Abstract
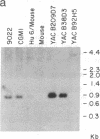
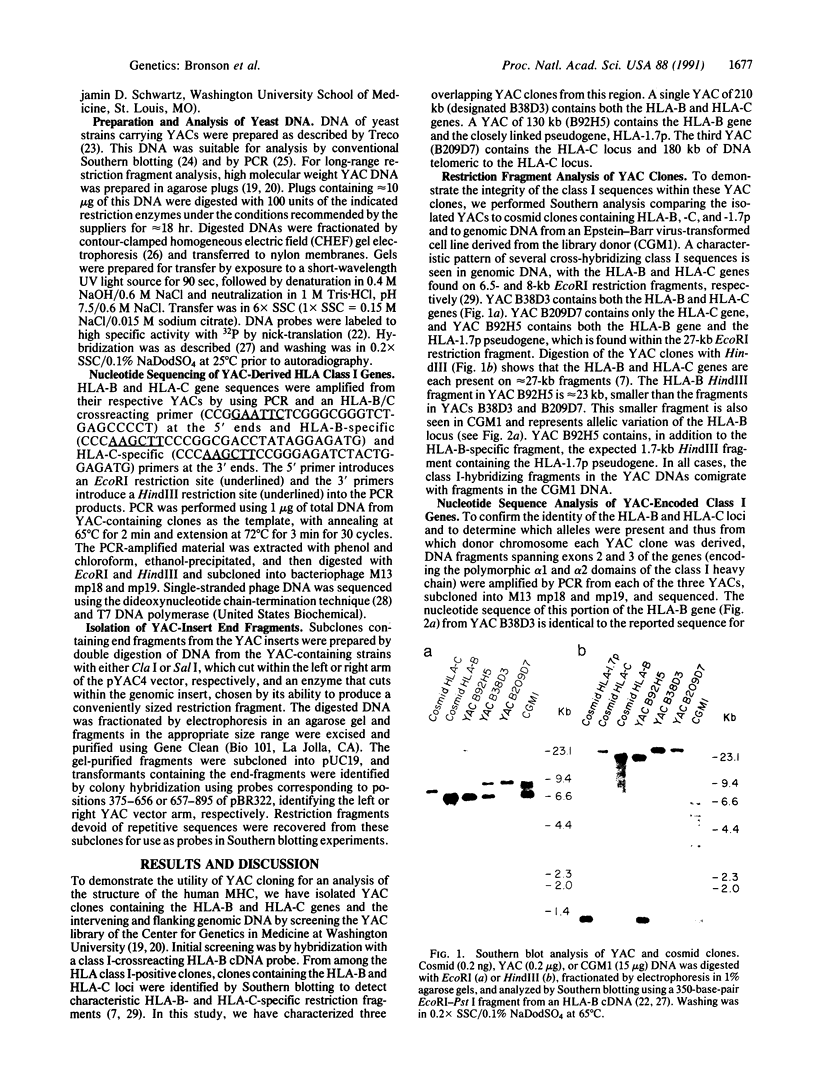
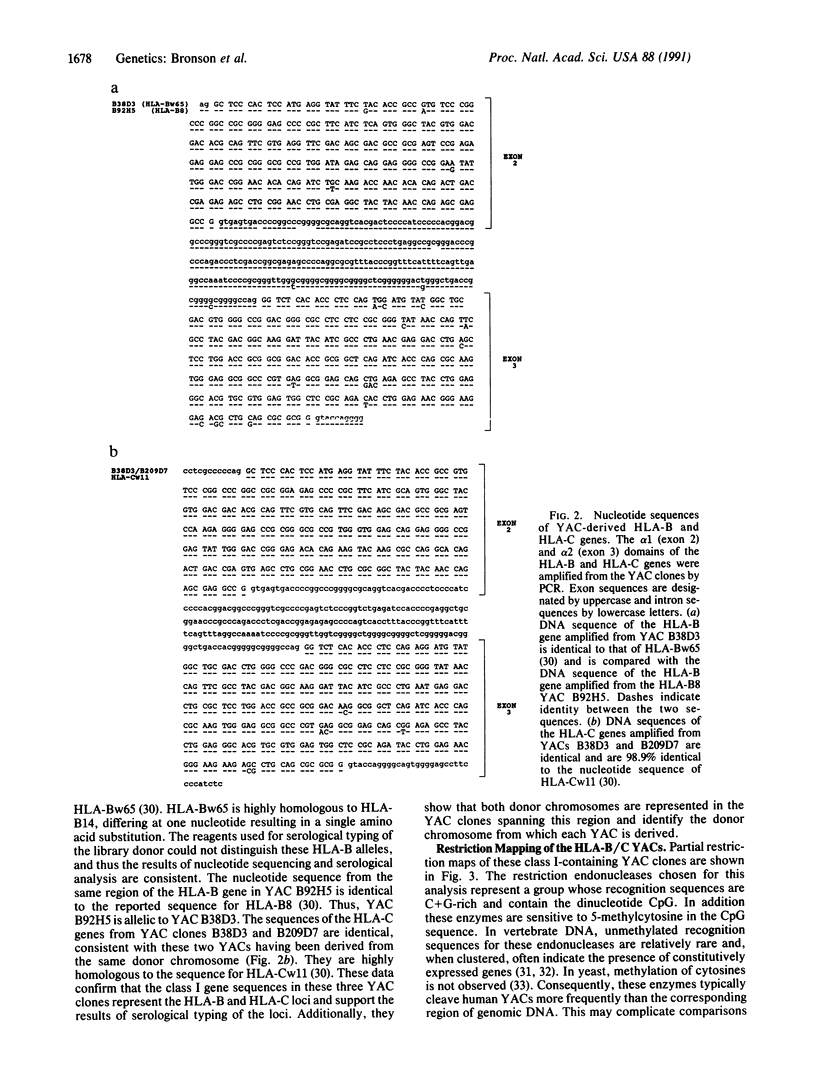
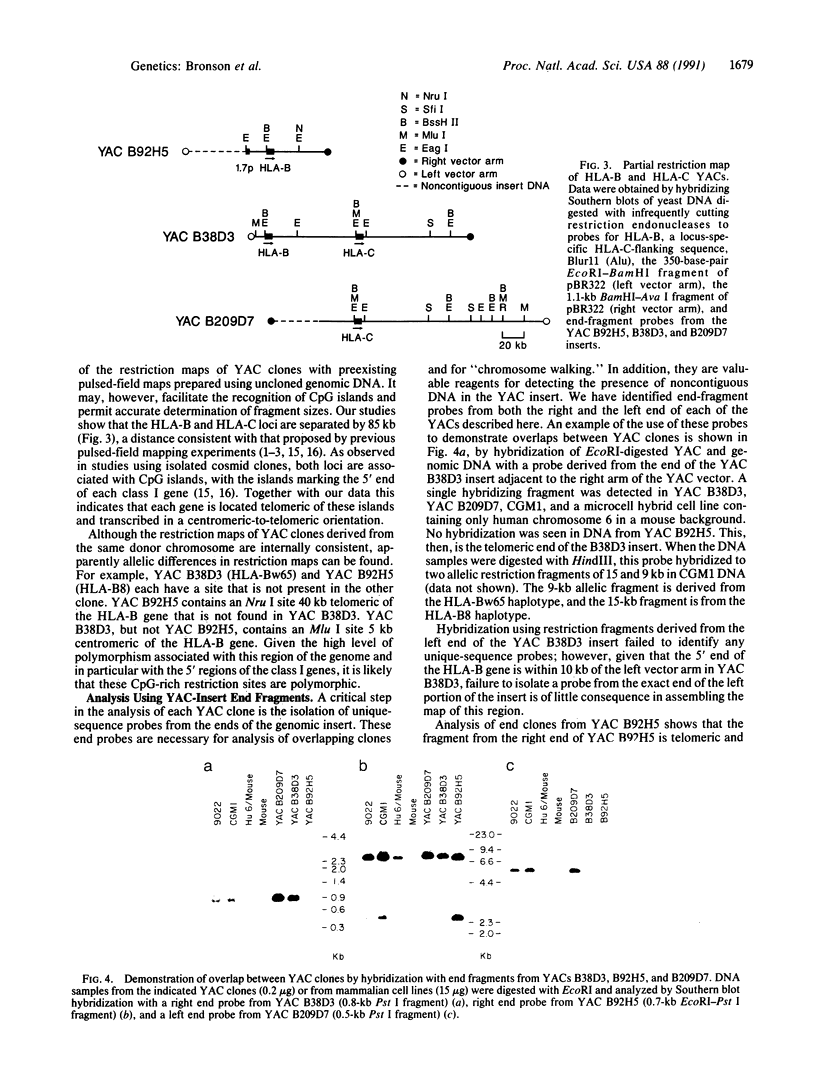
A 290-kilobase-pair chromosomal segment containing the genes encoding the human class I major histocompatibility complex molecules HLA-B and HLA-C as well as a class I pseudogene has been isolated on three overlapping yeast artificial chromosome (YAC) clones. One YAC clone contains both the HLA-B and HLA-C genes. These loci are located approximately 85 kilobase pairs apart, each in close association with a CpG island. Southern blotting and nucleotide sequencing showed no evidence of alteration of the structure of the cloned DNA in the YACs. End fragments from the YAC inserts have been isolated and used to confirm the overlaps between clones. These fragments can also serve as polymorphic markers for structural analysis of the major histocompatibility complex. Our data show that YAC cloning offers an attractive alternative for analysis of the structures of large gene complexes such as HLA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. R., Bird A. P. Long-range restriction site mapping of mammalian genomic DNA. 1986 Jul 31-Aug 6Nature. 322(6078):477–481. doi: 10.1038/322477a0. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Katzman P., Alicot E. M., Koller B. H., Geraghty D. E., Orr H. T., Strominger J. L., Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8535–8539. doi: 10.1073/pnas.84.23.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimini G., Pontarotti P., Nguyen C., Toubert A., Boretto J., Jordan B. R. The chromosome region containing the highly polymorphic HLA class I genes displays limited large scale variability in the human population. EMBO J. 1988 Feb;7(2):395–400. doi: 10.1002/j.1460-2075.1988.tb02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin H. L., Denny D. W., Jr, Weissman S. M., McDevitt H. O. HLA-B locus polymorphism: studies with a specific hybridization probe. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8614–8618. doi: 10.1073/pnas.82.24.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham I., Sargent C. A., Trowsdale J., Campbell R. D. Molecular mapping of the human major histocompatibility complex by pulsed-field gel electrophoresis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7237–7241. doi: 10.1073/pnas.84.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Wei X. H., Orr H. T., Koller B. H. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J Exp Med. 1990 Jan 1;171(1):1–18. doi: 10.1084/jem.171.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D. E., DeMars R., Duvick L., Rich S. S., Orr H. T. Chromosomal organization of the human major histocompatibility complex class I gene family. J Exp Med. 1989 Feb 1;169(2):469–480. doi: 10.1084/jem.169.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D. E., Shimizu Y., DeMars R., Orr H. T. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J Immunol. 1988 Aug 1;141(3):897–904. [PubMed] [Google Scholar]
- Koller B. H., Geraghty D., Orr H. T., Shimizu Y., DeMars R. Organization of the human class I major histocompatibility complex genes. Immunol Res. 1987;6(1-2):1–10. doi: 10.1007/BF02918100. [DOI] [PubMed] [Google Scholar]
- Kovats S., Main E. K., Librach C., Stubblebine M., Fisher S. J., DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990 Apr 13;248(4952):220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Lawrance S. K., Smith C. L., Srivastava R., Cantor C. R., Weissman S. M. Megabase-scale mapping of the HLA gene complex by pulsed field gel electrophoresis. Science. 1987 Mar 13;235(4794):1387–1390. doi: 10.1126/science.3029868. [DOI] [PubMed] [Google Scholar]
- Lindsay S., Bird A. P. Use of restriction enzymes to detect potential gene sequences in mammalian DNA. 1987 May 28-Jun 3Nature. 327(6120):336–338. doi: 10.1038/327336a0. [DOI] [PubMed] [Google Scholar]
- Little R. D., Porta G., Carle G. F., Schlessinger D., D'Urso M. Yeast artificial chromosomes with 200- to 800-kilobase inserts of human DNA containing HLA, V kappa, 5S, and Xq24-Xq28 sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1598–1602. doi: 10.1073/pnas.86.5.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Braciale V. L., Braciale T. J. Distinguishable pathways of viral antigen presentation to T lymphocytes. Immunol Res. 1986;5(4):294–304. doi: 10.1007/BF02935502. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Mapping of class I DNA sequences within the human major histocompatibility complex. Immunogenetics. 1983;18(5):489–502. doi: 10.1007/BF00364390. [DOI] [PubMed] [Google Scholar]
- Parham P., Lawlor D. A., Lomen C. E., Ennis P. D. Diversity and diversification of HLA-A,B,C alleles. J Immunol. 1989 Jun 1;142(11):3937–3950. [PubMed] [Google Scholar]
- Pontarotti P., Chimini G., Nguyen C., Boretto J., Jordan B. R. CpG islands and HTF islands in the HLA class I region: investigation of the methylation status of class I genes leads to precise physical mapping of the HLA-B and -C genes. Nucleic Acids Res. 1988 Jul 25;16(14B):6767–6778. doi: 10.1093/nar/16.14.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt J. H., Davie J. R., Swinton D., Hattman S. 5-Methylcytosine is not detectable in Saccharomyces cerevisiae DNA. Mol Cell Biol. 1984 May;4(5):985–988. doi: 10.1128/mcb.4.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Chorney M. J., Lawrance S. K., Pan J., Smith Z., Smith C. L., Weissman S. M. Structure, expression, and molecular mapping of a divergent member of the class I HLA gene family. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4224–4228. doi: 10.1073/pnas.84.12.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]