Abstract
Ethnopharmacological relevance
Lymphatic system plays an important role in maintaining the fluid homeostasis and normal immune responses, anatomic or functional obstruction of which leads to lymphedema, and treatments for therapeutic lymphangiogenesis are efficiency for secondary lymphedema. Total saponins of panaxnotoginseng (PNS) are a mixture isolated from Panaxnotoginseng (Burkill) F.H. Chen, which has been used as traditional Chinese medicine in China for treatment of cardio- and cerebro-vascular diseases. The aim of this study was to determine the effect and mechanism of PNS on lymphangiogenesis.
Methods
The Tg (fli1:egfp; gata1:dsred) transgenic zebrafish embryos were treated with different concentrations of PNS (10, 50, 100μM) for 48 hours with or without the 6 hours pretreatment of the 30μM Vascular endothelial growth factors receptor (VEGFR)-3 kinase inhibitor, followed with morphological observation and lympangiogenesis of thoracic duct assessment. The effect of PNS on cell viability, migration, tube formation and Vascular endothelial growth factors (VEGF)-C mRNA and protein expression of lymphatic endothelial cells (LECs) were determined. The role of phosphatidylinositol-3 (PI-3)-kinase (PI3K), extracellular signal-regulated kinase (ERK)1/2 pathways, c-Jun N-terminal kinase (JNK) and P38 mitogen activated protein kinases (MAPK) signaling in PNS-induced VEGF-C expression of LECs by using pharmacological agents to block each signal.
Results
PNS promotes lymphangiogenesis of thoracic duct in zebrafish with or without VEGFR3 Kinase inhibitor pre-impairment. PNS promotes proliferation, migration and tube formation of LECs. The tube formation induced by PNS could be blocked by VEGFR3 Kinase inhibitor. PNS induce VEGF-C expression of LEC, which could be blocked by ERK1/2, PI3K and P38MAPK signaling inhibitors.
Conclusion
PNS activates lymphangiogenesis both in vivo and in vitro by up-regulating VEGF-C expression and activation of ERK1/2, PI3K and P38MAPK signaling. These findings provide a novel insight into the role of PNS in lymphangiogenesis and suggest that it might be an attractive and suitable therapeutic agent for treating secondary lymphedema or other lymphatic system impairment related disease.
Keywords: Lymphangiogenesis, Saponins of panaxnotoginseng, Vascular endothelial growth factors C, Lymphedema
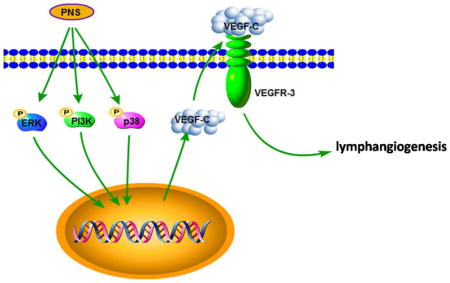
Graphical Abstract
1. Introduction
Lymphatic system plays an important role in maintaining the fluid homeostasis and normal immune responses, which transport extravasated fluid and macromolecules from peripheral tissues, filter lymphatic fluid and remove foreign material. Anatomic or functional obstruction of the lymphatic system leads to the progressive accumulation of protein-rich fluid in the interstitial spaces, which is named as lymphedema (de Almeida and Freedman, 1999; Szuba and Rockson, 1998). The condition can be inherited or resulting from trauma, surgery, radiotherapy, or parasitic infection (secondary lymphedema). In industrialized countries, cancer treatment is the leading cause of secondary lymphedema (DiSipio et al., 2013). Furthermore, 10%–30% of patients with malignant tumor develop lymphedema (Beesley et al., 2007; Cormier et al., 2010; Ohba et al., 2011; Tada et al., 2009). Despite significant progress in surgical and conservative techniques, therapeutic options for the treatment of lymphedema are limited (Ko et al., 1998; Saito et al., 2013; Szuba and Rockson, 1998). Although rarely lethal, lymphedema is a disfiguring and disabling condition, which reduces the quality of life (Girgis et al., 2011). There is no cure for lymphedema currently (Ostby and Armer, 2015). Several preclinical experiments demonstrated that treatments for therapeutic lymphangiogenesis are efficiency for secondary lymphedema (Cheung et al., 2006; Yoshida et al., 2015; Zhou et al., 2011). Enhancement of lymphangiogenesis in situations of lymph accumulation is considered as a promising strategy.
Lymphangiogenesis can be stimulated by various cytokines, but vascular endothelial growth factors (VEGF)-C is the most important and specific lymphatic vessel growth factors known. VEGF-C binds VEGF receptor-3 (VEGFR-3), which is expressed on lymphatic endothelial cells (LECs), and promotes mainly lymphangiogenesis (Khadim et al., 2015). It was reported that VEGF-C-deficient mice are unable to develop a functional lymphatic system (Karkkainen et al., 2004), transgenic expression of soluble VEGFR-3 results in inhibition of lymphangiogenesis and pronounced lymphedema (Makinen et al., 2001), and gene transfer of VEGF-C effectively augments lymphangiogenesis and ameliorates lymphedema in animal models (Yoon et al., 2003; Zhou et al., 2011). Therefore, VEGF-C is a valuable therapy target for lymphangiogenesis and lymphedema.
Panaxnotoginseng (Burkill) F.H. Chen, named as Sanqi in China, has been used as traditional Chinese medicine in China, for treatment of cardio- and cerebro-vascular diseases, such as Intracranial/intracerebral hemorrhage of stroke, ischemic heart and brain diseases, inflammation, trauma, and internal and external bleeding due to injury for thousands of years (Chen, 1987). Total saponins of panaxnotoginseng (PNS), a mixture isolated from Panaxnotoginseng, whose major components include ginsenosides and notoginsenosides (Yao et al., 2011), were the biologically active constituents responsible for the therapeutic action of this medicine (Park et al., 2009; Zhang et al., 2007). Previously, we screened several extracts from herbs for their ability to promote lymphangiogenesis in zebrafish, and found that PNS has such ability. Thus, the aim of our current study is to investigate that whether and why PNS could promote lymphangiogenesis.
2. Materials and Methods
2.1. Chemicals
Noto-G™ extracts from the root of Panaxnotoginseng (Burkill) F.H. Chen were supplied by National Institutes for Food and Drug Control (NIFDC) (Lot number: 110870-201002). Notoginseng was extracted from the root of the plant using ethanol and standardized to contain notoginsenoside R1 6.9%, ginsenosides Rg1 28%, ginsenoside Re 3.8%, ginsenoside Rb1 29.7%, ginsenoside Rd 7.3% of the whole extract, respectively. The quantification of total saponins of panaxnotoginseng in the notoginseng extract was determined by high-performance liquid chromatography analysis by NIFDC (Supplementary figure 1). The extract was dissolved in embryo water for zebrafish or culture grade DMSO (Sigma–Aldrich, St. Louis, MO) for murine LEC and subsequently sterile-filtered through a 0.22μM Millipore membrane. PD98059 (cat. #S1177), Wortmannin (cat. #S2758), SB203580 (cat. # S1076) and SP600125 (cat. # S1460) were purchased from selleckchem.
2.2. Animals
The transgenic zebrafish line (fli1:egfp; gata1:dsred), which expresses eGFP at endothelial cells and dsred at blood cells (Omae et al., 2013; Serbanovic-Canic et al., 2011), was kindly provided by Simon Ming Yuen Lee (Institute of Chinese Medical Sciences, Macau SAR.). It was maintained in zebrafish room of Longhua hospital, a controlled environment according to the description in the Zebrafish Handbook (Westerfield, 1995). Embryos were generated by natural pair wise mating, and were raised at 28.5°C in embryo water (13.7 mM NaCl, 540 μM KCl, pH 7.4, 25 μM Na2HPO4, 44 μM KH2PO4, 300 μM CaCl2, 100 μM MgSO4, 420 μM NaHCO3, pH 7.4). All animal experiments were conducted according to the ethical guidelines of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
2.3. Drug administration
At 48 hours post fecundation (hpf), healthy zebrafish embryos were picked out and were distributed into a 12-well microplate with 10 fishes per well. Following this, the embryos were treated with different concentrations (10, 50, 100μM) of PNS (National Institutes for Food and Drug Control, CAS No: 88105-29-7, Lot No: 110870-201002, purity>98.5, 80mg/kg) or 0.2%DMSO as a vehicle control for 48 hours with or without the pretreatment of the 30 μM VEGFR-3 kinase inhibitor (MAZ51, Calbiochem, La Jolla, CA, cat. #676492, lot. #D00152431) for 6 hours. Each group had more than 9–10 fishes.
2.4. Morphological observation and quantification of lymphatic phenotype of zebrafish
Zebrafish embryos were anesthetized, plated and oriented laterally on a coverslip after treatment. Image acquisition from zebrafish embryos was achieved using a Confocal Fluorescence Imaging Microscope (Leica TCS-SP5, Germany) and merged Z-stack images by visualization 3D projection. The length of developing lymphatic thoracic ducts (TD) of zebrafish embryos was individually counted from the trunk region spaning 10 somites, from somite boundary 7 or 8 to 18, on merged Z-stacked confocal images. Experiments were performed in triplicate. Quantification graphs were generated by Leica Application Suite Advanced Fluorescence 2.3.6 build 5381 program.
2.5. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)
A murine LEC cell line established from Freund’s adjuvant-induced benign lymphangiomas (Sironi et al., 2006) (Cells were provided by Dr. S. Ran from the University of Illinois, USA). Proliferation of LECs was examined by MTT assay (MTT based Cell Growth Determination Kit, Sigma) according to the manufacturer’s instructions. In brief, cells were seeded at a density of 1 × 104 cells/well in 96-well plates in quadruple. Twenty four hours later, cells were treated with different concentrations of PNS (0, 10, 50, 100μM) or VEGF-C (Sigma, cat. #SRP6020, lot. #MMS1713061, 0.34nM) for another 72 hours. Then cells were incubated with 20 μl of MTT solution at 37°C for 4 hours, followed by 200 μl of MTT solvent to terminate the reaction. The plates were read at 570 nm using a benchmark microplate reader (BioRad).
2.6. Wound healing assay
Murine LECs (2 ×105 cells/well) were cultured in 12-well plate. A confluent monolayer of Cells was wounded with a yellow pipet tip and the media was replaced by culture medium containing different concentration of PNS (0, 10, 50, 100μM) or VEGF-C (0.34nM). Closure of the wound was monitored and representative photomicrographs were taken at 24 hours after PNS or VEGF-C treatment. A reduction in the scraped area indicates a sign of migration. The migration length= scraped distance at 0 hour - scraped distance at 24 hours.
2.7. Tube formation assay
Reduced growth factor basement membrane Matrix (Gibco Life Technologies Corporation, Grand Island, NY, USA, Cat#A14132-02, 50μl) was pipetted into a 96-well plate and polymerized for 30 min at 37 °C. LECs were plated onto the layer of Matrigel at a density of 3×104 cells/well, followed by the addition of incubated with PNS (0, 10, 50, 100μM) or VEGF-C (0.34nM), or PNS (0, 100 μM) with VEGFR3 Kinase inhibitor (250nM) or VEGF-C (0.34nM) with VEGFR3 Kinase inhibitor (250nM). After 6 hours, tube formation was assayed by a microscope (Leica TCS-SP5, Germany).
2.8. Real time PCR
The total RNA of LECs was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA), and reverse transcribed to Complimentary DNA using Prime Script RT reagent Kit (Takara Bio, Dalian, CHI). Quantitative PCR amplification was performed in triplicate assays with gene-specific primers (primer sequence was listed in table 1) and iQ SYBR Green supermix (Takara Bio, Dalian, CHI) in BIO-RAD CFX96 touch q-PCR machine. The relative abundance of each gene was calculated by subtracting the CT value of each sample for an individual gene from the corresponding CT value of β-actin (ΔCT), and ΔΔCT was obtained by subtracting the ΔCT of the reference point. These values were then raised to the power two (2ΔΔCT) to yield the fold-expression relative to the reference point.
Table 1.
Sequences of Primers Used in Real-Time Polymerase Chain Reactions
| Genes | Sequences of primers | GenBank accession number | Annealing Tm(°C) | Product size (bp) |
|---|---|---|---|---|
| VEGF-C | F:5′ GCAATGCATGAACACCAGCA 3′ R:5′ AGTTTAGACATGCACCGGCA 3′ |
NM_005429.4 | 60 | 132 |
| NRP | F:5′ AAAGGTGAAGGCAGACGGAC 3′ R:5′ TGGGATCTCGTATAATGTCTTTGTG 3′ |
NM_030869.3 | 60 | 126 |
| FOXC2 | F:5′ GAAGGACGTGCCCAAGGATA 3′ R:5′ CGCTCTTGACCACCACTTTCT 3′ |
NM_005251.2 | 60 | 137 |
| Prox1 | F: 5′ AGCGCAATGAAGGGCTATCAC 3′ R: 5′ TGGGATGTGATGCATCTGTTG 3′ |
NM_001107201.1 | 58 | 133 |
| VEGFR3 | F:5′ CATTGGGGGCCTCTCCATAC 3′ R:5′ CAACTCTGCATGATGTGGCG 3′ |
XM_011534484.1 | 60 | 127 |
| β-actin | F: 5′ TTGCTGACAGGATGCAGAAGGAGA 3′ R: 5′ ACTCCTGCTTGCTGATCCACATCT 3′ |
NM_031144.3 | 60 | 159 |
Note: VEGF-C, Vascular endothelial growth factors-C; VEGFR3, Vascular endothelial growth factors receptor-3; NRP, Neuropilin-2; FOXC2, Forkhead transcription; Prox1, Prospero-related homeobox 1; F, Forward primer; R: Reverse primer
2.9. Western blot analysis
Whole cell lysates were harvested and samples (30μg protein/lane) were fractionated by SDS–PAGE and transferred to nitrocellulose membranes. Immunoblotting was carried out using antibodies to VEGF-C (Abcam, Cambridge, UK, Cat#ab191274) at dilution 1:100, and β-actin (Sigma-Aldrich Corp, cat. #A2228; lot. #052M4816V) at dilution 1:5000. Bands were visualized using ECL chemiluminescence (Amersham). All experiments were done in triplicate.
2.10. Statistical analysis
Data are presented as means ± SD. Statistical analyses were performed with SPSS 16.0 software. For more than two group comparisons, one-way ANOVA test followed by Bonferroni post-test was applied. When the value of P <0.05, the differences was considered statistically significant.
3. Results
3.1. PNS promotes lymphangiogenesis in zebrafish with or without VEGFR3 Kinase inhibitor injury
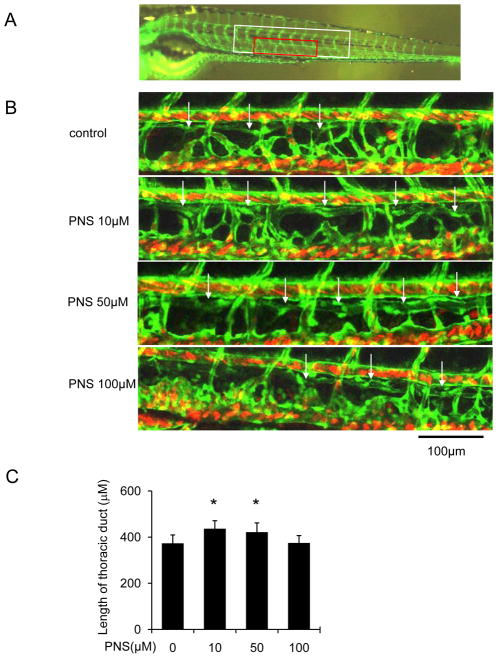
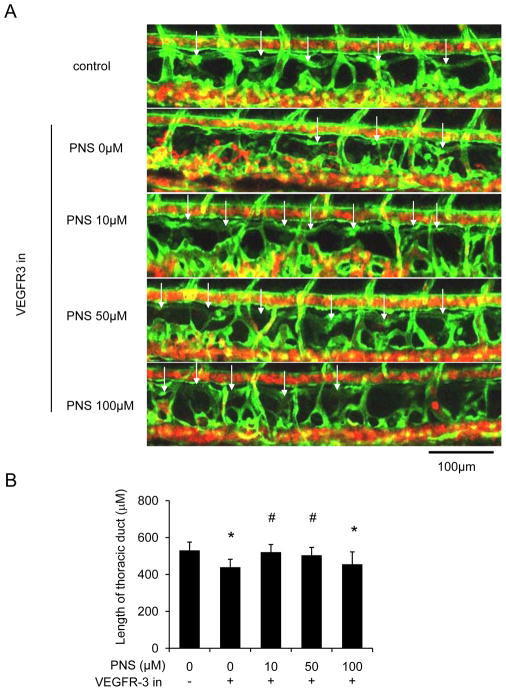
In order to determine the effect of PNS on lymphatic vessel, we used zeberafish screening system. At 48hpf, the zebrafish were treated with PNS (0, 10, 50, 100μM) for 48 hours, and we found that 10 and 50μM PNS significantly enhanced TD formation in normal situation (Figure 1A and B). Then, we added VEGFR-3 specific inhibitor (MAZ51, 30μM) for 6 hours at 48hpf, and then changed the medium with different concentrations of PNS (0, 10, 50, 100μM) for another 48 hours, and found that MAZ51 severely impaired the length of TD formation, but PNS (10 and 50μM) treatment significantly increased the length of TD formation in concentration dependent manner (Figure 2A and B).
Fig. 1.
The lymphatic thoracic duct formation of zebrafish was increased by PNS in concentration dependent manner. The 48 hpf zeberafish (fli1:egfp; gata1:dsred) was treated with different concentrations of PNS (10, 50, 100μM) for 48 hours. Embryos treated with 0.2% DMSO served as a vehicle control. (A) Confocal image of the 96 hpf. zebrafish (fli1:egfp) vascular system. White boxed region indicates ten segment of thoracic duct length for quantitation in C; Red boxed region shows approximate location of regions imaged in B. (B) Representative confocal images show that PNS increased lymphatic thoracic duct formation of zeberafish, white arrow indicates lymphatic thoracic duct, and white star indicates lack of lymphatic vessel. Scale bars, 100 μm. (C) Quantitation of the length of lymphatic thoracic duct. Values are mean ± SD of 9–11 zeberafishes. *P<0.05 vs. vehicle control group.
Fig. 2.
Impaired lymphatic thoracic duct formation induced by VEGFR-3 kinase inhibitor (MAZ51) was rescued by PNS in concentration dependent manner. The 48 hpf zeberafish (fli1:egfp; gata1:dsred) was treated with 30μM MAZ51 for 6 hours and then changed to be treated with different concentrations of PNS (10,50, 100 μM) for 48 hours. Embryos treated with 0.2% DMSO served as a vehicle control. (A) Representative confocal images show that PNS increased lymphatic thoracic duct formation of zeberafish, white arrow indicates lymphatic thoracic duct, and white star indicates lack of lymphatic vessel. Scale bars, 100 μm. (B) Quantitation of the length of lymphatic thoracic duct. Values are mean ± SD of 9–11 zeberafishes. *P<0.05 vs. control group; # P<0.05 versus MAZ51 treated group.
3.2. PNS promotes proliferation, migration and tube formation of LECs
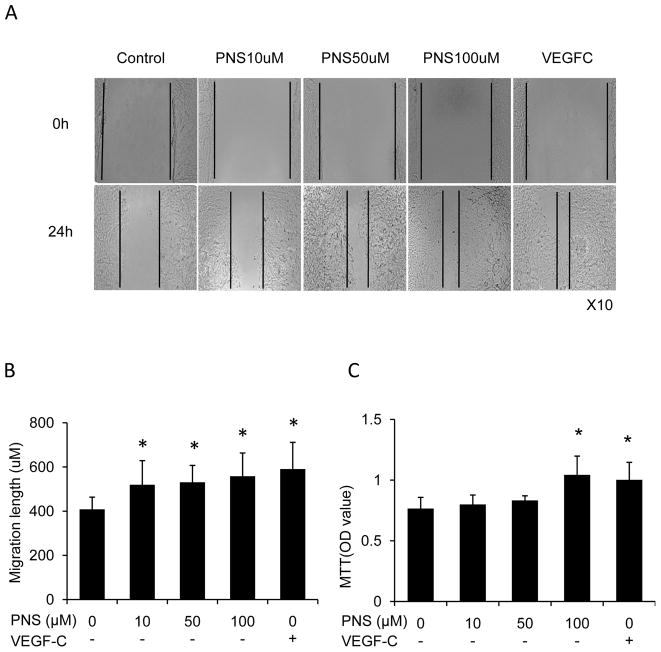
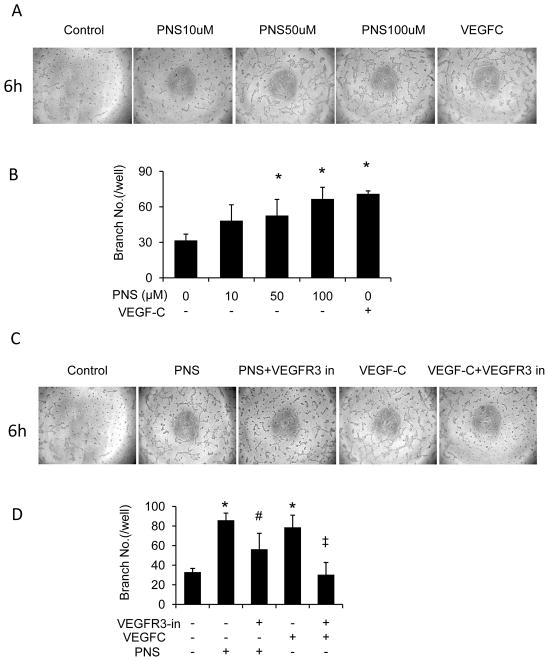
To determine whether PNS could regulate the lymphangiogenesis of LECs including cell growth, migration and tube formation, we performed MTT, wound healing and tube formation assay. We found that PNS significantly stimulated LECs migration (Figure 3A and B) in concentration dependent manner. 100μM PNS treatment for 72 hours significantly increased cell growth of LECs (Figure 3C), which is comparable to that of VEGF-C (0.34nM) treated cells. In addition, PNS significantly induced tube formation (Figure 4A and B) in concentration dependent manner. And the effect of PNS on LECs migration and tube formation is comparable to that of VEGF-C (0.34nM) treated cells.
Fig. 3.
PNS increased proliferation and cell migration of lymphatic endothelial cells (LECs). VEGF-C was considered as positive control. The group treated with PBS was considered as negative control. (A) Cell migration was assessed by wound healing assay. (B) Quantitation of migration length. Values are mean ± SD of 3 wells/treatment. *P<0.05 vs. control group. (C) LECs was treated with different concentrations of PNS (10, 50, 100μM) and VEGF-C (0.34nM) for 72 hours. Cell growth was determined by MTT assay. The values are the mean + SD of 4 wells. *P<0.05 vs. control group.
Fig. 4.
PNS induced tube formation of LECs, which was blocked by VEGFR3 Kinase inhibitor. (A) LECs cultured on 3-dimensional Matrigel in treatment of PNS (10, 50, and 100 μM) or VEGF-C (0.34nM). Cells receiving 0.1% DMSO served as vehicle control, and receiving VEGF-C served as positive control. (B) Number of branching points/well in different concentrations of PNS-treated LECs was calculated. Results are expressed as mean±SD (n=3 independent experiments), *P<0.05 versus control. (C) LECs on Matrigel in treatment of PNS (100 μM) or VEGF-C (0.34nM), with or without VEGFR3 Kinase inhibitor (250nM). (D) Number of branching points/well was expressed as mean±SD (n=3 independent experiments), *P<0.05 versus control group; # P<0.05 versus PNS group; ‡ P<0.05 versus VEGF-C group.
3.3. PNS promotes tube formation of LECs, which could be blocked by VEGFR3 Kinase inhibitor
To determine whether PNS affect lymphangiogenesis through VEGF-C/VEGFR3 signaling, we applied VEGFR3 Kinase inhibitor, and found that VEGFR3 Kinase inhibitor totally blocked VEGF-C induced tube formation of LECs, and partially inhibited the tube formation induced by PNS (Figure 4C and D). This result suggested that PNS induced lymphangiogenesis partially through VEGF-C/VEGFR3 signaling.
3.4. PNS induce VEGF-C expression of LECs, which could be blocked by ERK1/2, PI3K and P38MAPK signaling inhibitor
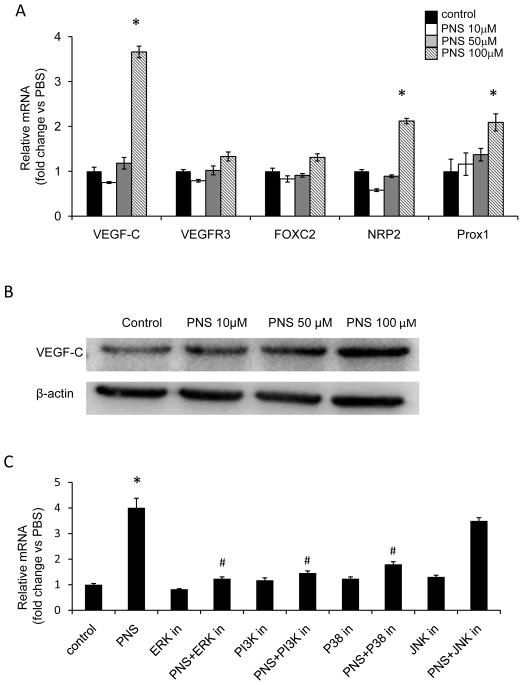
Next we examined several signaling involves lymphangiogenesis including VEGF-C and VEGFR3. And found that PNS induced 4 fold higher VEGF-C mRNA expression, but has no such significant effect on other factors, such as VEGFR3, Forkhead transcription factor FOXC2 (FOXC2) Neuropilin-2 (NRP2), or Prospero-related homeobox 1 (Prox 1) (Figure 5A). Furthermore, PNS increased VEGF-C protein level in concentration dependent manner (Figure 5B).
Fig. 5.
PNS stimulated VEGF-C expression of LECs, which was blocked by PI3K, ERK, and P38MAPK signaling inhibitor. (A) LECs were treated with different concentrations of PNS (10, 50, and 100 μM) for 24 hours, the mRNA expression of VEGF-C, VEGFR-3, FOXC2, NRP2 and Prox1 was analyzed by qPCR, Cells receiving 0.1% DMSO served as vehicle control. Results are represented as mean±SD (n = 3 independent experiments), * P<0.05 versus control. (B) The protein level of VEGF-C was assessed by westernblot. (C) LECs were incubated with or without PNS (100 μM) ± 25μM ERK inhibitor (PD98059), 50nM PI3K inhibitor (Wortmannin), 10μM P38MAPK inhibitor (SB203580), or 20μM JNK inhibitor (SP600125) for 24 hours. LECs treated with 0.1% DMSO served as a vehicle control. The mRNA expression of VEGF-C was examined by qPCR and the results are present as mean±SD (n = 3 independent experiments), * P<0.05 versus control group, and # P<0.05 versus PNS (100 μM) treated group.
To investigate the functional involvement of ERK1/2, PI3K, P38MAPK and JNK signaling, we treated LECs with 100 μM PNS plus or minus ERK inhibitor PD98059, PI3K inhibitor Wortmannin, P38MAPK inhibitor SB203580 or JNK inhibitor SP600125, and examined VEGF-C mRNA expression by qPCR. We found that the PNS-mediated increase in VEGF-C mRNA expression was significantly suppressed by ERK inhibitor, PI3K inhibitor and P38MAPK inhibitor, but not JNK inhibitor (Figure 5C). These data demonstrated that PNS induced VEGF-C expression through ERK, PI3Kand P38MAPK signaling.
4. Discussion
The aim of this study is to investigate the effect of PNS on lymphangiogenesis. Here, for the first time, we demonstrated that PNS promotes lymphangiogenesis in vivo and in vitro by stimulating VEGF-C expression of LECs. This is supported by the observations that 1) PNS promotes thoracic duct lymphangiogenesis of zeberafish with or without VEGFR3 Kinase inhibitor injury in vivo; 2) PNS induces tube formation of LECs in vitro, which can be blocked by VEGFR3 Kinase inhibitor; 3) PNS stimulates VEGF-C mRNA and protein expression of LECs, which can be blocked by ERK1/2 inhibitor, PI3K inhibitor and P38 MAPK inhibitor. These data are in line with the previous reports that VEGF-C stimulation by adenovirus recombinant VEGF-C (Szuba et al., 2002; Yan et al., 2011), VEGF-C protein injection (Zhou et al., 2011), a gelatin hydrogel containing VEGF-C (Hwang et al., 2011), or naked plasmid DNA encoding human VEGF-C (Yoon et al., 2003) augments lymphangiogenesis. Our results strongly support the notion that PNS is a new class of activating lymphangiogenic agents and that it may be explored for treatment of secondary lymphedema or other lymphatic system impairment related disease
In order to determine the effect of PNS on lymphatic vessel, we used zeberafish screening system. Zebrafish has been widely used in drug screening, assessment of efficacy and toxicity of any types of drugs, including multi-ingredient drugs, herbs and exacts (Tian LL, 2015). In current study, it is the first time for us to use zebrafish to determine the effect of PNS on lymphatic vessel formation. The lymphatic thoracic duct fully developed at 5 days after fertilization (Luo et al., 2011), thus we treated the 48hdf zebrafish, when the lymphatic thoracic duct was not fully developed, with PNS or VEGFR3 Kinase inhibitor. TG (gata1:DsRed; fli1:EGFP) zebrafish was used in this study, because its blood flow is visible in red (gata1:DsRed), its lymphatic and blood vessels is visible in green, which help us identify the lymphatic vessel (only green vessel without red cells) from blood vessel (red blood cells in the green vessel). By using zebrafish, we found that PNS could accelerate lymphangiogenesis at the thoracic duct of zebrafish no matter with or without VEGFR3 Kinase inhibitor impairment. These results indicated that PNS has good therapeutic effect on lymphatic vessel in vivo.
In our study, PNS induced LECs tube formation could not be totally blocked by VEGFR3 Kinase inhibitor, even though same concentration of VEGFR3 Kinase inhibitor abolished tube formation stimulated by VEGF-C. This data suggested that there might be other signaling involves lymphangiogenesis which could be affected by PNS. Besides of VEGF-C, FOXC2 and Prox1, NRP2 involves lymphangiogenesis. It was reported that FOXC2 is now recognized as a novel regulator of lymphatic vascular formation and remodeling, which controls later steps of lymphatic vascular development and establishing a collecting lymphatic vessel by regulating expression of PDGF-beta, Delta-like 4 (Dll4) and angiopoietin (Ang)-2(Hokari et al., 2008; Sasahira et al., 2014; Wu and Liu, 2011). Prox1 involves the first lymphatic sprouts and Prox1 deletion leads to a complete absence of the lymphatic vessel (Wigle et al., 2002; Wigle and Oliver, 1999). NRP2 has been suggested in promoting lymphangiogenesis via acting as an obligate co-receptor of VEGFR3 cooperatively enhancing the activity of VEGF-C (Ou et al., 2015; Zhang et al., 2015a). Therefore, we tested mRNA expression of FOXC2, Prox1, NRP2, VEGFR3 and VEGF-C. We found that except 4 fold higher mRNA expression of VEGF-C after treatment with PNS, NRP2 and Prox1 expression is about 2 fold higher after treatment with 100μM PNS, while FOXC2 and VEGFR3 are slightly increased. Those results suggested that there might be other signaling involves the mechanisms that PNS induced lymphangiogenesis, but PNS augments VEGF-C expression should be the major point.
It was reported that ERK1/2 (Hu et al., 2013; Yu et al., 2014; Zhang et al., 2015c), PI3K/AKT (Xiang et al., 2012; Zhang et al., 2012; Zhu et al., 2011), JNK (Naruishi et al., 2003), and P38MAPK (Tsai et al., 2003) pathway involved VEGF-C expression. In current study, PNS up-regulate VEGF-C expression of LECs through ERK, PI3K and P38MAPK signaling pathway. Our results are consistent with previous researches on other cell types. Previously, it was reported that PNS promote wound repair of anterior cruciate ligament through phosphorylation of PI3K, AKT and ERK (Yu et al., 2015), promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK (Linares and Gisbert, 2011), and activate PI3K/Akt pathway in cardiomyocytes (Chen et al., 2011). In addition, Its effective components Notoginsenoside R1-mediated neuroprotection through estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways (Meng et al., 2014), while Notoginsenoside Ft1 promotes fibroblast proliferation via PI3K/Akt/mTOR signaling pathway (Zhang et al., 2015b). Those above results suggested that PNS stimulate ERK, PI3K and P38MAPK signaling pathway to exert biological activity.
Previous reports demonstrated that PNS promotes angiogenesis of human umbilical vein endothelial cells and zebrafish (Hong et al., 2009). Its effective components Ginsenoside Rg1, Re, and Rb1, modulate angiogenesis, with a potential to be developed as angiotherapeutic agents (Cheung et al., 2011; Kawahira et al., 2008; Kimura et al., 2006; Leung et al., 2011; Yue et al., 2005). Rg1 stimulates hypoxia-induciblefactor-1α (HIF-1α) and induces VEGF expression (Cheung et al., 2011; Leung et al., 2011). Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways (Shen et al., 2012). Combined previous and our study, we found that PNS promotes both lymphangiogenesis and angiogenesis. These properties are expected to be useful for modulating those phenomena in diseases. In particular PNS could be applied to treat impaired angiogenesis and lymphangiogenesis of diabetes mellitus (Kononenkov et al., 2014; Lee et al., 2007; Liu et al., 2015; Uzayisenga et al., 2014; Wang et al., 2013).
In conclusion, our data suggest for the first time that PNS may perform activate-lymphangiogenic effects both in vivo and in vitro assay systems by up-regulating VEGF-C expression through ERK, PI3K and P38MAPK signaling pathway. These findings provide a novel insight into the role of PNS in lymphangiogenesis and suggest that it might be an attractive and suitable therapeutic agent for treating secondary lymphedema or other lymphatic system impairment related disease.
Supplementary Material
Acknowledgments
This work was sponsored by research grants from National Natural Science Foundation (81330085 to SQ, 81220108027 to WYJ, 81403417 to LQQ, 81403418 to XH), Special funding for the National Outstanding Doctoral Dissertation (201276 to LQQ), Ministry of Education, “Innovative Research Team”(IRT1270), NIH, USA (AR048697 and AR063650) and the lymphatic malformation institute, USA to XLP.
We are very grateful to Zhongyan Zhou, Longhua hospital affiliated to Shanghai University of traditional Chinese medicine, for his assistance on zebrafish culture.
Footnotes
Chemical compounds
Dimethyl sulfoxide (DMSO) (PubChem CID: 679); MAZ51, VEGF Receptor 3 Kinase inhibitor (PubChemCID:9839842); Ethanol (PubChem CID: 702); Chloroform (PubChemCID:6212); Methanol (PubChemCID:887); Acrylamide (PubChemCID:6579); Ammonium Persulfate (PubChemCID:62648); Glycine (PubChemCID:750); Sodium chloride (PubChemCID:5234); PD98059 (PubChemCID: 4713); Wortmannin (PubChemCID: 312145); SB203580 (PubChemCID: 176155); SP600125 (PubChemCID: 8515)
Author’s contributions
JLL performed most of the experiments, analyzed the data and participated in the manuscript draft. YC participated in zebrafish experiment and data analysis. LZ helped LEC culture and tube formation experiment. LPX helped with manuscript editing. HX helped real time PCR experiment. YJW and QS provided scientific input and helped with manuscript editing. QQL designed the study, and drafted and finalized the manuscript. All authors read and approved the final manuscript.
Competing Interests
None of the authors have any competing interests in the manuscript. And this manuscript/data, or parts thereof, has not been submitted or published elsewhere for publication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jinlong Li, Email: phjod@163.com.
Yan Chen, Email: 952026015@qq.com.
Li Zhang, Email: 15221152917@163.com.
Lianping Xing, Email: Lianping_xing@urmc.rochester.edu.
Hao Xu, Email: hoxu@163.com.
Yongjun Wang, Email: yjwang8888@126.com.
Qi Shi, Email: jzyjs200032@126.com.
References
- Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- Chen QS. Pharmacological studies on notoginseng saponins isolated from the fibrous root of Panax notoginseng. Zhong Yao Tong Bao. 1987;12:45–47. [PubMed] [Google Scholar]
- Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin Q, Zhu J, Mai L, Shan Z, Yu X, Yang M, Lin S. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. Journal of ethnopharmacology. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Cheung L, Han J, Beilhack A, Joshi S, Wilburn P, Dua A, An A, Rockson SG. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2006;20:363–370. doi: 10.2165/00063030-200620060-00007. [DOI] [PubMed] [Google Scholar]
- Cheung LW, Leung KW, Wong CK, Wong RN, Wong AS. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovascular research. 2011;89:419–425. doi: 10.1093/cvr/cvq300. [DOI] [PubMed] [Google Scholar]
- Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- de Almeida AB, Freedman DO. Epidemiology and immunopathology of bancroftian filariasis. Microbes and infection/Institut Pasteur. 1999;1:1015–1022. doi: 10.1016/s1286-4579(99)80519-x. [DOI] [PubMed] [Google Scholar]
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The Lancet. Oncology. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- Girgis A, Stacey F, Lee T, Black D, Kilbreath S. Priorities for women with lymphoedema after treatment for breast cancer: population based cohort study. BMJ. 2011;342:d3442. doi: 10.1136/bmj.d3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokari R, Kitagawa N, Watanabe C, Komoto S, Kurihara C, Okada Y, Kawaguchi A, Nagao S, Hibi T, Miura S. Changes in regulatory molecules for lymphangiogenesis in intestinal lymphangiectasia with enteric protein loss. Journal of gastroenterology and hepatology. 2008;23:e88–95. doi: 10.1111/j.1440-1746.2007.05225.x. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Wan JB, Zhang Y, Hu G, Lin HC, Seto SW, Kwan YW, Lin ZX, Wang YT, Lee SM. Angiogenic effect of saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytotherapy research: PTR. 2009;23:677–686. doi: 10.1002/ptr.2705. [DOI] [PubMed] [Google Scholar]
- Hu D, Fukuhara A, Miyata Y, Yokoyama C, Otsuki M, Kihara S, Shimomura I. Adiponectin regulates vascular endothelial growth factor-C expression in macrophages via Syk-ERK pathway. PloS one. 2013;8:e56071. doi: 10.1371/journal.pone.0056071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim IG, Lee JY, Piao S, Lee DS, Lee TS, Ra JC. Therapeutic lymphangiogenesis using stem cell and VEGF-C hydrogel. Biomaterials. 2011;32:4415–4423. doi: 10.1016/j.biomaterials.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature immunology. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kawahira K, Sumiyoshi M, Sakanaka M, Kimura Y. Effects of ginsenoside Rb1 at low doses on histamine, substance P, and monocyte chemoattractant protein 1 in the burn wound areas during the process of acute burn wound repair. Journal of ethnopharmacology. 2008;117:278–284. doi: 10.1016/j.jep.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Khadim MT, Ahmed SA, Khan FA, Ikram A, Shaikh SY. Evaluation of vascular endothelial growth factors A, C and D as indicators of lymphangiogenesis and angiogenesis in invasive and non-invasive urothelial carcinoma bladder. JPMA. The Journal of the Pakistan Medical Association. 2015;65:851–856. [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. British journal of pharmacology. 2006;148:860–870. doi: 10.1038/sj.bjp.0706794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DS, Lerner R, Klose G, Cosimi AB. Effective treatment of lymphedema of the extremities. Arch Surg. 1998;133:452–458. doi: 10.1001/archsurg.133.4.452. [DOI] [PubMed] [Google Scholar]
- Kononenkov VI, Klimontov VV, Kuznetsova IV. Impaired angiogenesis and lymphangiogenesis in diabetes mellitus. Arkhiv patologii. 2014;76:55–59. [PubMed] [Google Scholar]
- Lee WK, Kao ST, Liu IM, Cheng JT. Ginsenoside Rh2 is one of the active principles of Panax ginseng root to improve insulin sensitivity in fructose-rich chow-fed rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:347–354. doi: 10.1055/s-2007-976537. [DOI] [PubMed] [Google Scholar]
- Leung KW, Ng HM, Tang MK, Wong CC, Wong RN, Wong AS. Ginsenoside-Rg1 mediates a hypoxia-independent upregulation of hypoxia-inducible factor-1alpha to promote angiogenesis. Angiogenesis. 2011;14:515–522. doi: 10.1007/s10456-011-9235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares PM, Gisbert JP. Role of growth factors in the development of lymphangiogenesis driven by inflammatory bowel disease: a review. Inflammatory bowel diseases. 2011;17:1814–1821. doi: 10.1002/ibd.21554. [DOI] [PubMed] [Google Scholar]
- Liu T, Peng YF, Jia C, Yang BH, Tao X, Li J, Fang X. Ginsenoside Rg3 improves erectile function in streptozotocin-induced diabetic rats. The journal of sexual medicine. 2015;12:611–620. doi: 10.1111/jsm.12779. [DOI] [PubMed] [Google Scholar]
- Luo Y, Chen W, Zhou H, Liu L, Shen T, Alexander JS, Zheng S, Lu Y, Huang S. Cryptotanshinone inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3/ERK and small GTPase pathways. Cancer Prev Res (Phila) 2011;4:2083–2091. doi: 10.1158/1940-6207.CAPR-11-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature medicine. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Meng X, Sun G, Ye J, Xu H, Wang H, Sun X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free radical research. 2014;48:445–460. doi: 10.3109/10715762.2014.885117. [DOI] [PubMed] [Google Scholar]
- Naruishi K, Nishimura F, Yamada-Naruishi H, Omori K, Yamaguchi M, Takashiba S. C-jun N-terminal kinase (JNK) inhibitor, SP600125, blocks interleukin (IL)-6-induced vascular endothelial growth factor (VEGF) production: cyclosporine A partially mimics this inhibitory effect. Transplantation. 2003;76:1380–1382. doi: 10.1097/01.TP.0000085661.52980.95. [DOI] [PubMed] [Google Scholar]
- Ohba Y, Todo Y, Kobayashi N, Kaneuchi M, Watari H, Takeda M, Sudo S, Kudo M, Kato H, Sakuragi N. Risk factors for lower-limb lymphedema after surgery for cervical cancer. International journal of clinical oncology. 2011;16:238–243. doi: 10.1007/s10147-010-0171-5. [DOI] [PubMed] [Google Scholar]
- Omae M, Takada N, Yamamoto S, Nakajima H, Sato TN. Identification of inter-organ vascular network: vessels bridging between organs. PloS one. 2013;8:e65720. doi: 10.1371/journal.pone.0065720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby PL, Armer JM. Complexities of Adherence and Post-Cancer Lymphedema Management. Journal of personalized medicine. 2015;5:370–388. doi: 10.3390/jpm5040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou JJ, Wei X, Peng Y, Zha L, Zhou RB, Shi H, Zhou Q, Liang HJ. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer letters. 2015;358:200–209. doi: 10.1016/j.canlet.2014.12.046. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim SK, Shin IH, Kim HG, Choe JY. Effects of AIF on Knee Osteoarthritis Patients: Double-blind, Randomized Placebo-controlled Study. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2009;13:33–37. doi: 10.4196/kjpp.2009.13.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakagami H, Kaneda Y, Morishita R. Lymphedema and therapeutic lymphangiogenesis. BioMed research international. 2013;2013:804675. doi: 10.1155/2013/804675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahira T, Ueda N, Yamamoto K, Kurihara M, Matsushima S, Bhawal UK, Kirita T, Kuniyasu H. Prox1 and FOXC2 act as regulators of lymphangiogenesis and angiogenesis in oral squamous cell carcinoma. PloS one. 2014;9:e92534. doi: 10.1371/journal.pone.0092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbanovic-Canic J, Cvejic A, Soranzo N, Stemple DL, Ouwehand WH, Freson K. Silencing of RhoA nucleotide exchange factor, ARHGEF3, reveals its unexpected role in iron uptake. Blood. 2011;118:4967–4976. doi: 10.1182/blood-2011-02-337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y, Hou M, Wang Z. Notoginsenoside Ft1 promotes angiogenesis via HIF-1alpha mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochemical pharmacology. 2012;84:784–792. doi: 10.1016/j.bcp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Sironi M, Conti A, Bernasconi S, Fra AM, Pasqualini F, Nebuloni M, Lauri E, De Bortoli M, Mantovani A, Dejana E, Vecchi A. Generation and characterization of a mouse lymphatic endothelial cell line. Cell Tissue Res. 2006;325:91–100. doi: 10.1007/s00441-006-0171-y. [DOI] [PubMed] [Google Scholar]
- Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998;3:145–156. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- Tada H, Teramukai S, Fukushima M, Sasaki H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC cancer. 2009;9:47. doi: 10.1186/1471-2407-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian LL, ZG Application of zebra fishes in studies on traditional Chinese medicines. Zhongguo Zhong Yao Za Zhi. 2015 [PubMed] [Google Scholar]
- Tsai PW, Shiah SG, Lin MT, Wu CW, Kuo ML. Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa B signaling pathway. The Journal of biological chemistry. 2003;278:5750–5759. doi: 10.1074/jbc.M204863200. [DOI] [PubMed] [Google Scholar]
- Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytotherapy research: PTR. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- Wang J, Yin H, Huang Y, Guo C, Xia C, Liu Q, Zhang L. Panax Quinquefolius Saponin of Stem and Leaf Attenuates Intermittent High Glucose-Induced Oxidative Stress Injury in Cultured Human Umbilical Vein Endothelial Cells via PI3K/Akt/GSK-3 beta Pathway. Evidence-based complementary and alternative medicine: eCAM. 2013;2013:196283. doi: 10.1155/2013/196283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 3. Vol. 385 Eugene, OR: University of Oregon Press; 1995. The Zebrafish Book. [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. The EMBO journal. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Liu NF. FOXC2 transcription factor: a novel regulator of lymphangiogenesis. Lymphology. 2011;44:35–41. [PubMed] [Google Scholar]
- Xiang L, Xie G, Ou J, Wei X, Pan F, Liang H. The extra domain A of fibronectin increases VEGF-C expression in colorectal carcinoma involving the PI3K/AKT signaling pathway. PloS one. 2012;7:e35378. doi: 10.1371/journal.pone.0035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A, Avraham T, Zampell JC, Haviv YS, Weitman E, Mehrara BJ. Adipose-derived stem cells promote lymphangiogenesis in response to VEGF-C stimulation or TGF-beta1 inhibition. Future Oncol. 2011;7:1457–1473. doi: 10.2217/fon.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shi P, Shao Q, Fan X. Chemical fingerprinting and quantitative analysis of a Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chinese medicine. 2011;6:9. doi: 10.1186/1749-8546-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. The Journal of clinical investigation. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Hamuy R, Hamada Y, Yoshimoto H, Hirano A, Akita S. Adipose-derived stem cell transplantation for therapeutic lymphangiogenesis in a mouse secondary lymphedema model. Regenerative medicine. 2015;10:549–562. doi: 10.2217/rme.15.24. [DOI] [PubMed] [Google Scholar]
- Yu L, Xie J, Xin N, Wang Z. Panax notoginseng saponins promote wound repair of anterior cruciate ligament through phosphorylation of PI3K, AKT and ERK. International journal of clinical and experimental pathology. 2015;8:441–449. [PMC free article] [PubMed] [Google Scholar]
- Yu P, Tung JK, Simons M. Lymphatic fate specification: an ERK-controlled transcriptional program. Microvascular research. 2014;96:10–15. doi: 10.1016/j.mvr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue PY, Wong DY, Ha WY, Fung MC, Mak NK, Yeung HW, Leung HW, Chan K, Liu L, Fan TP, Wong RN. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg(1) in vivo and in vitro. Angiogenesis. 2005;8:205–216. doi: 10.1007/s10456-005-9000-2. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gao Z, Sun M, Li H, Fan H, Chen D, Zheng J. Prognostic significance of VEGF-C, semaphorin 3F, and neuropilin-2 expression in oral squamous cell carcinomas and their relationship with lymphangiogenesis. Journal of surgical oncology. 2015a;111:382–388. doi: 10.1002/jso.23842. [DOI] [PubMed] [Google Scholar]
- Zhang E, Gao B, Yang L, Wu X, Wang Z. Notoginsenoside Ft1 promotes fibroblast proliferation via PI3K/Akt/mTOR signaling pathway and benefits wound healing in genetically diabetic mice. The Journal of pharmacology and experimental therapeutics. 2015b doi: 10.1124/jpet.115.229369. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Wang JP, Wang HJ. Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine/Zhongguo Zhong xi yi jie he xue hui, Zhongguo Zhong yi yan jiu yuan zhu ban. 2007;27:589–592. [PubMed] [Google Scholar]
- Zhang P, Guo X, Li J, Yu S, Wang L, Jiang G, Yang D, Wei Z, Zhang N, Liu J, Sun Y. Immunoglobulin-like transcript 4 promotes tumor progression and metastasis and up-regulates VEGF-C expression via ERK signaling pathway in non-small cell lung cancer. Oncotarget. 2015c;6:13550–13563. doi: 10.18632/oncotarget.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ding L, Diao Z, Yan G, Sun H, Hu Y. CYR61 modulates the vascular endothelial growth factor C expression of decidual NK cells via PI3K/AKT pathway. Am J Reprod Immunol. 2012;67:216–223. doi: 10.1111/j.1600-0897.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang M, Hou C, Jin X, Wu X. Exogenous VEGF-C augments the efficacy of therapeutic lymphangiogenesis induced by allogenic bone marrow stromal cells in a rabbit model of limb secondary lymphedema. Japanese journal of clinical oncology. 2011;41:841–846. doi: 10.1093/jjco/hyr055. [DOI] [PubMed] [Google Scholar]
- Zhu C, Qi X, Chen Y, Sun B, Dai Y, Gu Y. PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in IGF-1-induced VEGF-C upregulation in breast cancer. Journal of cancer research and clinical oncology. 2011;137:1587–1594. doi: 10.1007/s00432-011-1049-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.