Abstract
Relapsing-remitting multiple sclerosis is commonly associated with motor impairments, neuropathic pain, fatigue, mood disorders, and decreased life expectancy. However, preclinical pharmacological studies predominantly rely on clinical scoring of motor deficit as the sole behavioral endpoint. Thus, the translational potential of these studies is limited. Here, we have assessed the therapeutic potential of a novel anti-inflammatory interleukin-10 (IL-10) non-viral gene therapy formulation (XT-101-R) in a rat relapsing remitting experimental autoimmune encephalomyelitis (EAE) model. EAE induced motor deficits and neuropathic pain as reflected by induction of low-threshold mechanical allodynia, suppressed voluntary wheel running, decreased social exploration, and was associated with markedly enhanced mortality. We also noted that voluntary wheel running was depressed prior to the onset of motor deficit, and may therefore serve as a predictor of clinical symptoms onset. XT-101-R was intrathecally dosed only once at the onset of motor deficits, and attenuated each of the EAE-induced symptoms and improved survival, relative to vehicle control. This is the first pharmacological assessment of such a broad range of EAE symptoms, and provides support for IL-10 gene therapy as a clinical strategy for the treatment of multiple sclerosis.
Keywords: neuroinflammation, myelin oligodendrocyte glycoprotein, dark agouti
1. Introduction
Approximately 85% of multiple sclerosis (MS) patients are affected by relapsing remitting neurological deficits (Trapp and Nave, 2008). Beyond motor impairments, MS is commonly associated with neuropathic pain, fatigue, mood disorders, and decreased life expectancy (Induruwa et al., 2012; Marrie et al., 2015; O’Connor et al., 2008; Ragonese et al., 2008; Truini et al., 2013). Yet, the vast majority of preclinical studies, which almost exclusively use the experimental autoimmune encephalomyelitis (EAE) model, rely solely on clinical scoring of motor deficit as the behavioral endpoint. This is also true of studies evaluating novel therapeutics. However, recommendations to improve therapeutic translation for central nervous system diseases have focused on assessment of a broad range of disease-related behaviors (Markou et al., 2009; Mogil, 2009). Thus, potential therapeutics should also be screened for reversal of other EAE/MS-related symptoms, beyond motor deficits.
Relapsing remitting MS and EAE are characterized by localized inflammatory lesions in the brain and spinal cord that contain auto-reactive T- and B-lymphocytes, and reactive dendritic cells and microglia. Demyelination, axonal transection and conduction block follow at these lesions, leading to motor deficits (Ciccarelli et al., 2014). Such central immune signaling is also known to underpin other symptoms of MS—neuropathic pain, fatigue, and mood disorders (Dantzer et al., 2008; Grace et al., 2014a; Morris et al., 2015; Salim et al., 2012). Thus, immuno-modulation is a viable and effective therapeutic approach for a range of EAE/MS-related symptoms.
We have developed a biodegradable microparticle formulation, which slowly releases plasmid DNA encoding interleukin 10 (IL-10) (XT-101-R) (Soderquist et al., 2010). The microparticles are taken up by meningeal macrophages, leading to sustained intrathecal delivery of the anti-inflammatory cytokine IL-10 (Soderquist et al., 2010). This formulation drives long-lasting action of IL-10, in contrast to other formulations that have been developed (Kwilasz et al., 2015). IL-10 has the potential to decrease disease severity by decreasing expression of pro-inflammatory cytokines, decreasing antigen presentation, inducing T cell anergy and promoting neuroprotection (Kwilasz et al., 2015). However, the effect of a single intrathecal administration of XT-101-R in EAE has not previously been explored, nor has such an approach been assessed for potential effects on broader symptomatology. Hence, the aim of this study was to determine whether a single intrathecal administration of XT-101-R could attenuate a broad range of EAE associated symptoms; namely, motor symptoms, neuropathic pain, fatigue, anxiety, and decreased life expectancy.
2. Methods
2.1. Animals
Male Dark Agouti rats (200–225 g; Harlan Labs) were used in all experiments, and housed in temperature (23 °C ± 3 °C) and light (12:12 light/dark) controlled rooms with standard rodent chow and water available ad libitum. All procedures were approved by the University of Colorado Boulder IACUC, and all behaviors were assessed by blinded experimenters.
2.2. EAE induction
Relapsing-remitting EAE was induced with rat myelin oligodendrocyte glycoprotein (MOG; amino acids 1–125) emulsion (4 μg for social exploration experiments; 17 μg for mechanical allodynia; 35 μg for all other experiments), as previously described (Sloane et al., 2009). MOG doses < 35 μg were used to decrease the severity of paralysis/paresis that might otherwise confound testing for mechanical allodynia and social interaction. The two low MOG doses were derived from different aliquots, but elicited a peak clinical score of < 3. Rats were briefly anesthetized with isoflurane and given a single intradermal injection (100 μl total volume) into the dorsal skin just rostral to the base of the tail.
2.3. IL-10 pDNA (XT-101-R) preparation and injection
The plasmid construct encoding for rat interleukin-10 (pDNA-IL-10F129S; previously described in detail (Milligan et al., 2006)) was encapsulated in PLGA microparticles as previously described (Soderquist et al., 2010), or commercially by Evonik Industries (Birmingham, AL) (for mechanical allodynia). PLGA microparticles without pDNA (blank) served as the vehicle control, as previous studies have shown no behavioral effect of foreign plasmid DNA per se on behavior at higher doses than used here (Milligan et al., 2006). Further, we have previously demonstrated that PLGA microparticles containing approximately the same or higher amount of non-coding, control plasmid DNA have no effect on behavior (Soderquist et al., 2010). On the first day that motor deficits were observed (clinical score ≥ 1; range across studies was 9–14 days post immunization), either 8 μg IL-10 pDNA or control was intrathecally administered in 20 μl DPBS, using methods previously described (Milligan et al., 1999). The dosing schedule allowed the immune response to be mounted in the absence of a therapeutic intervention, as well as allowing the effect of XT-101-R to be assessed on overt motor impairments in this aggressive model.
2.4. Motor score and body weight
Rats were monitored daily for body mass changes and motor symptoms, as previously described (Ledeboer et al., 2003; Loram et al., 2015; Sloane et al., 2009). Motor deficits were scored on a scale from 0 to 7 based on the degree of ascending paralysis: 0, no symptom expression; 1, partial tail paralysis; 2, full tail paralysis; 3, hindlimb weakness (unsteady gait while walking); 4, partial hindlimb paralysis (no weight bearing but observable movement of the limb); 5, full hindlimb paralysis; 6, partial forelimb paralysis; 7, euthanasia due to disease progression.
2.5. Mechanical allodynia
The von Frey test was performed as previously described (Grace et al., 2014b). Rats were excluded on a given testing day if they displayed a motor deficit score ≥ 3. This only occurred on day 18 post symptom onset (control group: n = 2; XT-101-R: n = 0).
2.6. Social interaction
Given the sensitivity of social interaction to anxiogenic and anxiolytic pharmacological agents, this behavior was used to assess anxiety as previously described in detail (Christianson et al., 2008). Exploratory behaviors initiated by the adult rat were timed. Tests were performed prior to MOG immunization, 3 days after MOG immunization, and 3 and 12 days after symptom onset. No rats were excluded from analysis due to motor deficit score ≥ 3 on testing days.
2.7. Voluntary wheel running
All rats were single housed and allowed voluntary, unrestricted access to in-cage running wheels, beginning the day of EAE induction, and continuing to study termination (day 16 post symptom onset). Daily wheel revolutions were recorded digitally using Vital View software (Mini Mitter, Bend, OR), and daily distance traveled calculated by multiplying number of revolutions by wheel circumference (1.081 m).
2.8. Survival
Death was recorded following spontaneous expiration or when animals were euthanized (clinical score = 7), relative to the onset of paralysis. The rats used here were from the same as those used for clinical scoring and body weights.
2.9. Statistics
Differences between treatment groups for motor scores were analyzed using a Mann-Whitney U test, corrected for multiple comparisons. Differences between treatment groups for all other endpoints were determined using unpaired t-test, one-way ANOVA, or repeated measures two-way ANOVA, with Holm-Sidak’s post hoc test, as appropriate. AUCs were calculated from the first day that motor deficits were observed (clinical score ≥ 1). Spearman rank correlation was used to determine the correlation between motor scores and distance traveled. Differences between Kaplan-Meier survival curves were determined using the log rank Mantel-Cox test. P < 0.05 was considered significant, and data are presented as mean ± SEM.
3. Results
3.1. XT-101-R attenuated EAE motor symptoms
A single intrathecal treatment of XT-101-R or vehicle control was administered on the first day that motor deficits were observed. The rats receiving vehicle control demonstrated the relapsing remitting motor impairment (Fig. 1A) as described previously (Ledeboer et al., 2003; Loram et al., 2015; Sloane et al., 2009). However, treatment with XT-101-R significantly attenuated the clinical score (Fig. 1A,B; P < 0.05). Loss of body weight was significantly attenuated (Fig. 1C,D; time × treatment: F20,360 = 2.38, P < 0.001; time: F20,360 = 26.38, P < 0.001; treatment: F1,18 = 2.64, P = 0.1).
Figure 1. XT-101-R therapy attenuated EAE-induced motor paralysis and loss of body weight.
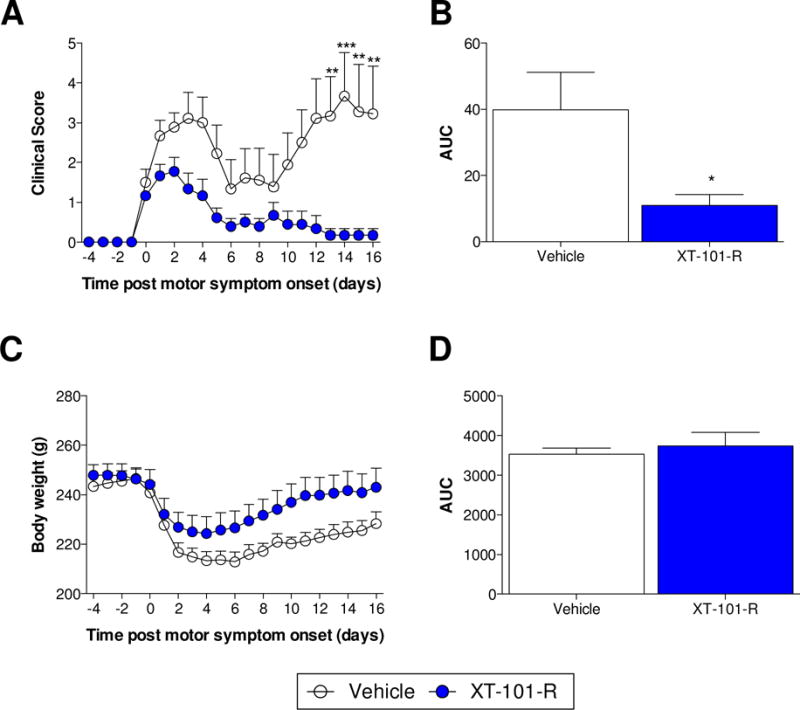
Motor deficits were scored and body weights recorded prior to and every day following MOG immunization. Scores are displayed relative to the first day that motor deficits were observed (clinical score ≥ 1). Intrathecal injections of XT-101-R or vehicle control occurred on the first day that motor deficits manifested (day 0). *P < 0.05, **P < 0.01, ***P < 0.001. n = 10/group.
3.2. XT-101-R attenuated a range of EAE-related behaviors
Mechanical allodynia thresholds were assessed as a measure of neuropathic pain. There was a significant interaction between time and treatment (Fig 2A; F10,60 = 4.89, P < 0.001), as well as significant effects of time (F5,60 = 9.72, P < 0.001) and treatment (F2,12 = 33.74, P < 0.001). Rats receiving vehicle control developed mechanical allodynia compared to naïve controls (P < 0.01), while XT-101-R significantly attenuated such allodynia (P < 0.01).
Figure 2. XT-101-R therapy attenuated a range of EAE-induced behaviors.
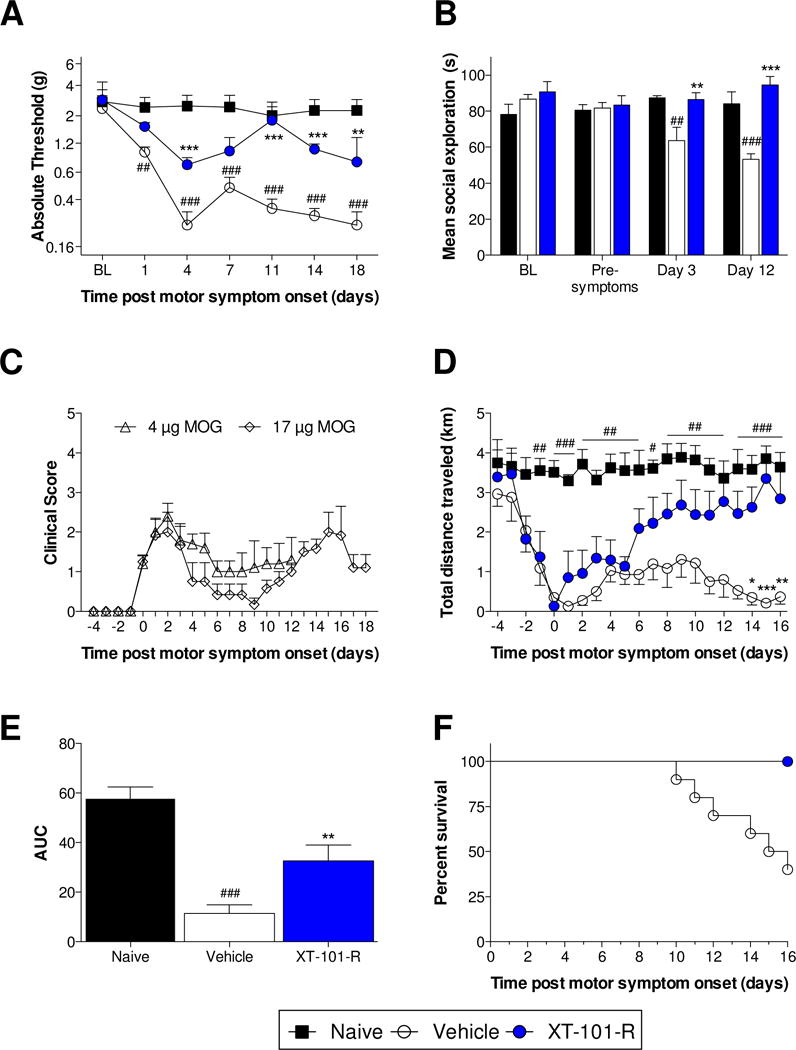
(A) Mechanical allodynia thresholds were assessed prior to (BL) and every 3–4 days following MOG immunization with the exception of rats that displayed a motor deficit score of 3 (unsteady hind limb gait) or higher on a given day (n = 6/group [n = 4 for control group on day 18]). (B) Social interaction was assessed by introducing a juvenile rat to the adult test cage for 3 minutes. Exploratory behaviors initiated by the adult rat were timed. Tests were performed prior to MOG immunization (BL), 3 days post MOG immunization (pre-symptoms), and days 3 and 12 after paralysis onset (Day 3; Day 12) (n = 6/group). (C) Clinical scores for the MOG doses used in nociceptive hypersensitivity (17 μg) and social exploration (4 μg) experiments (see Section 2.2). (D, E) Rats were single housed with access to a running wheel from the day of MOG immunization. Distance traveled was calculated daily prior to and every day following MOG immunization. Prior to the onset of hind limb paralysis, a significant fall in distance traveled was observed over several days (n = 9/group). (F) Death was recorded following spontaneous expiration or when animals were euthanized (clinical score = 7; n = 10/group). All data are displayed relative to the day of intrathecal injection of XT-101-R or vehicle control (onset of paralysis). **P < 0.01, ***P < 0.001: relative to vehicle control; ##P < 0.01, ###P < 0.001: relative to naïve control.
Social interaction was assessed as a measure of anxiety (Christianson et al., 2008). Tests were performed prior to MOG immunization (BL), 3 days post MOG immunization (pre-symptoms), and days 3 and 12 after paralysis onset (Day 3; Day 12), and presented in Figure 2B (time × treatment: F4,45 = 7.76, P < 0.001; time: F2,45 = 2.38, P = 0.1; treatment: F2,45 = 9.12, P < 0.001). No group differences were observed at either pre-immunization or pre-symptoms. However, EAE significantly attenuated social exploration time in the control group at day 3 (P < 0.01) and day 12 (P < 0.001), which was significantly restored by XT-101-R (P < 0.01). The motor deficits for the MOG doses used for nociceptive hypersensitivity (17 μg) and social exploration (4 μg) experiments (see Section 2.2) are presented in Figure 2C.
Voluntary wheel running has previously been used as a measure of fatigue/lethargy in both an endotoxemia and chemotherapy model, which are not associated with motor deficits (Harden et al., 2011; Ray et al., 2011). It is therefore possible that voluntary wheel running will be sensitive to fatigue, in addition to motor deficits. There was a significant interaction between time and treatment (F40,440 = 2.34, P < 0.001), as well as significant effects of time (F40,440 = 5.53, P < 0.001) and treatment (F2,22 = 19.22, P < 0.001) (Fig. 2D,E). Beginning at day -1, voluntary running was significantly suppressed in rats receiving vehicle control, compared to naïve controls (P < 0.01). Treatment with XT-101-R significantly reversed EAE-suppressed distance traveled (P < 0.05). Notably, daily distance traveled progressively fell in the days preceding symptom onset (P < 0.01). Distance traveled was negatively correlated with the motor score, irrespective of treatment group (rs = −0.84; P < 0.001).
The day of death, relative to paralysis onset, was recorded (Fig. 2F). XT-101-R significantly extended survival (P < 0.01), with no deaths recorded in this group for the study duration versus 65% mortality in the control group.
4. Discussion
Here, we demonstrate the efficacy of a novel IL-10 gene therapy formulation (XT-101-R) in a rat model of relapsing remitting EAE. XT-101-R reversed motor symptoms, and attenuated loss of body weight, the standard outcome measures for EAE. We also found that other behaviors were associated with EAE, including mechanical allodynia (reflecting neuropathic pain), decreased voluntary wheel running (reflecting fatigue and motor impairments), and decreased social exploration (reflecting anxiety). These symptoms are rarely assessed in rodent models, despite their frequent association with MS. Assessment of nociceptive hypersensitivity in relapsing remitting models is limited, but several studies have demonstrated pharmacological reversal of allodynia (Khan et al., 2014; Lu et al., 2012; Lynch et al., 2008; Ramos et al., 2010; Schmitz et al., 2014; Sloane et al., 2009; Thibault et al., 2011; Tian et al., 2013). While anxiety behaviors are a known feature in EAE models (Acharjee et al., 2013; Peruga et al., 2011; Pollak et al., 2000), only two studies have evaluated therapeutic modulation (Di Prisco et al., 2014; Haji et al., 2012). Our study is the first to quantify such a breadth of EAE-associated behaviors, and further introduces a measure of fatigue, as well as demonstrating reversal with XT-101-R.
Restored voluntary wheel running following XT-101-R treatment may be linked to improvements in motor scores, given the correlation between motor scores and voluntary wheel running. We have previously shown that voluntary running serves as a measure of evoked pain (Grace et al., 2014b). Furthermore, voluntary wheel running may confound study of EAE, as movement has been shown to both attenuate (Benson et al., 2015) and exacerbate disease progression (Arima et al., 2012). Nonetheless, we propose that it may also serve as a measure of fatigue or lethargy in EAE (induced by the sickness response (Harden et al., 2011; Ray et al., 2011)), as voluntary running declined to nearly zero before the onset of motor symptoms or neuropathic pain. This may reflect the pathological changes that are known to precede motor impairments (Constantinescu et al., 2011), and suggests that the measure is not completely confounded by paresis/paralysis. This finding is striking and provides a means to predict the onset of motor symptoms.
XT-101-R is a novel formulation of pDNA-IL-10F129S therapy. That XT-101-R could resolve several EAE associated symptoms is reflective of the broad range of disease-modifying actions of IL-10 (Kwilasz et al., 2015). XT-101-R has previously been used only in a rat neuropathic pain model (Soderquist et al., 2010). In this study, a single intrathecal injection of XT-101-R attenuated mechanical allodynia. Plasmid IL-10 mRNA was detected in both the CSF and lumbar spinal cord, and increased the number of CD163+ cells (a marker of M2 activation) in the lumbar spinal cord (Soderquist et al., 2010). Naked pDNA-IL-10F129S or lentivirally expressed IL-10 previously attenuated motor symptoms and mechanical allodynia in the rat model of relapsing remitting EAE (Sloane et al., 2009; van Strien et al., 2010). Furthermore, naked pDNA-IL-10F129S decreased GFAP expression (a marker for astrocyte activation) and the number of CD68+ cells (a marker of M1 activation) in the lumbar spinal cord (Sloane et al., 2009). A limitation of this study is the absence of histological and biochemical analysis of CNS regions relevant to neuropathic pain, fatigue, and mood disorders. However, such analyses will be the focus of future studies, and may shed insights into the mechanisms by which IL-10 normalizes these behaviors.
In summary, we have demonstrated that relapsing remitting EAE in rat is associated with neuropathic pain, fatigue, and anxiety, in addition to motor symptoms and decreased survival. These symptoms mirror the clinical manifestation of MS. Furthermore, these symptoms were normalized by a IL-10 gene therapy. This is the first pharmacological assessment of such a broad range of EAE symptoms, and provides support for IL-10 gene therapy as a clinical approach for MS.
Highlights.
Rat EAE was associated with neuropathic pain, anxiety and fatigue
These symptoms were attenuated by a novel IL-10 gene therapy formulation
Depressed wheel running preceded motor deficit, and may predict symptom onset
Acknowledgments
This work was generously supported by a National Multiple Sclerosis Society grant (L.R.W., P.M.G.), and an NIH SBIR grant R43NS81878 (R.A.C.). P.M.G. is an Australian National Health and Medical Research Council C. J. Martin Fellow (ID 1054091; 2013-). J.R.F., R.A.C. and L.R.W. are co-founders of Xalud Therapeutics, a start up company working toward bringing XT-101 to human clinical trials. R.A.C. is the Vice President of Research and Development of Xalud; J.R.F. and L.R.W. serve on the Scientific Advisory Board of Xalud.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- IL-10
interleukin 10
- IFA
incomplete Freund’s adjuvant
- MS
multiple sclerosis
- MOG
myelin oligodendrocyte glycoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharjee S, Nayani N, Tsutsui M, Hill MN, Ousman SS, Pittman QJ. Altered cognitive-emotional behavior in early experimental autoimmune encephalitis–cytokine and hormonal correlates. Brain Behav Immun. 2013;33:164–172. doi: 10.1016/j.bbi.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, Kawamoto T, Iwakura Y, Betz UA, Marquez G, Blackwell TS, Ohira Y, Hirano T, Murakami M. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2015;271:279–290. doi: 10.1016/j.expneurol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Barkhof F, Bodini B, De Stefano N, Golay X, Nicolay K, Pelletier D, Pouwels PJ, Smith SA, Wheeler-Kingshott CA, Stankoff B, Yousry T, Miller DH. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol. 2014;13:807–822. doi: 10.1016/S1474-4422(14)70101-2. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco S, Merega E, Lanfranco M, Casazza S, Uccelli A, Pittaluga A. Acute desipramine restores presynaptic cortical defects in murine experimental autoimmune encephalomyelitis by suppressing central CCL5 overproduction. Br J Pharmacol. 2014;171:2457–2467. doi: 10.1111/bph.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014a;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Maier SF, Watkins LR. Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hind paw-evoked pain. J Pain. 2014b;15:121–128. doi: 10.1016/j.jpain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji N, Mandolesi G, Gentile A, Sacchetti L, Fresegna D, Rossi S, Musella A, Sepman H, Motta C, Studer V, De Chiara V, Bernardi G, Strata P, Centonze D. TNF-alpha-mediated anxiety in a mouse model of multiple sclerosis. Exp Neurol. 2012;237:296–303. doi: 10.1016/j.expneurol.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Roth J, Loram LC, Poole S, Laburn HP. Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1beta and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior. Psychoneuroendocrinology. 2011;36:608–622. doi: 10.1016/j.psyneuen.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis - a brief review. J Neurol Sci. 2012;323:9–15. doi: 10.1016/j.jns.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Khan N, Woodruff TM, Smith MT. Establishment and characterization of an optimized mouse model of multiple sclerosis-induced neuropathic pain using behavioral, pharmacologic, histologic and immunohistochemical methods. Pharmacol Biochem Behav. 2014;126:13–27. doi: 10.1016/j.pbb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96:55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Wierinckx A, Bol JG, Floris S, Renardel de Lavalette C, De Vries HE, van den Berg TK, Dijkstra CD, Tilders FJ, van dam AM. Regional and temporal expression patterns of interleukin-10, interleukin-10 receptor and adhesion molecules in the rat spinal cord during chronic relapsing EAE. J Neuroimmunol. 2003;136:94–103. doi: 10.1016/s0165-5728(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Loram LC, Strand KA, Taylor FR, Sloane E, Van Dam AM, Rieger J, Maier SF, Watkins LR. Adenosine 2A receptor agonism: A single intrathecal administration attenuates motor paralysis in experimental autoimmune encephalopathy in rats. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Kurejova M, Wirotanseng LN, Linker RA, Kuner R, Tappe-Theodor A. Pain in experimental autoimmune encephalitis: a comparative study between different mouse models. J Neuroinflammation. 2012;9:233. doi: 10.1186/1742-2094-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JL, Gallus NJ, Ericson ME, Beitz AJ. Analysis of nociception, sex and peripheral nerve innervation in the TMEV animal model of multiple sclerosis. Pain. 2008;136:293–304. doi: 10.1016/j.pain.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, Cutter G, Reider N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Mult Scler. 2015;21:305–317. doi: 10.1177/1352458514564487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137:96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Peruga I, Hartwig S, Thone J, Hovemann B, Gold R, Juckel G, Linker RA. Inflammation modulates anxiety in an animal model of multiple sclerosis. Behav Brain Res. 2011;220:20–29. doi: 10.1016/j.bbr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Ovadia H, Goshen I, Gurevich R, Monsa K, Avitsur R, Yirmiya R. Behavioral aspects of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;104:31–36. doi: 10.1016/s0165-5728(99)00257-x. [DOI] [PubMed] [Google Scholar]
- Ragonese P, Aridon P, Salemi G, D’Amelio M, Savettieri G. Mortality in multiple sclerosis: a review. Eur J Neurol. 2008;15:123–127. doi: 10.1111/j.1468-1331.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169:1888–1900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med. 2011;61:119–130. [PMC free article] [PubMed] [Google Scholar]
- Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- Schmitz K, de Bruin N, Bishay P, Mannich J, Haussler A, Altmann C, Ferreiros N, Lotsch J, Ultsch A, Parnham MJ, Geisslinger G, Tegeder I. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol Med. 2014;6:1398–1422. doi: 10.15252/emmm.201404168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist RG, Sloane EM, Loram LC, Harrison JA, Dengler EC, Johnson SM, Amer LD, Young CS, Lewis MT, Poole S, Frank MG, Watkins LR, Milligan ED, Mahoney MJ. Release of plasmid DNA-encoding IL-10 from PLGA microparticles facilitates long-term reversal of neuropathic pain following a single intrathecal administration. Pharm Res. 2010;27:841–854. doi: 10.1007/s11095-010-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault K, Calvino B, Pezet S. Characterisation of sensory abnormalities observed in an animal model of multiple sclerosis: a behavioural and pharmacological study. Eur J Pain. 2011;15:231 e231–216. doi: 10.1016/j.ejpain.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Tian DH, Perera CJ, Apostolopoulos V, Moalem-Taylor G. Effects of vaccination with altered Peptide ligand on chronic pain in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Front Neurol. 2013;4:168. doi: 10.3389/fneur.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Truini A, Barbanti P, Pozzilli C, Cruccu G. A mechanism-based classification of pain in multiple sclerosis. J Neurol. 2013;260:351–367. doi: 10.1007/s00415-012-6579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien ME, Mercier D, Drukarch B, Breve JJ, Poole S, Binnekade R, Bol JG, Blits B, Verhaagen J, van Dam AM. Anti-inflammatory effect by lentiviral-mediated overexpression of IL-10 or IL-1 receptor antagonist in rat glial cells and macrophages. Gene Ther. 2010;17:662–671. doi: 10.1038/gt.2010.8. [DOI] [PubMed] [Google Scholar]


