine healthcare visits and their cost. It can provide patients with continuous self-monitoring of their health status, personalized healthcare, improved adherence, better communication to health provider, self-management of their diseases, and better quality of life with both time and cost effectiveness.14,15 As shown in Figure 1, remote monitoring and remote consultation mHealth app category hold the potential for revenue growth in the next 5 years.
Figure 1.
mhealth app category business potential in next 5 years showing remote monitoring apps have the biggest market potential of all mHealth app categories. With permission from Search2-guidance, mHealth app developer Economics 2014.
The uses of RPM are getting support from gradually building scientific evidence. In Tobacco, Exercise and Diet Messages (TEXT ME) trial published recently in JAMA, Chow et al16 reported the impact of personalized texting and feedback for risk factor modification in patients with coronary heart disease. At 6 months, levels of low-density lipoprotein–cholesterol complex were significantly lower in intervention participants (mean difference, −5 mg/dL; P = .04), with significant reduction in smoking (26% vs 44%; P < .001) as well as reductions in systolic blood pressure (−7.6 mm Hg) and body mass index (−1.3). Similar to Tobacco, Exercise and Diet Messages program, BlueStar from WellDoc Communications Inc (Baltimore, MD) offers automated semi-personalized guidance messages but also combines them with medication and blood sugar logs, health coach support, and report-sending capabilities via smartphones to provide an integrated remote monitoring program for diabetes.17 Patients using BlueStar saw a greater mean hemoglobin A1c decline than those receiving usual care, 1.2% (1.9% vs 0.7%) during a 12-month period, and this app was the first mobile app to gain approval from the Food and Drug Administration for better diabetes management.17
Several systematic reviews show evidence in support of RPM. When mortality was measured as a primary outcome for 3337 chronic heart failure patients in a meta-analysis of 13 studies (2003–2013), RPM was found to significantly reduce chronic heart failure mortality (risk ratio, 0.76%) as compared with conventional care.18 McLean et al19 performed a meta-analysis to evaluate effectiveness of telemedicine for chronic obstructive pulmonary disease patients and included 10 randomized controlled trials measuring quality of life, risk of hospitalization, and death as primary outcomes. A significant reduction in emergency department visits (odds ratio, 0.27; 95% confidence interval, 0.11–0.66) and hospitalization (odds ratio, 0.46; 95% confidence interval, 0.33–0.65) was reported with telehealthcare.
RPM also has potential for distant monitoring of patients with asthma to check their adherence to inhalers,20 dementia to prevent them getting lost,21 stroke to anticipate and assess fall,22 obstructive sleep apnea for diagnosis and treatment response,23 parkinsonism for independent daily living,24 bipolar disorders to anticipate mania and depression,25 postsurgical monitoring of artificial kidney transplants,26 and sensing pressure readings after aneurysm repair to predict leakage.27 RPM has improved intensive care unit (ICU) care with real-time monitoring that is easily dispersible to distant ICU physicians to provide in time interventions.28 RPM is now evolving toward providing patients with complete ambient monitoring resulting in independent living while minimizing the health-related risks of living alone at the same time, especially for the elderly and for those with chronic conditions.29,30
Smartphones and Mobile Apps
Currently, more than two-thirds of Americans have smartphones.31 Americans are also increasingly technology-laden, with an average of 4 devices per person; one-third of them have an Internet tablet versus just 5% two years ago.31 Since 1985, many low-income patients qualify for the LifeLine assistance program (http://www.fcc.gov/lifeline) that provides free smartphones and free monthly airtime. Most patients at all socioeconomic levels have smartphone or Internet access.32 Rising trends of smartphone usage are being observed for healthcare providers as well.33 One study found that almost all of those physicians who are in the Accreditation Council for Graduate Medical Education training program are currently using smartphones.34
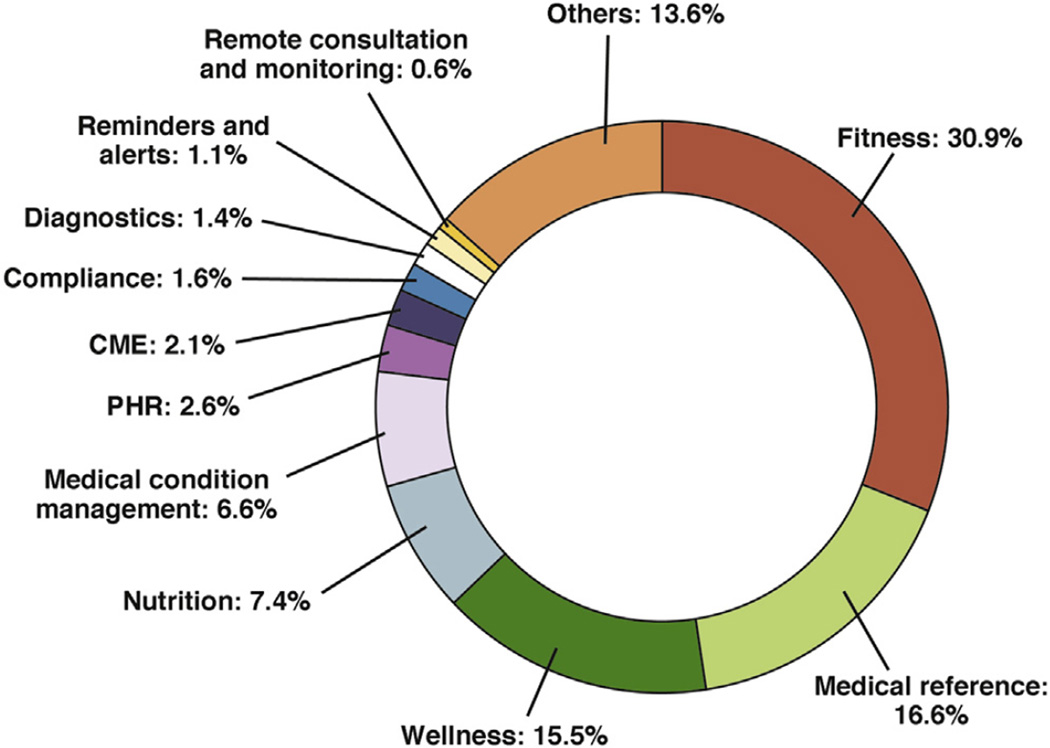
With increased use of smartphones, Internet tablets, and access to high-speed data networks, more patients are able to use mobile applications (apps) than ever before. Thus, smartphones with health apps provide a good strategy to integrate all the efforts so far because of their innate qualities and acceptability to patients and physicians. Majority of patients having smartphones report using mobile apps for health needs ranging from health information search to wellness to managing their diseases through remote monitoring.35,36, According to the Fifth Annual Pulse of Online Health Survey, two-thirds of Americans are interested in using mobile phones and health apps to maintain their health, and 79% are willing to use wearable devices and remote health sensors.37 At present, there are more than 165,000 apps dedicated to mobile health that are available for Android and iOS, a figure that has doubled during the last 2 years. Figure 2 shows the current mHealth app landscape, with most of the apps targeting fitness (30.9%), wellness (15.5%), medical reference (16.6%), and nutrition categories (7.4%), whereas apps providing diagnosis (1.4%), remote consultation (0.6%), and medical conditions management (6.6%) are still less than 10% of the current market. In this review, we will discuss in detail the dynamics of the current market and how things are changing for the future.
Figure 2.
mHealth app category share. CME, continuing medical education; PHR, patient health record. With permission from Search2guidance, mHealth app developer Economics 2014.
Remote Sensor Technologies
Development of remote health sensors has brought about a revolution in RPM. Sensors could be in smartphones, implantable, wearables (like Fitbit or Apple Watch), or placed in the surroundings. New sensors are being built that are small in size, better synchronized, energy efficient, wireless, and equipped with better biosensing technologies,38,39 thus providing unobtrusive and ambient monitoring.40 This has led to the concept of Internet of Things,41 which is a scenario in which objects, animals, or people are provided with unique identifiers and the ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. We are on a continuum from standard sensors to partially passive sensors to fully passive sensor development. Fully passive sensors can both collect and transmit information to the destined server on their own without any required participation from user or patient.42,43
The new age of health sensors can automatically sample blood to measure key biomarkers (eg, glucose to electrolytes), be implanted in different tissues such as heart and muscle to monitor their activity, be incorporated into pharmaceutical products to monitor patient’s compliance. and become part of the patient’s environment in the form of wearable accessories such as watches, shoes, or clothing itself43 or in chairs, beds, toilet seats, mirrors, etc. A compelling example is that of sensors incorporated into car steering40 mechanisms with the ability to measure body acceleration, position, orientation, gait, emotions, location, behavior, sleep patterns, and vital signs including pulse, respiratory rate, blood pressure, oxygen saturation, and temperature.40,42–44 Adopting these sensors in RPM marks a transition from a manual world to an automated one in which sensors collect and disseminate information in real time to healthcare providers who can use these data for surveillance and timely interventions.45 Technologies like Apple HealthKit (Apple Computer, Cupertino, CA) are allowing data from multiple sensors to be aggregated and delivered to providers integrated with electronic health records. Ongoing pilots are testing how best to present data from these sensors to providers without creating information overload.46
Mobile Telemedicine
Telemedicine is defined as the use of telecommunication and information technologies to provide clinical healthcare at a distance. Traditionally, telemedicine has been thought to be beneficial mainly to patients living in isolated communities and remote regions who can receive care from doctors or specialists far away without the patient having to travel to visit them. Until recently, telemedicine required use of dedicated hardware to be successful, and this limited the widespread acceptance of this technology. However, recent developments in mobile technology and broadband availability that have allowed smartphones and mobile apps to serve as a tool for telemedicine interventions have revolutionized this field because of its user friendliness and wider acceptance.46–48 Now patients, caregivers, and healthcare professionals in multiple locations can share information and discuss care issues to support urgent consults, remote coaching for fitness, care coordination in chronic diseases, as well as to augment remote patient monitoring through mobile technology to reduce the need for outpatient and emergency department visits, significantly reducing the overall cost of medical care.18,49–58
The role of telemedicine has been well-studied, and evidence with respect to efficacy, effectiveness, economics, and clinical preferences has been building.18,50 The failure to generate evidence for non-mobile telemedicine in the past was related to the problems of complex outcomes comparison and inadequate power in different studies.18,50–52 Recent research (especially around mobile telemedicine models) is showing promising results for telemedicine effectiveness in decreasing readmission rates and mortality in heart failure,53–55 better glycemic and hypertension control in diabetics and hypertensive patients, respectively,56,57 and decreased hospital stay and mortality in ICU patients.58
Project ECHO that is funded by Agency for Healthcare Research and Quality is also one of the success stories for telemedicine-based consulting. It was started in New Mexico to provide guided care to underserved hepatitis C patients through video conferencing tool controlled from tertiary healthcare center and enabling primary health care providers to provide specialist care to their patients in prisons and distant rural areas.59,60 In a cohort study of 407 patients comparing outcome results from 21 ECHO sites with the University of New Mexico Hepatitis Clinic, the sustained viral response was found to be equal in both groups, proving effectiveness of telemedicine.61 There are now attempts to expand telemedicine care and integrate it with RPM for hepatitis C patients through smartphone app, physician dashboard, and wireless adherence tracking pill bottles.62
Convergence of Smartphones, Mobile Apps, Sensors, and Telemedicine for Chronic Digestive Disorders
Chronic gastrointestinal diseases such as inflammatory bowel disease (IBD) or functional gastrointestinal disorders are especially suitable for RPM through a combination of mobile apps, sensor-based technologies, and telemedicine. Medical treatment has improved significantly in recent years; however, current efforts are largely ameliorative rather than curative.63 Many studies have reported increased variation in standards of care in patients with IBD and functional gastrointestinal disorders. Irrespective of the strength of a patient’s care team or treatment plan, most patients with these gastrointestinal conditions spend less than a few hours per year in face-to-face communication with their provider(s). The remainder of the year is spent in “self-management.” Indeed, the dominance of self-management is evident in outcomes. The risk of symptom flare, as well as the interventions required to control disease, is directly influenced by patient behaviors that take place outside a healthcare setting and that we often do not adequately assess or track (eg, adherence to medication, stress levels, depression, health literacy, smoking). This quality makes patients with chronic gastrointestinal disorders the ideal candidates to target for improved self-managed or collaborative management strategies incorporating remote monitoring and consultation.64
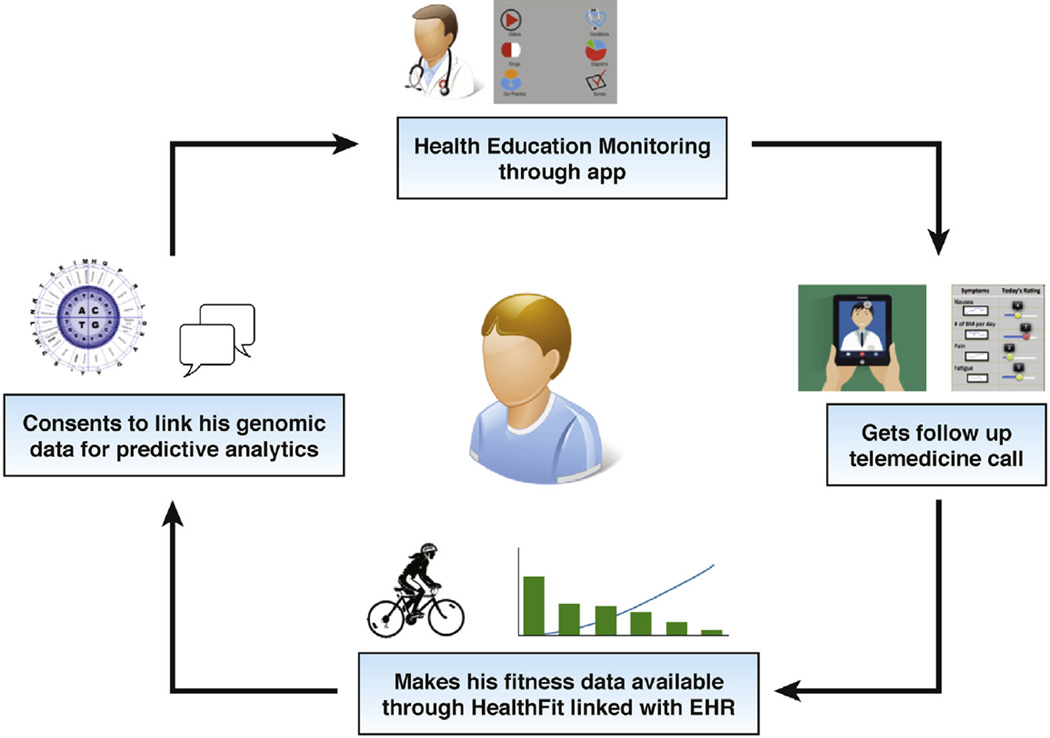
Figure 3 shows a schematic of a newly diagnosed IBD patient’s journey and how she/he is able to access various resources seamlessly delivered remotely through mobile apps, telemedicine, and sensors, enabling him/her to become a more engaged health consumer. Although this patient’s journey may seem fictitious at first look, many aspects of remote care highlighted in this figure are actually being delivered in a few centers today or are being evaluated in clinical trials and will likely become mainstream in the next few years. Table 1 lists a wide range of apps available in the Apple and Google Play stores for IBD patients from apps related to health education, symptom diaries, nutritional guides, weight trackers, preparing guides for procedures such as colonoscopy, to chronic disease management. Some of the general purpose apps that are not specifically designed for IBD (such as personal health records, care coordination) can also prove to be useful in providing comprehensive care.
Figure 3.
A newly diagnosed IBD patient’s care coordination and follow-up in remote monitoring platform through a combination of mobile app, telemedicine, and wearables devices. EHR, electronic health record.
Table 1.
Overview of Common Apps for Patients With IBD Available in Apple and Google Play Stores and Their Purpose
| General purpose | |
| Bathroom scout | Provides information about nearest restroom facilities. |
| Care zone | Organizes various aspects of disease management for patients and their care providers. |
| Colonoscopy Prep Assistant |
Helps patients remember details of preparation for procedure and also keeps record of due appointments. |
| Lisa’s Diet | Diet logging app to find possible correlation between specific foods ingested and associated symptoms severity. |
| Personal Health Record | This app allows patient’s access to their own medical charts including medications, preventive reminders, upcoming appointments, lab results, and enables secure communication with their providers. |
| MyCrohnsandColitisTeam Mobile |
Social network app that helps people with the same disease to stay connected and share their experiences. |
| Health education | |
| AnswersIn Crohn’s Disease |
Provides authentic information about different aspects of Crohn’s disease from clinical features to investigations and treatment. |
| AnswersIn Ulcerative Colitis |
A similar app for ulcerative colitis like Crohn’s disease app. |
| Crohn’s Disease by AZoMedical |
Provides regularly updated information and news about Crohn’s disease. |
| Inform Health | IBD health education app developed at Sinai App Lab. This has IBD health education content categorized into multimedia videos, drug information, conditions, or images. |
| Symptom tracking | |
| GI Monitor | Keeps real-time record of symptoms and helps to find possible links among lifestyle, diet, and symptoms. |
| Poop Time | Keeps track of bathroom time. |
| Poo Log | Handy tool to keep poo log with humorous twists. |
| myIBD—sickkids | Records details about symptoms, appetite, and trips to the bathroom with graphical presentation of record as well. |
| My Pain Diary | Keeps record of level of pain and helps to find precipitating factors such as weather, diet, and activities to pinpoint triggers. |
| GI Buddy | Enables patients to track their food, symptoms, exercise, treatments, and overall quality of life with presented reports showing general trends of their health as well. |
| Chronic disease management | |
| IBD Circle | Provides a wide range of tools and educational material including a symptom diary, journals for lifestyles and nutrition and quality of life index to help patients track trends in their own health. |
| UCLA eIBD App | Patients can view their upcoming labs, clinic visits, scheduled home care, medications, and procedures and can contact healthcare team through this platform. |
| HealthPromise App | This app developed at Sinai App Lab. It helps to track patient’s symptoms, quality of life, follow-up, and interventions in real time and provides point-of- care intervention facility from physicians at the same time. |
Limitations and Challenges
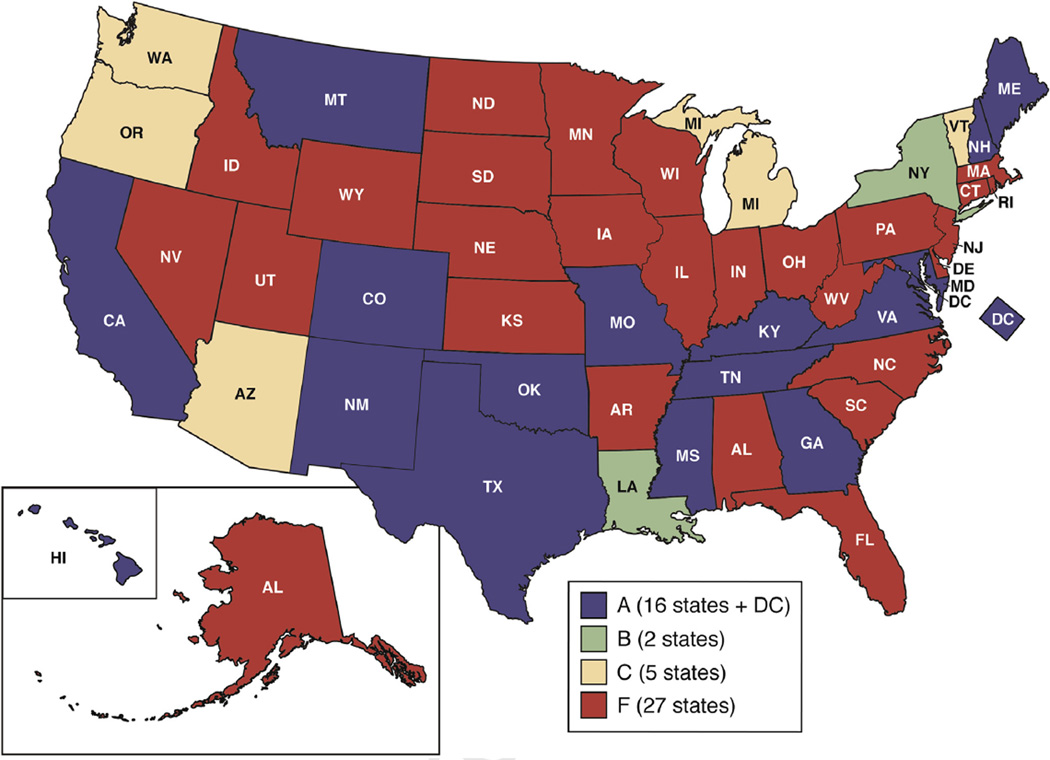
Acceptability by providers and healthcare systems seems to be the biggest block for widespread use of RPM. Patients lose their follow-up and adherence to remote monitoring if their physicians are not using them in a required, expected, and desired manner.65 The reason for lack of widespread adoption among health systems is multifactorial. First, there is inertia to adoption of new technologies, especially considering changes in workflow required in already busy schedules of care providers. Second, there are reservations among physicians regarding insurance reimbursements for healthcare for remote monitoring and telemedicine. Recently, the Center for Medicare and Medicaid Services has approved a Current Procedural Terminology code for chronic care management that can support remote monitoring beginning 2015. Recent gap analysis done by American Telemedicine Association for 50 states reveals positive trends, because almost half of the states are moving toward resolving reimbursement issues for telemedicine in the form of policy making and legislation at state level. Figure 4 shows favorable parity laws and policies regarding reimbursements for telemedicine services adopted by private insurances across all states, dividing the states into 4 groups. These groups were categorized by American Telemedicine Association by using 13 indicator scores, with group A having the full parity, meaning comparable reimbursement policy for telemedicine services when compared with in-person services and group F showing the poorest scores.
Figure 4.
States with parity laws for private insurance coverage of telemedicine (May 2015) classified into 4 groups on the basis of their corresponding scores, with group A having full parity, meaning comparable reimbursement policy for telemedicine services when compared with in-person services, and group F showing the poorest scores. With permission from American Telemedicine Association (ATA).
Another important factor that causes physicians to refrain from adopting digital health interventions is the limited evidence published to date to support effectiveness and efficiency of these interventions in actual clinical practice (outside pilot interventions). Many of the recently published and ongoing trials aim to fill in this gap in digital health evidence and aim to share the evidence with the health community through conferences, journals, workshops, social media, news, and blogs.43–45,66–69
Privacy, security, and confidentiality of the information are a challenge and prerequisite to share health information.67 Data should maintain their integrity, authenticity, confidentiality, and availability during storage and exchange. This exchange should be Health Insurance Portability and Accountability Act compliant as well.68 A wide variety of available cloud-hosted solutions aim to protect and secure devices and networks for data sharing, recommend use of personalized pins and logins to use the networks, have encryption and decryption steps for preventing leaked data from unauthorized use, and maintain ongoing risk analysis by a structured risk management system that eliminates risks and vulnerabilities. Still, more remains to be done to prevent against data thefts and breaches.70 Continuous uncontrolled gathering and sharing of information via modern remote sensors, especially those that are embedded in clothes and environment, can compromise patients’ privacy.64 Physicians on their side must ensure the security and safety of their smartphones and digital devices to be used as a medical tool.71,72 On a similar note, confidentiality of the data needs to be maintained to prevent inappropriate use. All the parties whose data are going to be collected and shared must be informed about the potential uses of their data. The American Medical Association has focused on issues of complementarity and bipartite responsibility of shared information in its proposal.73 This transparency will act as a confidence building measure and will address an important factor for decreased compliance as well.74
In regard to return on investment, effective business models have been lacking to ensure adequate return on investment for remote monitoring and consultation. Different tools have been proposed to address this issue,75 and efforts are now underway to fill this gap of evidence.46,76–78 The major proposed economic benefit of mobile apps and future mobile health in this model will not be served by downloads. In fact, it is expected that costs of technology investment will be overshadowed by health services being provided by digital health companies that will generate revenue by reducing cost of alternate system of consultations, decreasing readmission rates, increasing efficiency in monitoring, and promoting population-based well-being.14,78–92 In contrast, most of the current app usage is limited only to individual unmonitored and single-ended use. That is why health fitness apps that are disengaged with rest of the ecosystem will lose major share of the future market and be replaced by the apps sharing the burden of healthcare in a structured and scientific manner as presented in Figure 1.
Conclusion
The shift from volume-based to value-based healthcare is bound to increase in the next few years. RPM through combination of mobile apps, remote sensors, and telemedicine has potential to bend the cost curve by bringing efficiency and improving the effectiveness of healthcare delivery. RPM is likely to become mainstream not only for chronic disease management (such as for IBD, irritable bowel syndrome, or liver diseases) but also during episodes of care such as procedure preparation or immediate post-discharge care. By engaging with digital health technologies such as RPM, providers can be effective change agents and positively impact the rapidly evolving healthcare ecosystem.
Acknowledgments
Funding
Supported in part by the Crohn’s & Colitis Foundation of America (grant #253624) and the National Institutes of Health (5 K23 DK97451-02) with Ashish Atreja as the principal investigator.
Abbreviations used in this paper
- IBD
inflammatory bowel disease
- ICU
intensive care unit
- RPM
remote patient monitoring
Footnotes
Conflicts of interest
The authors disclose no conflicts. The authors disclose that they are part of the innovation team at Sinai Applab that has developed 2 of the mobile apps references in the article: “HealthPROMISE” and “HepCure”.
References
- 1.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 2.Patient protection and affordable care act. Public Law. :111–148. [Google Scholar]
- 3.Burwell SM. Setting value-based payment goals: HHS efforts to improve US health care. N Eng J Med. 2015;10:897–899. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Disease Prevention and Health Promotion. Available at: http://www.cdc.gov/chronicdisease/. 2014. Accessed.
- 5.DeVol R, Bedroussian A, Charuworn A, et al. An unhealthy America: the economic burden of chronic disease—charting a new course to save lives and increase productivity and economic growth. Available at: http://www.milkeninstitute.org/publications/view/321. 2007. Accessed. [Google Scholar]
- 6.Natarajan Y, Kanwal F. Pay for performance in chronic liver disease. Clin Gastroenterol Hepatol. 2015;13:2042–2047. doi: 10.1016/j.cgh.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Dorn SD. Quality measurement in gastroenterology: confessions of a realist. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Fortune BE, Golus A, Barsky CL, et al. Linking a hepatology clinical service line to quality improvement. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Allen JI. Patient safety counterpoint: systems approaches and multidisciplinary strategies at the centerpiece of error prevention. Clin Gastroenterol Hepatol. 2015;13:824–826. doi: 10.1016/j.cgh.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Siegel CA, Allen JI, Melmed GY. Translating improved quality of care into an improved quality of life for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:908–912. doi: 10.1016/j.cgh.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein JD, Castillo NE, Siddique SS, et al. Poor documentation of inflammatory bowel disease quality measures in academic, community, and private practice. Clin Gastroenterol Hepatol. 2016;14:421–428. doi: 10.1016/j.cgh.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 12.Sonnier P. The digital health update: story of digital health. Available at: http://storyofdigitalhealth.com/definition. 2015. Accessed. [Google Scholar]
- 13.Bayliss EA, Steiner JF, Fernald DH, et al. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med. 2003;1:15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Research outcomes: telemedicine’s impact on healthcare cost and quality. American Telemedicine Association. Available at: http://www.americantelemed.org/docs/default-source/policy/examples-of-research-outcomes—telemedicine’s-impact-on-healthcare-cost-and-quality.pdf. 2015. Accessed. [Google Scholar]
- 15.Seto E. Cost comparison between telemonitoring and usual care of heart failure: a systematic review. Telemed J E Health. 2008;14:679–686. doi: 10.1089/tmj.2007.0114. [DOI] [PubMed] [Google Scholar]
- 16.Chow CK, Redfern J, Hillis GS. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314:1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 17.Quinn CC, Shardell MD, Terrin ML. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934–1942. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura N, Koga T, Iseki H. A meta-analysis of remote patient monitoring for chronic heart failure patients. J Telemed Telecare. 2014;20:11–17. doi: 10.1177/1357633X13517352. [DOI] [PubMed] [Google Scholar]
- 19.McLean S, Nurmatov U, Liu JL. Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta-analysis. Br J Gen Pract. 2012;62:e739–e749. doi: 10.3399/bjgp12X658269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AH, Harrison J, Black PN, et al. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3:335–349. doi: 10.1016/j.jaip.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Sposaro F, Danielson J, Tyson G. iWander: an Android application for dementia patients. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:3875–3888. doi: 10.1109/IEMBS.2010.5627669. [DOI] [PubMed] [Google Scholar]
- 22.Edgar S, Swyka T, Fulk G, et al. Wearable shoe-based device for rehabilitation of stroke patients. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:3772–3775. doi: 10.1109/IEMBS.2010.5627577. [DOI] [PubMed] [Google Scholar]
- 23.Bsoul M, Minn H, Tamil L. Apnea MedAssist: real-time sleep apnea monitor using single-lead ECG. IEEE Trans Inf Technol Biomed. 2011;15:416–427. doi: 10.1109/TITB.2010.2087386. [DOI] [PubMed] [Google Scholar]
- 24.Kostikis N, Hristu-Varsakelis D, Arnaoutoglou M, et al. Towards remote evaluation of movement disorders via smartphones. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5240–5243. doi: 10.1109/IEMBS.2011.6091296. [DOI] [PubMed] [Google Scholar]
- 25.Puiatti A, Mudda S, Giordano S, et al. Smartphone-centred wearable sensors network for monitoring patients with bipolar disorder. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3644–3647. doi: 10.1109/IEMBS.2011.6090613. [DOI] [PubMed] [Google Scholar]
- 26.Rosner MH, Ronco C. Remote monitoring for the wearable artificial kidney. Contrib Nephrol. 2011;171:243–247. doi: 10.1159/000327229. [DOI] [PubMed] [Google Scholar]
- 27.Parsa CJ, Daneshmand MA, Lima B. Utility of remote wireless pressure sensing for endovascular leak detection after endovascular thoracic aneurysm repair. Ann Thorac Surg. 2010;89:446–452. doi: 10.1016/j.athoracsur.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 28.Field MJ, Grigsby J. Telemedicine and remote patient monitoring. JAMA. 2002;288:423–425. doi: 10.1001/jama.288.4.423. [DOI] [PubMed] [Google Scholar]
- 29.Boulos MNK, Wheeler S, Tavares C, et al. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. BioMedical Engineering OnLine. 2011;10:24. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvaney D, Woodward B, Datta S, et al. Development of m-health monitoring systems in India and Iraq. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:288–291. doi: 10.1109/EMBC.2012.6345926. [DOI] [PubMed] [Google Scholar]
- 31.The US Digital Consumer Report. Nielsen. Available at: http://www.nielsen.com/us/en/insights/reports/2014/the-us-digital-consumer-report.html. 2014. Accessed. [Google Scholar]
- 32.McInnes DK, Li AE, Hogan TP. Opportunities for engaging low-income, vulnerable populations in health care: a systematic review of homeless persons’ access to and use of information technologies. Am J Public Health. 2013;103:e11–e24. doi: 10.2105/AJPH.2013.301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiser K. 25 ways to use your smartphone: physicians share their favorite uses and apps. Minn Med. 2011;94:22–29. [PubMed] [Google Scholar]
- 34.Franko OI, Tirrell TF. Smartphone app use among medical providers in ACGME training programs. J Med Syst. 2012;36:3135–3139. doi: 10.1007/s10916-011-9798-7. [DOI] [PubMed] [Google Scholar]
- 35.Cohen R, Stussmen B. Health information technology use among men and women aged 18–64: early release of estimates from the national health interview survey. Health E-Stats. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/hestat/healthinfo2009/healthinfo2009.pdf. 2010. Accessed. [Google Scholar]
- 36.Atreja A, Khan S, Rogers JD, et al. HealthPROMISE Consortium Group: impact of the Mobile HealthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease—study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc. 2015;18:e23. doi: 10.2196/resprot.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fifth Annual “Pulse of Online Health” survey finds 66% of Americans eager to leverage digital tools to manage personal health. Available at: http://www.makovsky.com/insights/articles/733. 2015. Accessed. [Google Scholar]
- 38.Valdastri P, Rossi S, Menciassi A, et al. An implantable ZigBee ready telemetric platform for in vivo monitoring of physiological parameters. Sens Actuators A Phys. 2008;142:369–378. [Google Scholar]
- 39.Pantelopoulos A, Bourbakis NG. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans Syst Man Cybern Syst. 2010;40:1–12. [Google Scholar]
- 40.Zheng YL, Ding XR, Poon CC, et al. Unobtrusive sensing and wearable devices for health informatics. IEEE Trans Biomed Eng. 2014;61:1538–1554. doi: 10.1109/TBME.2014.2309951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Internet of Things global standards initiative. Available at: http://www.itu.int/en/ITU-T/gsi/iot/Pages/default.aspx. 2015. Accessed. [Google Scholar]
- 42.Alwan M. Passive in-home health and wellness monitoring: overview, value and examples. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4307–4310. doi: 10.1109/IEMBS.2009.5333799. [DOI] [PubMed] [Google Scholar]
- 43.Sarasohn-Kahn J. Making sense of sensors: how new technologies can change patient care. California Healthcare Foundation. Available at: http://www.chcf.org/publications/2013/02/making-sense-sensors. 2013. Accessed. [Google Scholar]
- 44.Intille SS, Lester J, Sallis JF, et al. New horizons in sensor development. Med Sci Sports Exerc. 2012;44:24–31. doi: 10.1249/MSS.0b013e3182399c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skubic M, Guevara RD, Rantz M. Automated health alerts using in-home sensor data for embedded health assessment. JTEHM. 2015;3:1–11. doi: 10.1109/JTEHM.2015.2421499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchet KD. Remote patient monitoring. Telemed J E-Health. 2008;14:127–130. doi: 10.1089/tmj.2008.9989. [DOI] [PubMed] [Google Scholar]
- 47.Demiris G, Afrin L, Speedie S, et al. Patient-centered applications: use of information technology to promote disease management and wellness—a white paper by the AMIA knowledge in motion working group. J Am Med Inform Assoc. 2008;15:8–13. doi: 10.1197/jamia.M2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demiris G, Finkelstein SM, Speedie S, et al. Considerations for the design of a web-based clinical monitoring and educational system for elderly patients. J Am Med Inform Assoc. 2001;8:468–472. doi: 10.1136/jamia.2001.0080468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung D, Hinze A. First European Conference on Mobile Government (EURO mGOV) Brighton, UK: A mobile alerting system for the support of patients with chronic conditions. Available at: http://www.mgov.cn/lab/Archives/EuromGov2005/PDF/27_S040JD-S08.pdf. 2005. Accessed. [Google Scholar]
- 50.Jakobsen NK, Jensen LS, Kayser L. Collaborative efforts are needed to ensure proper knowledge dissemination of telemedicine projects. Dan Med J. 2014;61:A4896. [PubMed] [Google Scholar]
- 51.van Beugen S, Ferwerda M, Hoeve D, et al. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res. 2014;16:e88. doi: 10.2196/jmir.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eysenbach G, Powell J, Englesakis M, et al. Health related virtual communities and electronic support groups: systematic review of the effects of online peer to peer interactions. BMJ. 2004;328:1166. doi: 10.1136/bmj.328.7449.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raikhelkar J, Raikhelkar JK. The impact of telemedicine in cardiac critical care. Crit Care Clin. 2015;31:305–317. doi: 10.1016/j.ccc.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 55.Widmer RJ, Collins NM, Collins CS, et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clinic Proc. 2015;90:469–480. doi: 10.1016/j.mayocp.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhai YK, Zhu WJ, Cai YL, et al. Clinical and cost effectiveness of telemedicine in type2 diabetes mellitus: a systematic review and meta-analysis. J Medicine. 2014;93:e312. doi: 10.1097/MD.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verberk WJ, Kessels AG, Thien T. Telecare is a valuable tool for hypertension management, a systematic review and meta-analysis. Blood Press Monit. 2011;16:149–155. doi: 10.1097/MBP.0b013e328346e092. [DOI] [PubMed] [Google Scholar]
- 58.Lilly CM, Zubrow MT, Kempner KM, et al. Critical care telemedicine: evolution and state of the art. Crit Care Med. 2014;42:2429–2436. doi: 10.1097/CCM.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 59.Project ECHO: bringing specialty care to rural New Mexico. Available at: https://healthit.ahrq.gov/ahrq-funded-projects/transforming-healthcare-quality-through-health-it/project-echo-bringing. 2014. Accessed.
- 60.Arora S, Thornton K, Jenkusky SM, et al. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122:74–77. doi: 10.1177/00333549071220S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Project HepCure. Available at: http://hepcure.org. 2015. Accessed. [Google Scholar]
- 63.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Deen WK, van der Meulen-de Jong AE, Parekh NK, et al. Development and validation of an inflammatory bowel diseases monitoring index for use with mobile health technologies. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 65.Jethwani K, Ling E, Mohammed M, et al. Diabetes Connect: an evaluation of patient adoption and engagement in a Web-based remote glucose monitoring program. Journal of Diabetes Science and Technology. 2012;6:1328–1336. doi: 10.1177/193229681200600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misra S, Lewis TL, Aungst TD. Medical application use and the need for further research and assessment for clinical practice: creation and integration of standards for best practice to alleviate poor application design. JAMA Dermatol. 2013;149:661–662. doi: 10.1001/jamadermatol.2013.606. [DOI] [PubMed] [Google Scholar]
- 67.Hall JL, McGraw D. For telehealth to succeed, privacy and security risks must be identified and addressed. Health Aff (Millwood) 2014;33:216–221. doi: 10.1377/hlthaff.2013.0997. [DOI] [PubMed] [Google Scholar]
- 68.Luxton DD, Kayl RA, Mishkind MC. mHealth data security: the need for HIPAA-compliant standardization. Telemed J E Health. 2012;18:284–288. doi: 10.1089/tmj.2011.0180. [DOI] [PubMed] [Google Scholar]
- 69.Gordon CR, Rezzadeh KS, Li A, et al. Digital mobile technology facilitates HIPAA-sensitive perioperative messaging, improves physician-patient communication, and streamlines patient care. Patient Saf Surg. 2015;9:21. doi: 10.1186/s13037-015-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallwiener M, Wallwiener CW, Kansy JK, et al. Impact of electronic messaging on the patient-physician interaction. J Telemed Telecare. 2009;15:243–250. doi: 10.1258/jtt.2009.090111. [DOI] [PubMed] [Google Scholar]
- 71.Aungst TD, Clauson KA, Misra S, et al. How to identify, assess and utilise mobile medical applications in clinical practice. Int J Clin Pract. 2014;68:155–162. doi: 10.1111/ijcp.12375. [DOI] [PubMed] [Google Scholar]
- 72.Manning ML, Davis J, Sparnon E, et al. iPads, droids, and bugs: infection prevention for mobile handheld devices at the point of care. Am J Infect Control. 2013;41:1073–1076. doi: 10.1016/j.ajic.2013.03.304. [DOI] [PubMed] [Google Scholar]
- 73.Opinion 5.026: the use of electronic mail. American Medical Association. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5026.page. 2003. Accessed. [Google Scholar]
- 74.HONcode, Health on the Net Foundation. Available at: http://www.hon.ch/HONcode/Patients/Conduct.html. 2013. Accessed. [Google Scholar]
- 75.Kazanjian A, Green CJ. Beyond effectiveness: the evaluation of information systems using a comprehensive health technology assessment framework. Comput Biol Med. 2002;32:165–177. doi: 10.1016/s0010-4825(02)00013-6. [DOI] [PubMed] [Google Scholar]
- 76.Featherly K, Garets D, Davis M, et al. Sharpening the case for returns on investment from clinical information systems. Healthc Q. 2007;10:101–110. [PubMed] [Google Scholar]
- 77.Whitten P, Kuwahara E. Telemedicine from the payor perspective: considerations for reimbursement decisions. Dis Manag Health Outcomes. 2003;11:291–298. [Google Scholar]
- 78.mHealth App Developer Economics. research2guidance mobile Health Economics. Available at: http://mhealtheconomics.com/mhealth-developer-economics-report/. 2014. Accessed. [Google Scholar]
- 79.Ryan D, Price D, Musgrave SD, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ. 2012;344:e1756. doi: 10.1136/bmj.e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holopainen A, Galbiati F, Voutilainen K. Use of smart phone technologies to offer easy-to-use and cost-effective telemedicine services; First International Conference on the IEEE; Available at: http://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=4063766&isnumber=4063753. 2007. Accessed. [Google Scholar]
- 81.Kaplan WA. Can the ubiquitous power of mobile phones be used to improve health outcomes in developing countries? Globalization and Health. 2006;2:9. doi: 10.1186/1744-8603-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de la Torre-Díez, López-Coronado M, Vaca C, et al. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015;21:81–85. doi: 10.1089/tmj.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Free C, Phillips G, Watson L, et al. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013;10:e1001363. doi: 10.1371/journal.pmed.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vo AH. The telehealth promise: better health care and cost savings for the 21st century. AT&T Center for Telehealth Research and Policy. Available at: http://telehealth.utmb.edu/presentations/The%20Telehealth%20Promise-Better%20Health%20Care%20and%20Cost%20Savings%20for%20the%2021st%20Century.pdf. 2008. Accessed. [Google Scholar]
- 85.Rosenberg CN, Peele P, Keyser D, et al. Results from a patient-centered medical home pilot at UPMC Health Plan hold lessons for broader adoption of the model. Health Aff (Millwood) 2012;31:2423–2431. doi: 10.1377/hlthaff.2011.1002. [DOI] [PubMed] [Google Scholar]
- 86.Cryer L, Shannon SB, Van Amsterdam M, et al. Costs for ’hospital at home’ patients were 19 percent lower, with equal or better outcomes compared to similar inpatients. Health Aff (Millwood) 2012;31:1237–1243. doi: 10.1377/hlthaff.2011.1132. [DOI] [PubMed] [Google Scholar]
- 87.Baker LC, Johnson SJ, Macaulay D, et al. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Aff (Millwood) 2011;30:1689–1697. doi: 10.1377/hlthaff.2011.0216. [DOI] [PubMed] [Google Scholar]
- 88.Bardach NS, Wang JJ, De Leon SF, et al. Effect of pay-for-performance incentives on quality of care in small practices with electronic health records: a randomized trial. JAMA. 2013;310:1051–1059. doi: 10.1001/jama.2013.277353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Epstein AM, Lee TH, Hamel MB. Paying physicians for high-quality care. N Engl J Med. 2004;350:406–410. doi: 10.1056/NEJMsb035374. [DOI] [PubMed] [Google Scholar]
- 90.Iglehart JK. Linking compensation to quality: Medicare payments to physicians. N Engl J Med. 2005;353:870–872. doi: 10.1056/NEJMp058194. [DOI] [PubMed] [Google Scholar]
- 91.Thomas L, Capistrant G. State telemedicine gaps analysis, coverage & reimbursement. American Telemedicine Association. Available at: http://www.americantelemed.org/policy/state-policy-resource-center#.VbuKxPmqqko. 2015. Accessed. [Google Scholar]
- 92.Thomas L, Capistrant G. State telemedicine gaps analysis physician practice standards & licensure. American Telemedicine Association. Available at: http://www.americantelemed.org/policy/state-policy-resource-center#.VbJEs7Oqqko. 2015. Accessed. [Google Scholar]