Abstract
Background
Zinc (Zn) and copper (Cu) may play a pivotal role in the pathogenesis of diabetes and diabetic complications by mediating oxidative stress. Both Zn deficiency and excess of Cu are associated with an increased risk of type 2 diabetes and cardiovascular disease. We aimed to investigate the relationships between serum Zn/Cu ratio and glycemic status, renal function, and metabolic parameters in patients with and without type 2 diabetes.
Methods
We conducted a cross-sectional study on 355 subjects (149 type 2 diabetic and 206 non-diabetic) in whom serum Zn and Cu levels were measured at the same time. Associations between serum Zn/Cu ratio and clinical data were evaluated using multiple regression analysis. We also evaluated associations between serum Zn/Cu ratio and the prevalence of type 2 diabetes and glycemic control by multivariate logistic regression analysis.
Results
Serum Zn/Cu ratio was positively associated with estimated glomerular filtration rate after adjustment for body mass index (BMI) (β = 0.137, p = 0.014). Plasma B-type natriuretic peptide levels were negatively associated with serum Zn/Cu ratio after adjustment for age, sex, and BMI (β = −0.258, p = 0.032). In patients with type 2 diabetes, serum Zn/Cu ratio was negatively associated with plasma HbA1c levels after adjustment for age, sex, and BMI (β = −0.239, p = 0.003). In addition, multivariate logistic regression analysis revealed that the highest quartile of serum Zn/Cu ratio was associated with a reduced risk of poor (HbA1c ≥ 7%) glycemic control (odds ratio = 0.382; 95% confidence interval, 0.165–0.884; p = 0.025) in patients with type 2 diabetes.
Conclusion
Serum Zn/Cu ratio was favorably associated with renal function in all subjects and glycemic control in patients with type 2 diabetes. The Zn/Cu ratio, in addition to the individual serum levels of trace elements, is important for metabolism in humans.
Keywords: type 2 diabetes, zinc, copper, eGFR, B-type natriuretic peptide
Introduction
Diabetes is a metabolic disease characterized by defects in insulin secretion, insulin sensitivity, or both. Trace elements, such as zinc (Zn) and copper (Cu), may play a pivotal role in the pathogenesis of diabetes and diabetic vascular complications by mediating oxidative stress (1–4). Several studies have shown that both Zn deficiency and excess of Cu are associated with an increased risk of type 2 diabetes and cardiovascular disease (CVD) (2, 3, 5–9).
Zn is a critical trace element in human health. Zn has a potential to be utilized for the treatment of type 2 diabetes; however, the epidemiologic evidence suggests that the effect of Zn on type 2 diabetes remains unclear (10). Up to 85% of the whole body Zn content is found in muscle and bones, with 11% in the skin and liver (11). Zn is an indispensable cofactor for more than 300 enzymes involved in metabolism and also reportedly plays a role in aging, immune system, apoptosis, and oxidative stress (11). Although the effect of zinc supplementation in the improvement of oxidative stress is controversial, one of the causes that the oxidative stress is present in patients with type 2 diabetes is the change in zinc metabolism (4). Moreover, a number of studies have suggested that matrix metalloproteinases which include a Zn ion-binding site are associated with the progression of diabetic microvascular complications and diabetic tendon disorders (12). Recent studies have demonstrated that the islet-restricted zinc transporter, ZnT8 (SLC30A8), regulates insulin secretion (13) and hepatic insulin clearance (14), suggesting that Zn is a key biological factor in glucose homeostasis and the risk of developing type 2 diabetes (15).
Cu has an integral role in many enzymatic activities involved in modifying oxidative stress. Free Cu ions have catalytic activity in the generation of highly reactive hydroxyl radicals (16). Disruption of Cu homeostasis induces oxidative damage by free radicals; such Cu toxicity is associated with disrupted lipid metabolism, hepatic disorders, neurodegenerative disorders, and atherogenesis (17, 18). Cu ion may also play a protective role in the accumulation of human islet amyloid peptide, which is the major component of amyloid deposits in pancreatic β-cells of type 2 diabetic patients; however, whether or not Cu have a protective role in the etiology of type 2 diabetes is not clarified (19, 20). Excess of Cu under inflammatory conditions trigger oxidative stress which are present in chronic diseases (21). On the other hand, increased Zn ion levels may provide a protective effect against Cu toxicity by competing for Cu binding sites (16).
Epidemiological and biological studies have indicated that an imbalance between serum Zn and Cu levels is a causative factor for various diseases, particularly diabetes and CVD (2, 3, 5–9). However, there is a lack of evidence regarding the association between serum Zn/Cu ratio and metabolic parameters in humans. In this study, we aimed to investigate the relationships between serum Zn/Cu ratio and various parameters, such as hematological parameters, glycemic status, lipid profile, renal function, and body composition, in patients with and without type 2 diabetes.
Materials and Methods
Subjects
Between April 2010 and November 2014, a total of 355 individuals (149 type 2 diabetic patients and 206 non-diabetic patients) who measured both serum Zn and Cu at our hospital were investigated retrospectively. Exclusion criteria were type 1 diabetes, malnutrition [serum albumin (Alb) < 3.0 g/dl], and anemia [plasma hemoglobin (Hb) levels < 10 g/dl]. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2. Patients were diagnosed as having type 2 diabetes according to the Japanese diagnostic criteria for type 2 diabetes (22). Briefly, diabetic type was diagnosed if subjects met the following criteria: fasting plasma glucose (PG) level of ≥126 mg/dl or casual PG level of ≥200 mg/dl or HbA1c level ≥6.5%. Patients with type 1 diabetes were excluded. If such conditions were confirmed more than once in the past or the presence of typical symptoms of diabetes and definite diabetic retinopathy were detected, patients were diagnosed as having type 2 diabetes. However, HbA1c level ≥6.5% alone cannot be defined as diabetes (22).
The patients were anonymized to protect their personal information. The study protocol was approved by the Medical Ethics Committee of the National Center for Global Health and Medicine Kohnodai Hospital (Reference No. NCGM-G-001912), and the study was performed in accordance with the Declaration of Helsinki.
Anthropometric Measurement
Height and weight were measured using a rigid stadiometer and calibrated scales (seca 764, seca Co., Ltd., Birmingham, UK). Body mass index (BMI) was calculated as body weight in kilograms divided by the square of body height in meters.
Blood Examination
We measured blood cell count, Alb, blood urea nitrogen, creatinine, eGFR, Zn, and Cu. Serum Zn and Cu levels were measured by atomic absorption spectrophotometry (Z6100, Hitachi Power Solutions Co., Ltd., Ibaraki, Japan) (23, 24). eGFR was calculated using the revised equation adjusted for the Japanese population (25). We also measured PG, hemoglobin A1c (HbA1c), B-type natriuretic peptide (BNP), serum total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and insulin levels. Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula (26). Plasma BNP levels were measured using a specific immunoradiometric assay for human BNP (ARCHITECT BNP-JP®, ABBOTT JAPAN Co., Ltd., Tokyo, Japan).
Body Composition Analysis
Body composition was analyzed using a bioelectrical impedance analysis device (InBody720/S10, Biospace Co., Ltd., Tokyo, Japan). This method is based on the principle that lean body mass contains higher water and electrolyte content than fat tissue; hence, these tissues can be distinguished by electrical impedance. Segmental body composition was estimated using a patented 8-point tactile electrode system. The device uses six frequencies (1, 5, 50, 250, 500, and 1000 kHz) and produces 30 impedance values for five body segments: the right and left upper extremities, trunk, and right and left lower extremities (27). A previous validation study demonstrated that both fat mass and lean body mass content measured using this device were highly correlated with measurements using dual-energy X-ray absorptiometry (28).
Statistical Analysis
Statistical analyses were performed using SPSS version 23 (IBM Co., Ltd., Chicago, IL, USA). All values are expressed as mean ± SD. Pearson’s correlation coefficient was calculated to analyze the association of serum Zn/Cu ratio with physical, biochemical, and physiological data. Multiple regression analysis was performed to test independent correlations between serum Zn/Cu ratio and clinical data. Differences in clinical data between patients with and without type 2 diabetes were analyzed using the paired t-test. Furthermore, multivariate logistic regression analyses were performed to analyze the association between the quartile of serum Zn/Cu ratio and glycemic control (good: HbA1c < 7.0%; poor: HbA1c ≥ 7.0%) in patients with type 2 diabetes and to determine odds ratios and 95% confidence intervals (CI). p values < 0.05 were considered statistically significant.
Results
Characteristics of Subjects
A total of 355 subjects (151 men and 204 women) were included in this study. The mean age was 61.1 ± 17.6 years. The number of patients with type 2 diabetes was 149 (42.0%). The number of patients with dyslipidemia, hypertension, CKD, and liver diseases such as fatty liver were 117 (33.0%), 94 (26.5%), 87 (24.5%), and 35 (9.9%), respectively. Seventy two patients (20.3%) suffered from autoimmune diseases such as Hashimoto disease, Sjögren’s Syndrome, and rheumatoid arthritis. Thirty-three patients (9.3%) suffered from endocrine disorders such as adrenal deficiency and hyper/hypothyroidism. Eighteen patients (5.1%) had malignant diseases, such as lung cancer, prostate cancer, breast cancer, gastric cancer, and colon cancer. Eleven patients (3.1%) had neurodegenerative diseases, such as Parkinson disease and Alzheimer dementia. The number of patients who had a history of CVD was 29 (8.2%). Patient characteristics are summarized in Table 1. The prevalence of dyslipidemia (p < 0.001), hypertension (p < 0.001), and CVD (p < 0.001) was higher and the prevalence of endocrine disorders (p = 0.005) and autoimmune diseases (p < 0.001) was lower in patients with type 2 diabetes than in those without (by chi-square test).
Table 1.
Patient clinical characteristics.
| Demographics | |
|---|---|
| n | 355 |
| Age, years | 61.1 (17.6) |
| Sex (male/female) | 151/204 |
| Height, cm | 159.4 (9.4) |
| Weight, kg | 61.6 (18.1) |
| BMI, kg/m2 | 24.1 (5.7) |
| Blood data | |
| Red blood cell, ×104/μl | 430 (55.3) |
| Hemoglobin, g/dl | 13.1 (1.5) |
| Hematocrit, % | 38.8 (5.2) |
| Biochemical data | |
| Albumin, g/dl | 4.2 (0.5) |
| Blood urea nitrogen, mg/dl | 15.1 (7.2) |
| Creatinine, mg/dl | 0.8 (0.3) |
| eGFR, ml/min/1.73 m2 | 75.4 (26.9) |
| Total cholesterol, mg/dl | 187.4 (39.9) |
| Triglycerides, mg/dl | 135.1 (91.5) |
| HDL cholesterol, mg/dl | 54.5 (15.8) |
| LDL cholesterol, mg/dl | 105.5 (31.5) |
| Plasma glucose, mg/dl | 127 (60.6) |
| HbA1c, % | 6.8 (1.8) |
| Serum insulin, μU/ml (n = 42) | 11.6 (7.7) |
| Plasma BNP, pg/ml (n = 80) | 53.9 (71.1) |
| Serum Zn, μg/dl | 72.6 (15.4) |
| Serum Cu, μg/dl | 110.8 (26.4) |
| Serum Zn/Cu ratio | 0.69 (0.21) |
| Body composition (n = 119) | |
| Skeletal muscle mass (whole body), kg | 26.1 (6.7) |
| Right upper extremity muscle mass, kg | 2.5 (0.8) |
| Left upper extremity muscle mass, kg | 2.5 (0.8) |
| Right lower extremity muscle mass, kg | 7.3 (1.9) |
| Left lower extremity muscle mass, kg | 7.2 (1.9) |
| Body fat mass, kg | 22.8 (12.5) |
| Body fat percentage, % | 30.8 (10.7) |
Values are expressed as means (SD) except for number of subjects and sex.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, hemoglobin A1c; BNP, B-type natriuretic peptide.
Associations between Serum Zn/Cu Ratio and Clinical Parameters in All Subjects
Serum Zn/Cu ratio was inversely correlated with age and plasma BNP levels, whereas it was positively correlated with height, weight, serum Alb levels, and eGFR. Serum Zn/Cu ratio was also positively correlated with red blood cell count, Hb, Ht, and skeletal muscle mass (Table 2).
Table 2.
Correlations between serum Zn/Cu ratio and clinical parameters in all subjects.
| Correlation coefficient | p-Value | |
|---|---|---|
| Demographics | ||
| Age | −0.284 | <0.001 |
| Height | 0.219 | <0.001 |
| Weight | 0.152 | 0.005 |
| BMI | 0.07 | 0.2 |
| Blood data | ||
| Red blood cell | 0.192 | 0.003 |
| Hemoglobin | 0.302 | <0.001 |
| Hematocrit | 0.197 | 0.003 |
| Biochemical data | ||
| Albumin | 0.357 | <0.001 |
| eGFR | 0.144 | 0.008 |
| Total cholesterol | −0.046 | 0.44 |
| Triglycerides | 0.108 | 0.062 |
| HDL cholesterol | −0.063 | 0.3 |
| LDL cholesterol | −0.039 | 0.5 |
| Plasma glucose | −0.047 | 0.42 |
| HbA1c | −0.085 | 0.16 |
| Serum insulin | 0.123 | 0.44 |
| Plasma BNP | −0.383 | <0.001 |
| Body composition | ||
| Skeletal muscle mass (whole body) | 0.189 | 0.039 |
| Right upper extremity muscle mass | 0.183 | 0.046 |
| Left upper extremity muscle mass | 0.193 | 0.036 |
| Right lower extremity muscle mass | 0.214 | 0.019 |
| Left lower extremity muscle mass | 0.224 | 0.014 |
| Body fat mass | −0.064 | 0.49 |
| Body fat percentage | −0.152 | 0.099 |
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, hemoglobin A1c; BNP, B-type natriuretic peptide.
Positive associations of serum Zn/Cu ratio with serum Alb levels and Hb remained after adjustment for age, sex, and BMI (β = 0.335, p < 0.001 and β = 0.237, p = 0.002, respectively). Plasma BNP levels were also negatively associated with serum Zn/Cu ratio (β = −0.258, p = 0.032). In addition, eGFR was positively associated with serum Zn/Cu ratio after adjustment for BMI (β = 0.137, p = 0.014). However, independent associations between serum Zn/Cu ratio and other biochemical parameters were not detected.
To investigate differences in clinical parameters between patients with and without type 2 diabetes, patients with cancers, autoimmune diseases, endocrine disorders, and liver dysfunction, which were known to affect serum levels of Zn and Cu, were excluded from the analysis. Height, weight, BMI, red blood cell count, and Hb were significantly higher in patients with type 2 diabetes than those without type 2 diabetes. Serum levels of HDL-C were significantly lower in patients with type 2 diabetes than those without type 2 diabetes (Table 3).
Table 3.
Comparison of clinical data between patients with and without type 2 diabetes.
| Type 2 diabetic patients (n = 113) | Non-diabetic subjects (n = 110) | p-Value | |
|---|---|---|---|
| Age, years | 64.4 (14.7) | 60.7 (20.4) | 0.12 |
| Duration of diabetes, years | 10.5 (10) | – | – |
| Sex (male/female) | 64/49 | 36/74 | <0.001 |
| Height, cm | 161 (8.8) | 157.5 (10.2) | 0.009 |
| Weight, kg | 68.8 (17.9) | 57.9 (17.7) | <0.001 |
| BMI, kg/m2 | 26.4 (5.6) | 23.2 (5.6) | <0.001 |
| Red blood cell, ×104/μl | 445.6 (61.2) | 423.5 (50.2) | 0.02 |
| Hemoglobin, g/dl | 13.7 (1.7) | 13 (1.4) | 0.013 |
| Hematocrit, % | 40.1 (5.8) | 38.2 (4.8) | 0.2 |
| Albumin, g/dl | 4.1 (0.5) | 4.3 (0.5) | 0.028 |
| eGFR, ml/min/1.73 m2 | 71.2 (25.1) | 79 (32.9) | 0.054 |
| Total cholesterol, mg/dl | 184.9 (37.4) | 192.7 (41.5) | 0.18 |
| Triglycerides, mg/dl | 138.6 (88.7) | 130.6 (85.2) | 0.53 |
| HDL cholesterol, mg/dl | 51.5 (16.3) | 58.1 (15.2) | 0.007 |
| LDL cholesterol, mg/dl | 104.1 (30.8) | 108.3 (31.5) | 0.35 |
| Plasma glucose, mg/dl | 151.5 (62.2) | 99.5 (21) | <0.001 |
| HbA1c, % | 7.7 (1.9) | 5.7 (0.4) | <0.001 |
| Serum insulin, μU/ml | 10.9 (7.1) | 13.1 (10) | 0.56 |
| Plasma BNP, pg/ml | 55.5 (97.3) | 62.5 (59.6) | 0.76 |
| Serum Zn, μg/dl | 75.8 (13.7) | 72.7 (18.7) | 0.15 |
| Serum Cu, μg/dl | 109.9 (21) | 109.4 (23) | 0.42 |
| Serum Zn/Cu ratio | 0.72 (0.19) | 0.69 (0.23) | 0.87 |
| Skeletal muscle mass (whole body), kg | 26.9 (6.4) | 25.3 (7.2) | 0.28 |
| Right upper extremity muscle mass, kg | 2.7 (0.8) | 2.4 (0.9) | 0.13 |
| Left upper extremity muscle mass, kg | 2.6 (0.8) | 2.4 (0.9) | 0.16 |
| Right lower extremity muscle mass, kg | 7.6 (1.9) | 7.2 (2.1) | 0.36 |
| Left lower extremity muscle mass, kg | 7.5 (1.9) | 7.1 (1.9) | 0.33 |
| Body fat mass, kg | 23.9 (13.2) | 23 (11.8) | 0.76 |
| Body fat percentage, % | 31.2 (10.1) | 31.9 (10.2) | 0.75 |
Values are expressed as means (SD) except for sex.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, hemoglobin A1c; BNP, B-type natriuretic peptide.
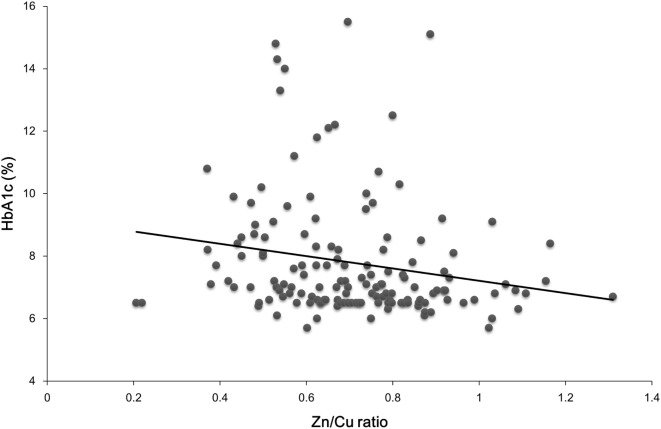
The prevalence of dyslipidemia was higher in patients with type 2 diabetes than that in patients without diabetes (p < 0.001). However, there were no differences in the prevalence of other diseases, such as hypertension (p = 0.065) and CKD (p = 0.23), between patients with and without type 2 diabetes. In patients with type 2 diabetes, serum Zn/Cu ratio was positively correlated with height (r = 0.178, p = 0.031), serum levels of Alb (r = 0.46, p < 0.001), Hb (r = 0.41, p < 0.001), and Ht (r = 0.277, p = 0.016) and negatively correlated with age (r = −0.223, p = 0.006), plasma HbA1c levels (r = −0.193, p = 0.019) (Figure 1), and plasma BNP levels (r = −0.392, p = 0.022). Positive associations of serum Zn/Cu ratio with serum Alb levels and Hb were observed after adjustment for age, sex, and BMI (β = 0.494, p < 0.001 and β = 0.476, p < 0.001, respectively), and serum Zn/Cu ratio remained negatively associated with plasma HbA1c levels after the same adjustments (β = −0.239, p = 0.003). However, significant associations between serum Zn/Cu ratio and other biochemical data disappeared following statistical adjustments.
Figure 1.
An inverse correlation between serum Zn/Cu ratio and plasma HbA1c levels in patients with type 2 diabetes.
The multivariate logistic regression analysis revealed that the highest quartile of serum Zn/Cu ratio was significantly associated with a decreased risk of poor glycemic control (odds ratio = 0.382; 95% CI, 0.165–0.884; p = 0.025) in patients with type 2 diabetes (Table 4).
Table 4.
Logistic regression analysis of glycemic control in patients with type 2 diabetes.
| Adjusted odds ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.003 | 0.980–1.021 | 0.78 |
| Sex | |||
| Male | 0.844 | 0.440–1.618 | 0.61 |
| Female | (reference) | ||
| BMI | 1.045 | 0.982–1.111 | 0.16 |
| Serum Zn/Cu ratio | |||
| <0.5461 | (reference) | ||
| 0.5462–0.6721 | 0.638 | 0.298–1.364 | 0.25 |
| 0.6722–0.8 | 0.604 | 0.281–1.295 | 0.19 |
| >0.8 | 0.382 | 0.165–0.884 | 0.025 |
The odds ratio of good glycemic control (HbA1c < 7.0%) to poor glycemic control (HbA1c ≧ 7.0%) was calculated.
HbA1c, hemoglobin A1c; CI, confidence interval; BMI, body mass index.
Discussion
To the best of our knowledge, this is to be the first study to demonstrate the associations of serum Zn/Cu ratio with renal function, plasma BNP levels, and glycemic control in a relatively large population.
A positive correlation between serum Zn/Cu ratio and serum Alb and Hb levels is expected. Plasma Zn concentrations are reduced by hypoalbuminemia, as zinc is bound to Alb in the circulation, and Zn deficiency is a known cause of anemia (29). Although Cu deficiency is a potential cause of hematologic abnormalities (30), no significant associations between serum Cu levels and blood cell count were observed in this study.
An imbalance between Zn and Cu levels leads to increased oxidative damage in humans, contributing to the pathogenesis of diabetes and diabetic complications (1, 2, 31). The dysregulation of matrix metalloproteinases which include a Zn ion-binding site are associated with the progression of diabetic complications via the interaction with advanced glycation end products in patients with type 2 diabetes, although the relationship between matrix metalloproteinases and serum Zn levels were not investigated in the present study (12). Furthermore, these trace elements are necessary for the activity of mitochondrial antioxidant enzymes, such as Cu/Zn superoxide dismutase (SOD), that protect the cell from reactive oxygen species toxicity (32). Both Zn and Cu are essential for metabolism; however, an imbalance in Zn/Cu (or Cu/Zn) ratio may be a better indicator of metabolic disturbance than Zn or Cu status alone. Indeed, previous studies have demonstrated the utility of Cu/Zn ratio as a biomarker for vascular complications in type 2 diabetes (33) and a predictor of mortality in elderly individuals (34).
Renal dysfunction has been shown to be induced by Cu toxicity in both rats (35) and humans (36). On the other hand, Zn may contribute to the preservation of renal function. Kurihara et al. reported that Zn deficiency decreased renal blood flow and increased renal vascular resistance, which may be attributable to decreased nitric oxide activity due to the presence of increased concentrations of superoxide anions through low SOD activity in the kidneys of rats (37). Yanagisawa et al. suggested that Zn deficiency aggravates tubulointerstitial nephropathy in rats due to an increase in the action of angiotensin II and endothelin (38). Recently, Sun et al. demonstrated that renal function was improved by Zn supplementation in diabetic mice (39). Zn supplementation has also been shown to reduce Alb excretion in patients with diabetic nephropathy (40, 41). The role of Zn is of importance in preserving renal function. However, there is a lack of evidence regarding the association between Zn and/or Cu and renal function in humans. It is notable that the results of this study indicate the potential utility of serum Zn/Cu ratio as a biomarker for renal function.
The negative association observed between serum Zn/Cu ratio and plasma BNP levels in this study may be mediated by insulin sensitivity. An inverse association between natriuretic peptides and insulin resistance has been reported by previous studies (42–44). BNP stimulates lipolysis (45) and promotes muscle mitochondrial biogenesis and fat oxidation through upregulation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (46) and increases adiponectin secretion (47), thereby improving insulin resistance. In addition to previous reports of the association of an imbalance in Zn and Cu levels with increased oxidative stress and inflammation (34, 48), which impair insulin secretion and action (1), Zn deficiency may lower SOD1 activity in pancreatic islets, which has been shown to increase insulin resistance (49, 50). Zn has also been shown to be associated with insulin secretion via metallothionein synthesis (51) and the zinc transporter ZnT8 (15). Recently, Zn has been observed to promote insulin secretion independently on metallothionein synthesis (52). The inverse association between serum Zn/Cu ratio and plasma BNP levels observed in this study after adjustment for age, sex, and BMI indicates that a higher Zn/Cu ratio may be associated with improved insulin sensitivity. However, we were unable to detect a significant association between serum Zn/Cu ratio and insulin levels. This inconsistency may be because of the following study limitations: the small sample size (n = 42) for investigating the association between serum Zn/Cu and insulin and confounding effects of oral hypoglycemic agents and diet. We are unable to confidently explain the negative association observed between serum Zn/Cu ratio and BNP in this study. Thus, further studies are required to elucidate the mechanisms underlying the association between serum Zn/Cu ratio and BNP.
Several studies have demonstrated decreased serum/plasma Zn levels in patients with type 2 diabetes compared with healthy individuals (53–55); on the contrary, serum Zn levels were not lower in patients with type 2 diabetes in this study. As mentioned above, serum Zn levels were found to be strongly affected by nutritional status and Hb levels; thus, no significant difference in serum Zn levels between patients with and without type 2 diabetes was observed.
Low HDL-C levels in patients with type 2 diabetes may have been observed as serum HDL-C levels have been shown to decrease with impaired function in type 2 diabetes (56). Patients with type 2 diabetes were more obese than those without type 2 diabetes in this study. Although no significant differences in body composition between patients with and without type 2 diabetes were found, obesity may be attributable to lower HDL-C levels in patients with type 2 diabetes.
The observed associations of serum Zn/Cu ratio with lower HbA1c levels and reduced risk of poor glycemic control (HbA1c ≥ 7.0%) in a population of patients with type 2 diabetes are noteworthy findings of this study. Although excess of intracellular Zn can trigger oxidative stress from mitochondria and lead to neuronal degeneration (57), Zn has previously been shown to have beneficial effects on glycemic control as well as aging, immunity, and oxidative stress (58), whereas serum Cu is associated with higher HbA1c levels (31, 59). Experimental studies have shown that the amino acids residues involved in Cu coordination complexes have a key role in the formation of human islet amyloid peptide aggregation, which alters the autophagy pathway in pancreatic β-cells and leads to the development of diabetes (19, 20). The actions of Zn and Cu in glucose metabolism appear to be antagonistic; hence, the balance between Zn and Cu ion concentrations is important. In this study, higher Zn levels were not associated with good glycemic control (odds ratio = 0.991; 95% CI, 0.968–1.014; p = 0.436), whereas serum Cu levels were associated with glycemic control (odds ratio = 1.024; 95% CI, 1.007–1.041, p = 0.006). However, the high serum Zn/Cu ratio was associated with good glycemic control in patients with type 2 diabetes. This result suggests that not only Zn and Cu status alone but also Zn/Cu ratio should be considered when evaluating the relationship between these trace elements in patients with diabetes.
This study had several limitations. First, a causal relationship between serum Zn/Cu ratio and renal function and/or glycemic control could not be evaluated because of the retrospective and observational nature of this study. Second, we were unable to evaluate the utility of other biomarkers in assessing Zn and Cu status, such as urinary and other tissue concentrations of Zn and Cu. Zn and Cu concentrations are known to be affected by other factors, such as inflammation, and fasting or postprandial states (60, 61). However, blood Zn and Cu levels are currently considered to be useful and reliable biomarkers (60, 61). Third, we are unable to generalize the results of this study to healthy individuals or other populations. The subjects included in this study had a high prevalence of comorbid diseases, such as hypertension, dyslipidemia, fatty liver, autoimmune disease, CKD, and CVD, besides type 2 diabetes. Neurodegenerative disorders could also undergo changes in serum Zn and Cu levels. Although we excluded subjects with malnutrition and anemia, comorbidities and medications may have affected the observed associations of Zn and Cu status with other parameters. We should also perform a further investigation in healthy controls. Fourth, other confounding factors, such as dietary intake, smoking and drinking habits, and physical activity, should also be considered. Fifth, we were unable to provide a value for the most desirable balance between Zn and Cu from the results of this study. Despite these limitations, we were able to demonstrate significant associations of serum Zn/Cu ratio with renal function, glycemic control, and a number of metabolic parameters, providing new insights in the management of type 2 diabetes.
Conclusion
The findings of this study demonstrate that serum Zn/Cu ratio is significantly associated with renal function in all subjects and glycemic control in patients with type 2 diabetes. Serum Zn/Cu ratio was negatively associated with plasma BNP levels. Imbalance between Zn and Cu levels induces oxidative stress and insulin resistance, which may lead to progression of diabetes and diabetic complications. Zn/Cu ratio, in addition to individual levels of each trace element status alone, appears to have an important effect on metabolism, indicating that these trace elements may play a key role in the pathogenesis of metabolic diseases. We, clinicians should note that serum Zn/Cu ratio could be a better indicator for human metabolism as compared with Zn or Cu status alone.
Author Contributions
HH performed the study, conducted the data analyses, and drafted and revised the manuscript. YK contributed to the data collection and analyses. HY critically reviewed the manuscript and the scientific interpretations of study results. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate the supporting of Tomoko Kaga who cooperated to collect the data.
References
- 1.Ceriello A. Oxidative stress and glycemic regulation. Metabolism (2000) 49(2 Suppl 1):27–9. 10.1016/S0026-0495(00)80082-7 [DOI] [PubMed] [Google Scholar]
- 2.Soinio M, Marniemi J, Laakso M, Pyörälä K, Lehto S, Rönnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care (2007) 30(3):523–8. 10.2337/dc06-1682 [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Kaneto H, Miyatsuka T, Yamamoto K, Yoshiuchi K, Yamasaki Y, et al. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr J (2009) 56(5):699–706. 10.1507/endocrj.K09E-051 [DOI] [PubMed] [Google Scholar]
- 4.Cruz KJ, de Oliveira AR, Marreiro Ddo N. Antioxidant role of zinc in diabetes mellitus. World J Diabetes (2015) 6(2):333–7. 10.4239/wjd.v6.i2.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Białkowska M, Hoser A, Szostak WB, Dybczyński R, Sterliński S, Nowicka G, et al. Hair zinc and copper concentration in survivors of myocardial infarction. Ann Nutr Metab (1987) 31(5):327–32. 10.1159/000177287 [DOI] [PubMed] [Google Scholar]
- 6.Kok FJ, Van Duijn CM, Hofman A, Van der Voet GB, De Wolff FA, Paays CH, et al. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol (1988) 128(2):352–9. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES. Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol (2000) 151(12):1182–8. 10.1093/oxfordjournals.aje.a010168 [DOI] [PubMed] [Google Scholar]
- 8.Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition (2010) 26(11–12):1050–7. 10.1016/j.nut.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Yary T, Virtanen JK, Ruusunen A, Tuomainen TP, Voutilainen S. Serum zinc and risk of type 2 diabetes incidence in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. J Trace Elem Med Biol (2016) 33:120–4. 10.1016/j.jtemb.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Ruz M, Carrasco F, Sánchez A, Perez A, Rojas P. Does zinc really “metal” with diabetes? The epidemiologic evidence. Curr Diab Rep (2016) 16(11):111. 10.1007/s11892-016-0803-x [DOI] [PubMed] [Google Scholar]
- 11.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol (2012) 86(4):521–34. 10.1007/s00204-011-0775-1 [DOI] [PubMed] [Google Scholar]
- 12.Abreu BJ, de Brito Vieira WH. Metalloproteinase changes in diabetes. Adv Exp Med Biol (2016) 920:185–90. 10.1007/978-3-319-33943-6_17 [DOI] [PubMed] [Google Scholar]
- 13.Rutter GA. Think zinc: new roles for zinc in the control of insulin secretion. Islets (2010) 2(1):49–50. 10.4161/isl.2.1.10259 [DOI] [PubMed] [Google Scholar]
- 14.Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest (2013) 123(10):4513–24. 10.1172/JCI68807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab (2009) 11(Suppl 4):202–14. 10.1111/j.1463-1326.2009.01110.x [DOI] [PubMed] [Google Scholar]
- 16.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology (2003) 189(1–2):147–63. 10.1016/S0300-483X(03)00159-8 [DOI] [PubMed] [Google Scholar]
- 17.Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol (2014) 88(11):1929–38. 10.1007/s00204-014-1355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinecke JW, Rosen H, Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest (1984) 74(5):1890–4. 10.1172/JCI111609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magrì A, La Mendola D, Nicoletti VG, Pappalardo G, Rizzarelli E. New insight in copper-ion binding to human islet amyloid: the contribution of metal-complex speciation to reveal the polypeptide toxicity. Chemistry (2016) 22(37):13287–300. 10.1002/chem.201602816 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-López C, Cortés-Mejía R, Miotto MC, Binolfi A, Fernández CO, Del Campo JM, et al. Copper coordination features of human islet amyloid polypeptide: the type 2 diabetes peptide. Inorg Chem (2016). 10.1021/acs.inorgchem.6b01963 [DOI] [PubMed] [Google Scholar]
- 21.Pereira TC, Campos MM, Bogo MR. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J Appl Toxicol (2016) 36(7):876–85. 10.1002/jat.3303 [DOI] [PubMed] [Google Scholar]
- 22.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig (2010) 1(5):212–28. 10.1111/j.2040-1124.2010.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meret S, Henkin RI. Simultaneous direct estimation by atomic absorption spectrophotometry of copper and zinc in serum, urine, and cerebrospinal fluid. Clin Chem (1971) 17(5):369–73. [PubMed] [Google Scholar]
- 24.Abe A, Yamashita S, Noma A. Sensitive, direct colorimetric assay for copper in serum. Clin Chem (1989) 35(4):552–4. [PubMed] [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis (2009) 53(6):982–92. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem (1972) 18(6):499–502. [PubMed] [Google Scholar]
- 27.Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res (2012) 32(7):479–85. 10.1016/j.nutres.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 28.Faria SL, Faria OP, Cardeal MD, Ito MK. Validation study of multi-frequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes Surg (2014) 24(9):1476–80. 10.1007/s11695-014-1190-5 [DOI] [PubMed] [Google Scholar]
- 29.Wieringa FT, Dijkhuizen MA, Fiorentino M, Laillou A, Berger J. Determination of zinc status in humans: which indicator should we use? Nutrients (2015) 7(5):3252–63. 10.3390/nu7053252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol (2008) 80(6):523–31. 10.1111/j.1600-0609.2008.01050.x [DOI] [PubMed] [Google Scholar]
- 31.Viktorínová A, Toserová E, Krizko M, Duracková Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism (2009) 58(10):1477–82. 10.1016/j.metabol.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 32.Reddi AR, Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell (2013) 152(1–2):224–35. 10.1016/j.cell.2012.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karahan SC, Değer O, Orem A, Uçar F, Erem C, Alver A, et al. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med (2001) 39(2):109–15. 10.1515/CCLM.2001.019 [DOI] [PubMed] [Google Scholar]
- 34.Malavolta M, Giacconi R, Piacenza F, Santarelli L, Cipriano C, Costarelli L, et al. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology (2010) 11(3):309–19. 10.1007/s10522-009-9251-1 [DOI] [PubMed] [Google Scholar]
- 35.Galhardi CM, Diniz YS, Faine LA, Rodrigues HG, Burneiko RC, Ribas BO, et al. Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol (2004) 42(12):2053–60. 10.1016/j.fct.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 36.Iyanda AA, Anetor J, Adeniyi FA. Altered copper level and renal dysfunction in Nigerian women using skin-whitening agents. Biol Trace Elem Res (2011) 143(3):1264–70. 10.1007/s12011-011-8962-8 [DOI] [PubMed] [Google Scholar]
- 37.Kurihara N, Yanagisawa H, Sato M, Tien CK, Wada O. Increased renal vascular resistance in zinc-deficient rats: role of nitric oxide and superoxide. Clin Exp Pharmacol Physiol (2002) 29(12):1096–104. 10.1046/j.1440-1681.2002.03783.x [DOI] [PubMed] [Google Scholar]
- 38.Yanagisawa H, Nodera M, Wada O. Zinc deficiency aggravates tubulointerstitial nephropathy caused by ureteral obstruction. Biol Trace Elem Res (1998) 65(1):1–6. 10.1007/BF02784109 [DOI] [PubMed] [Google Scholar]
- 39.Sun W, Wang Y, Miao X, Wang Y, Zhang L, Xin Y, et al. Renal improvement by zinc in diabetic mice is associated with glucose metabolism signaling mediated by metallothionein and Akt, but not Akt2. Free Radic Biol Med (2014) 68:22–34. 10.1016/j.freeradbiomed.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parham M, Amini M, Aminorroaya A, Heidarian E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud (2008) 5(2):102–9. 10.1900/RDS.2008.5.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan MI, Siddique KU, Ashfaq F, Ali W, Reddy HD, Mishra A. Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. J Nat Sci Biol Med (2013) 4(2):336–40. 10.4103/0976-9668.117002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension (2005) 46(4):660–6. 10.1161/01.HYP.0000179575.13739.72 [DOI] [PubMed] [Google Scholar]
- 43.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol (2013) 62(8):752–60. 10.1016/j.jacc.2013.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamasaki H, Yanai H, Kakei M, Noda M, Ezaki O. The association between daily physical activity and plasma B-type natriuretic peptide in patients with glucose intolerance: a cross-sectional study. BMJ Open (2015) 5(1):e006276. 10.1136/bmjopen-2014-006276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J (2000) 14(10):1345–51. 10.1096/fj.14.10.1345 [DOI] [PubMed] [Google Scholar]
- 46.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes (2009) 58(12):2880–92. 10.2337/db09-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol (2009) 53(22):2070–7. 10.1016/j.jacc.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 48.Guo CH, Chen PC, Yeh MS, Hsiung DY, Wang CL. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin Biochem (2011) 44(4):275–80. 10.1016/j.clinbiochem.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, et al. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal (2011) 14(3):391–401. 10.1089/ars.2010.3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal (2011) 14(3):489–503. 10.1089/ars.2010.3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Islam MS, Loots du T. Diabetes, metallothionein, and zinc interactions: a review. Biofactors (2007) 29(4):203–12. 10.1002/biof.5520290404 [DOI] [PubMed] [Google Scholar]
- 52.Hardy AB, Prentice KJ, Froese S, Liu Y, Andrews GK, Wheeler MB. Zip4 mediated zinc influx stimulates insulin secretion in pancreatic beta cells. PLoS One (2015) 10(3):e0119136. 10.1371/journal.pone.0119136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med (1983) 75(2):273–7. 10.1016/0002-9343(83)91205-6 [DOI] [PubMed] [Google Scholar]
- 54.Walter RM, Jr, Uriu-Hare JY, Olin KL, Oster MH, Anawalt BD, Critchfield JW, et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care (1991) 14(11):1050–6. 10.2337/diacare.14.11.1050 [DOI] [PubMed] [Google Scholar]
- 55.Aguilar MV, Saavedra P, Arrieta FJ, Mateos CJ, González MJ, Meseguer I, et al. Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab (2007) 51(5):402–6. 10.1159/000108108 [DOI] [PubMed] [Google Scholar]
- 56.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab (2009) 5(3):150–9. 10.1038/ncpendmet1066 [DOI] [PubMed] [Google Scholar]
- 57.Granzotto A, Sensi SL. Intracellular zinc is a critical intermediate in the excitotoxic cascade. Neurobiol Dis (2015) 81:25–37. 10.1016/j.nbd.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 58.Jansen J, Karges W, Rink L. Zinc and diabetes – clinical links and molecular mechanisms. J Nutr Biochem (2009) 20(6):399–417. 10.1016/j.jnutbio.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 59.Naka T, Kaneto H, Katakami N, Matsuoka TA, Harada A, Yamasaki Y, et al. Association of serum copper levels and glycemic control in patients with type 2 diabetes. Endocr J (2013) 60(3):393–6. 10.1507/endocrj.EJ12-0342 [DOI] [PubMed] [Google Scholar]
- 60.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr (2009) 89(6):2040S–51S. 10.3945/ajcn.2009.27230G [DOI] [PubMed] [Google Scholar]
- 61.Harvey LJ, Ashton K, Hooper L, Casgrain A, Fairweather-Tait SJ. Methods of assessment of copper status in humans: a systematic review. Am J Clin Nutr (2009) 89(6):2009S–24S. 10.3945/ajcn.2009.27230E [DOI] [PubMed] [Google Scholar]