Abstract
Purpose
Adjuvant high-dose-rate brachytherapy (HDRBT) offers advantages over low dose rate brachytherapy (LDRBT), although there are little data on local tumor control and treatment related toxicity. We report outcome in patients with primary, recurrent, and metastatic extremity and superficial trunk soft tissue sarcoma.
Material and methods
Eleven patients (12 sites) with intermediate or high grade sarcoma were treated with adjuvant HDRBT following surgical resection. Patients were treated at 3.4 Gy fractions delivered twice daily to a total dose of 34 Gy (1 patient received 9 fractions).
Results
With median follow-up of 20.8 months, 1 patient developed a local recurrence. 2-year local control and overall survival are 89% and 71%, respectively. Wound complications occurred in 3 sites. Two of the wound complications developed in the area of previous external beam radiotherapy (EBRT).
Conclusion
Surgical resection followed by HDRBT is associated with excellent early local tumor control and acceptable wound complication.
Keywords: sarcoma, brachytherapy, high dose rate, radiotherapy
Purpose
Radiation therapy played a pivotal role in the transition from the traditional amputation to the current management of limb-preservation in treatment of soft tissue sarcomas (STS) of the extremity. External beam radiotherapy (EBRT) is the most common modality to deliver radiation treatments for soft tissue sarcomas. While this modality achieves a high local control rate, toxicity remains problematic as the size of the treatment fields are large, particularly in the post-operative setting. In cases of recurrent tumors, reirradiation with external beam techniques may result in significant toxicity. Low dose rate brachytherapy (LDRBT) is an established alternative to external beam radiation for adjuvant treatment of extremity STS [1]. Brachytherapy enables delivery of higher radiation doses directly to the tumor bed with less effect on nearby structures due to rapid dose fall off and smaller treatment volumes. However, LDR brachytherapy has disadvantages. Patients are required to stay in the hospital in an isolated, lead-lined room for the duration of treatment, placing caregivers at risk for radiation exposure. High dose rate brachytherapy (HDRBT) offers the advantages of LDRBT, with the additional benefit of potentially outpatient treatment, less radiation exposure to the patient’s caregivers, and greater control over the dose distribution through the ability to alter dwell positions and dwell times. Despite these potential advantages, the effectiveness of HDRBT for extremity and superficial trunk STS has not been well established in the literature.
The goal of this retrospective study was to determine the toxicity and local control of soft tissue sarcomas in the extremity and superficial trunk treated with wide local excision and adjuvant HDR brachytherapy.
Material and methods
This research was reviewed and approved by the Medical College of Wisconsin Institutional Review Board (IRB) and all investigators completed training in both human research and patient privacy. From October 2005 through February 2010, 11 patients (12 disease sites) with intermediate to high grade soft tissue sarcoma were treated with surgical resection followed by HDR brachytherapy only. Patient age ranged from 26 to 76. Median maximum tumor size was 5.0 cm (range 2.2 cm to 13.7 cm). One patient was treated with adjuvant HDR brachytherapy to 2 sites of metastatic disease (Case No. 10, 11). Five patients were treated to a metastatic site, 3 were treated for local recurrence, and 3 presented with localized primary sarcoma and were treated definitively with surgery and adjuvant HDR brachytherapy. No patient was treated with EBRT in addition to the HDRBT. The 3 patients with recurrent disease had received previous EBRT (1 patient had a combination of EBRT and LDRBT), with disease free intervals of 1.5, 3.5, and 9.5 years, respectively. One patient with metastatic disease (Case No. 10) developed locally recurrent disease in the thigh after previous treatment with neoadjuvant chemotherapy and EBRT approximately 1.5 years earlier. Patient characteristics, treatment and outcome are summarized in Table 1.
Table 1.
Patient characteristics and outcome
| Case No. | Histology | Primary site | HDR site | Max tumor size (cm) | Initial stage | Stage prior to HDR | Prior RT treatment | Margin status | Type of wound closure | Postop start day | Followup | Recurrence (mo) | Complication (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Round cell liposarcoma | Right groin | Thigh | 5.1 | T2bN0M0G3 | T2bN0M0G3 | None | Negative | Rotational flap | 6 | NED/28.0 | None | Grade 3 Wound Toxicity (2) |
| 2 | Synovial | Scapula | Scapula | 2.6 | T2bN0M0G3 | T2bN0M0G3 | None | Negative | Rotational flap | 6 | NED/6.0 | None | Grade 2 ROM (1) |
| 3 | High grade spindle cell | Axilla | Axilla | 4.4 | T2bN0M0G3 | T2bN0M0G3 | None | Close | Primary | 6 | NED/5.6 | None | None |
| 4 | Fibrosarcoma | Thigh | Thigh | 5.2 | T2bN0M0G3 | Local recurrence | LDRBT/EBRT to thigh | Negative | Primary | 4 | NED/46.2 | None | Grade 2 Wound Toxicity (1), Grade 1 Joint Stiffness (7), Grade 2 ROM (7) |
| 5 | Malignant fibrous histiocytoma | Thigh | Thigh | 5 | T2bN0M0G3 | Local recurrence | EBRT to thigh | Negative | Primary | 4 | NED/26.9 | LR (14.2) | Grade 3 Wound Toxicity (1), Grade 1 fibrosis (25) |
| 6 | Pleomorphic liposarcoma | Proximal upper extremity | Proximal upper extremity | 3.4 | T2bN0M0G3 | Local recurrence | EBRT to upper extremity | Negative | Primary | 6 | NED/18.5 | None | Grade 1 Pain (1), Grade 2 Desquamation (1), Grade 1 Fibrosis (12.25), Grade 1 Joint Stiffness (12.25), Grade 3 Weakness (12.25) |
| 7 | MPNST | Thigh | Thigh | 3 | T2bN0M0G4 | Metastatic | None | Close | Primary | 5 | Deceased/24.6 | DM (14) | None |
| 8 | Leiomyosarcoma | Uterus | Thigh | 8 | T2bN0M0G3 | Metastatic | EBRT to pelvis | Close | Primary | 7 | Deceased /22.0 | DM (2) | None |
| 9 | Myxofibrosarcoma | Thigh | Forearm | 2.2 | T2bN0M0G3 | Metastatic | EBRT to pelvis and thigh | Negative | Primary | 10 | Deceased/52.8 | DM (11.75) | Grade 1 Telangectasia (15) |
| 10 | Myxofibrosarcoma | Thigh/pelvis | Thigh | 6 | T2bN0M1G4 | Metastatic | Neoadjuvant chemo and EBRT to thigh | Close | Primary | 6 | Deceased/19.4 | DM (1.5) | None |
| 11 | Myxofibrosarcoma | Thigh/ pelvis | Thigh | 13.7 | T2bN0M1G4 | Metastatic | None | Positive | Primary | 6 | Deceased/19.6 | DM (1.5) | None |
| 12 | Leiomyosarcoma | Retroperitoneal | Thigh | 5 | T2bN0M0G3 | Metastatic | EBRT to retroperitoneum | Negative | Primary | 6 | NED/10.3 | None | Grade 2 Weakness (2.25) |
Cases 1-3 received definitive adjuvant HDRBT for primary treatment; Cases 4-6 received adjuvant HDRBT for local recurrence; Cases 7-12 received adjuvant HDRBT for sites of metastatic disease.
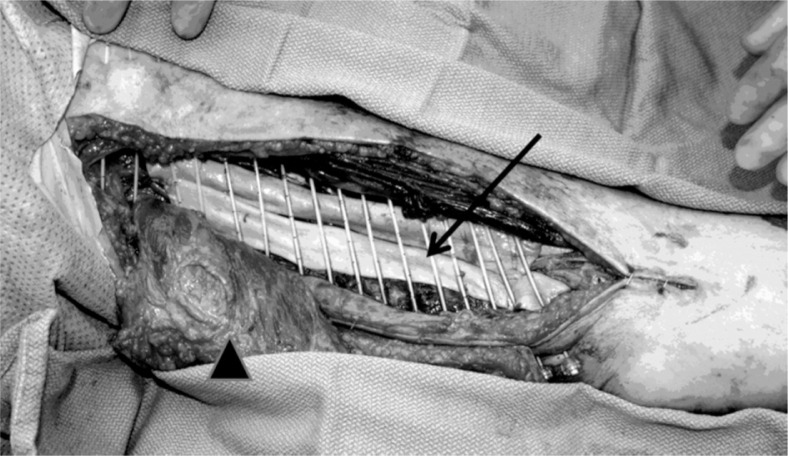
After a gross total resection with attempt at wide margins, the surgeon demarcated the tumor bed with surgical clips. A piece of Alloderm (Lifecell, Branchburg, New Jersey) was placed directly over the surface of the neurovascular bundle or bone if applicable (Fig. 1). Plastic afterload catheters were placed tangentially over the tumor bed plus 2 cm margin in the superior and inferior directions with 1 cm spacing between catheters. The number of catheters ranged from 7 to 21 (median 12). Catheters were secured with absorbable sutures as needed, and the surgeon placed the drains and proceeded with wound closure. All patients had a single plane implant.
Fig. 1.
Intraoperative placement of catheters in the patient after a wide resection and latissimus dorsi myocutaneous rotational flap reconstruction. “→” identifies the Alloderm graft placed over neurovascular bundle. “▲” identifies the reconstructed flap which was subsequently rotated into the defect
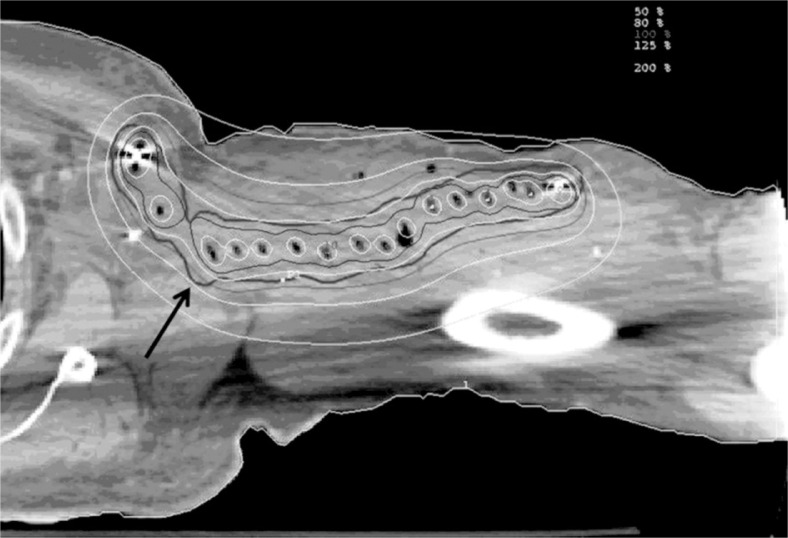
CT simulation was performed on post-operative day (POD) numbers 1-4. The tumor bed was determined based on the radiopaque clips placed during surgery and pre-treatment MRI images. The planning target volume (PTV), defined as tumor bed plus 1 cm radial margin and 2 cm superior-inferior margin, was then contoured on the CT images. Drain sites, incision sites, and skin surface were not included in the PTV. A total dose of 34 Gy was prescribed to > 90% of the PTV. Maximal skin dose was constrained to less than 80% of the prescription dose. Fifty percent or more of the PTV was constrained to less than 200% of the prescription dose (Fig. 2). Between POD 4 and 10, the first HDR brachytherapy treatment was delivered using Iridium-192 (10 Ci initial activity, dose rate 1.101 cGy/Uh at 1 cm distance from the source). Ten patients received a total dose of 34 Gy delivered at 3.4 Gy per fraction BID, with ≥ 6 hours between treatments. This dose is biologically equivalent to 50 Gy of EBRT using conventional fractionation as determined by the linear quadratic model, and has demonstrated efficacy in post-operative treatment of soft tissue sarcomas [2]. One patient received 30.6 Gy, delivered in the same manner, but for a total of 9 rather than 10 fractions, due to the patient’s scheduling conflict. The interstitial catheters were removed in the HDR brachytherapy suite immediately following the last treatment. The patients returned to radiation oncology for follow-up 4 weeks after the completion of radiotherapy, and follow-up was then continued according to a standard regimen. Acute and late toxicities were recorded with the CTCAE v3.0 scale. The Kaplan-Meier curves were generated for local control and overall survival.
Fig. 2.
Dose distribution for treatment of a left upper extremity sarcoma. Arrow denotes the PTV (thick black line). The outermost isodose line denotes 50% isodose line
Results
All patients completed treatment as initially planned. One patient (Case No. 11) had a focally positive surgical margin and 4 patients (Case No. 3, 7, 8, 10) had close margins (≤ 0.1 cm). In the other 7 surgeries, negative margins (> 0.1 cm) were achieved. HDR treatment started between POD four and seven for all but 1 patient (Table 1). Due to tension on the skin from the wound closure and the proximity of the catheters to the skin surface in the patient with metastatic high-grade sarcoma of the dorsal forearm (Case No. 9), the initiation of radiotherapy was postponed until POD ten in this patient. Two patients (Case No. 1, 2) had a flap reconstruction while the other 10 operations had primary closures. Patients were treated with HDR brachytherapy alone for a variety of reasons, including noncompliance, travel distance, patient desire to complete treatment quickly, and decreased burden of treatment time in patients with metastatic disease.
Toxicity
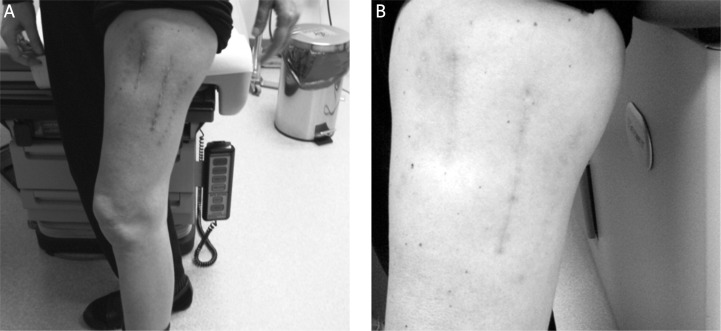
No patients developed acute reactions during the week of HDR brachytherapy delivery. Three patients (25%) developed wound healing complications, one grade 2 (Case No. 4) and two grade 3 (Case No. 1, 5). One patient with high grade sarcoma of the groin (Case No. 1) experienced wound dehiscence and infection 2 months after the completion of adjuvant brachytherapy, with resolution after the third incision and drainage. The second patient suffered from locally recurrent high grade sarcoma of the thigh (Case No. 4) and developed recurrent wound infections after HDRBT, requiring hospitalization on two occasions. The third patient (Case No. 5) who also had locally recurrent high grade sarcoma of the thigh developed wound dehiscence 5 weeks after completion of HDR brachytherapy. The patient was treated with intravenous antibiotics and a wound VAC with subsequent successful healing. The latter 2 patients had previous radiotherapy to this site (combination of LDR brachytherapy and EBRT and EBRT alone, respectively). Nine of 12 wounds (75%) had no wound healing issues (Table 1). Figure 3 is a representative picture demonstrating complete wound healing at 1 and 4 months after the completion of HDRBT.
Fig. 3.
Clinical appearance of treated thigh (A) 1 month and (B) 4 months after completion of brachytherapy. This patient had a primary closure of the surgical wound
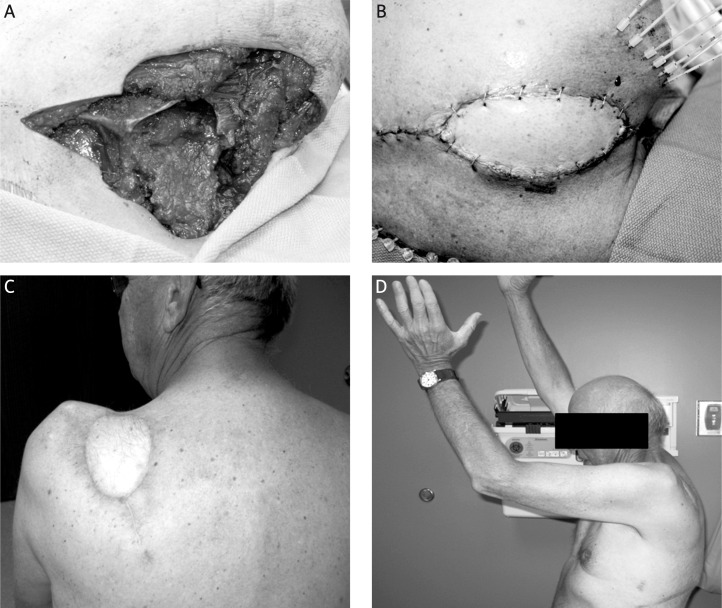
For the entire duration of follow-up, no patients developed edema. One patient (Case No. 6) experienced grade 1 pain in the treated area. Two patients (Case No. 5, 6) developed fibrosis, both grade 1, in the radiation field. One patient had grade 2 desquamation (Case No. 6) and one patient had grade 1 telangectasias (Case No. 9). Two patients (Case No. 4, 6) reported occasional joint stiffness (grade 1), while two (Case No. 2, 4) experienced decreased range of motion (both grade 2) (Fig. 4). Two patients (Case No. 6, 12) complained of weakness of the treated extremity, grade 3 and grade 2, respectively. There were no grade 4 or 5 toxicities.
Fig. 4.
Patient (Case No. 2) treated with surgical resection and flap reconstruction followed by adjuvant HDRBT for treatment of a primary left sub-scapular synovial sarcoma. (A) Resection defect of left scapula. (B) Catheter placement and latissimus dorsi myocutaneous rotational flap reconstruction. (C) 6 month follow-up appointment. The patient did not experience any wound healing toxicity. (D) Grade 2 joint-function toxicity, with > 25% impairment in range of motion and no interference in activities of daily living 6 months after completion of HDRBT
Local control
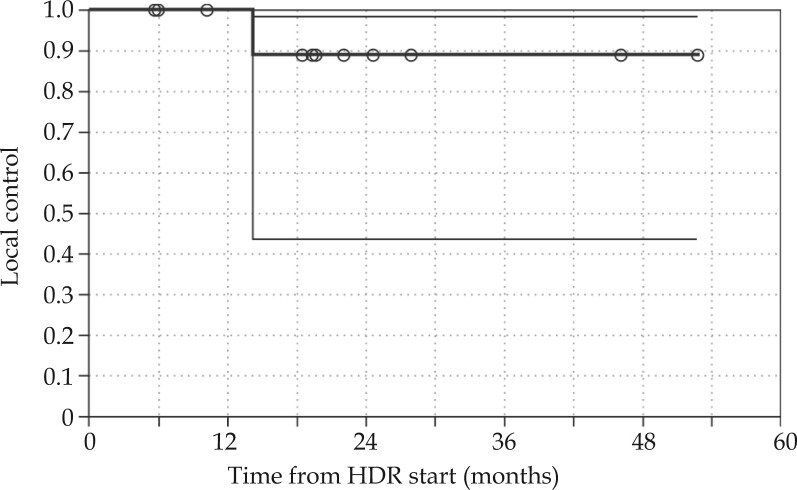
One patient (Case No. 5) developed a local recurrence 14 months after the completion of adjuvant HDR brachytherapy. This recurrence constituted the patient’s third local recurrence since his initial presentation in 1999 at which time he had a subtotal resection followed by re-excision and post-operative external beam radiotherapy. He had locally recurrent disease in 2003 treated with surgical resection and flap reconstruction, locally recurrent disease in April 2006 treated with complete surgical resection, and a third local recurrence in May 2008 in the left groin for which the patient had a complete surgical resection and adjuvant HDRBT. Of note, negative margins were achieved after surgical resection prior to the HDRBT, and the patient received 9 fractions instead of the standard 10 fractions of brachytherapy. The patient again developed locally recurrent disease in July 2009 treated with surgery including abdominal wall reconstruction. This recurrence presented as a mass in the medial aspect of the previous surgical field and superior to the incision consistent with a marginal recurrence. Less than 1 year later in April 2010, disease again presented locally in the inguinal region and lower abdominal wall treated with surgery with positive margins. The patient is undergoing post-operative external beam radiotherapy, which comprises his third course of radiation. He has no distant disease. None of the patients with close or positive margins developed a local recurrence. The Kaplan-Meier 2 year local control is 89% (Fig. 5).
Fig. 5.
Kaplan-Meier curve for local control for the cohort, with 95% confidence intervals. Local control at 2 years is 89%
Overall survival
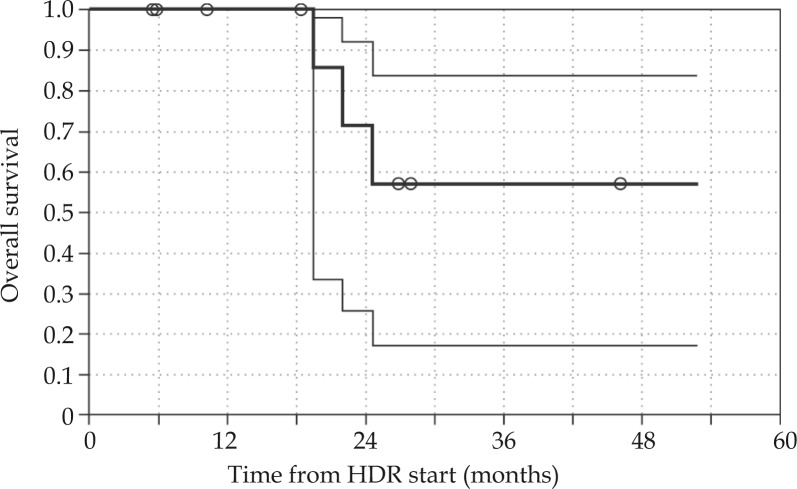
Five patients (Case No. 7-12) had sites of metastatic disease treated with HDR brachytherapy following surgical resection. Four patients (Case No. 7-11) died from progressive metastatic disease and one (Case No. 12) remains alive. This patient (Case No. 12) has not had further disease progression since completion of adjuvant HDR brachytherapy 10.3 months ago. Despite systemic disease, none of these patients developed local recurrence in the region treated with adjuvant HDR brachytherapy. The Kaplan-Meier 2-year overall survival is 71% (Fig. 6).
Fig. 6.
Kaplan-Meier curve for overall survival for the cohort, with 95% confidence intervals. Overall survival is 100% at 1 year, 71% at 2 years, and 57% at 4 year
Discussion
The goal of this retrospective study was to determine the toxicity and local control of soft tissue sarcomas in the extremity and superficial trunk treated with wide local excision and adjuvant HDR brachytherapy. The most frequent toxicity was poor wound healing and infection, especially in the setting of previous external beam radiation treatment. Local control was high with a rate similar to that of other studies reporting outcomes of post-operative radiation therapy [3, 4]. The HDR brachytherapy was well-tolerated by all patients. The wound healing complication rate of 25% (3 of 12 treated sites) is comparable to that in the published literature for other forms of radiotherapy. O’Sullivan et al. [5] reported a lower rate of major wound complications with post-operative EBRT compared to pre-operative EBRT, 17% vs. 35% (p = 0.01), respectively. This study also reported that anatomic location, specifically the proximal lower extremity, was associated with increased frequency of wound healing complications. All 3 patients in our study with difficulty wound healing had a lower extremity tumor, each approximately 5 cm in size (range 5.0-5.2 cm). Controversy exists regarding reconstruction of sarcoma defects which require brachytherapy. Concerns arise over margin status and radiating the flap [6, 7]. In this series, Case 1 and 2 had immediate reconstruction to provide viable tissue for wound closure. No seromas, wound separation or flap necrosis developed in these patients. Two patients had a previous course of external beam radiotherapy. Late complications were infrequent and compared favorably to the late morbidity reported for the NCIC trial [8], given the limitations of different toxicity scales and retrospective versus prospective data collection. No patients had grade 2 or greater fibrosis and no patients had edema of the treated region in our study. Grade 3 late toxicity was reported by one patient with extremity weakness.
The benefit of radiotherapy in the treatment of soft tissue sarcomas has been well-established. EBRT following surgical resection was shown to result in equivalent disease-free survival and overall survival compared to amputation in the landmark study by Rosenberg et al. [9]. With a median follow-up of 56 months, 15% of patients in the limb-sparing arm developed a local recurrence. Yang et al. [10] further established the benefit of post-operative radiotherapy in a phase III trial in which patients were randomized to limb-sparing surgery alone or with adjuvant EBRT. Although radiotherapy did not provide a survival benefit in this study, it improved local control for high and low grade soft tissue sarcomas, with only 1 of 70 patients treated with radiotherapy developing a local recurrence with a median follow-up of 9.6 years. Chun et al. [11] reported 17 patients treated with post-operative HDR brachytherapy (12-18 Gy in 6 fractions delivered BID) followed 3 weeks later by EBRT (36-60 Gy). With a median follow-up of 31 months, no patients had local failure. Skin toxicity included 1 patient with wound dehiscence, 1 with wound infection, and 1 with delayed healing that required further operative intervention. Pohar et al. [3] retrospectively compared outcomes of EBRT combined with either LDR or HDR brachytherapy. Two year local control was comparable for both techniques (90% with LDR boost vs. 94% with HDR boost). Wound healing complications were significantly less with HDR brachytherapy.
Pisters et al. [1] reported improved local control for high-grade tumors with adjuvant brachytherapy alone (no EBRT) compared to observation following surgical resection of soft tissue sarcomas. In this randomized phase III trial, LDR brachytherapy was employed. Doses of 42-45 Gy were delivered over 4-6 days using Iridium-192 implant, requiring hospital admissions of approximately 2 weeks. 5-year actuarial local control for all patients in the brachytherapy arm was 82%, significantly better than 69% local control for patients who received surgery alone. Wound toxicity was initially high, occurring in 48% of patients receiving brachytherapy. The technique was modified to postpone radiation treatment to greater than 4 days after surgery, and wound toxicity in the brachytherapy arm subsequently decreased to 14% which was not significantly different from the rate of wound toxicity in the surgery alone arm. A prospective, single-institution study by Rosenblatt et al. [12] reported a local control rate of 85% with mean follow-up of 27 months, and 5 year survival of 90% using adjuvant LDR brachytherapy with or without an EBRT component. 55% developed chronic radiation changes at the primary site, including fibrosis and telangectasias. 15% developed wound healing complications, including 1 patient who died from septic shock and 1 requiring hyperbaric oxygen treatment for soft tissue necrosis.
No prospective randomized trial has been performed to establish the benefit of HDR brachytherapy in the treatment of soft tissue sarcomas. However, with the advantages afforded by HDR brachytherapy, this technique deserves further consideration. Treatment time is significantly shorter with brachytherapy compared to EBRT, a consideration that may be especially important for palliative treatment or patients who have a substantial travel time to come for treatment. The radiation field is smaller and the surgical specimen can be examined without interference from pre-operative radiation changes, which could influence consideration of other treatments such as chemotherapy [13]. The temporary presence of the radiation source simplifies patient care issues that arise with LDR brachytherapy and hospital admission is not mandatory. For example, hospitalized patients can interact with medical staff and/or caregivers, including postoperative physical therapy and rehabilitation, during the interval between HDR treatments without concern for radiation exposure to staff or visitors.
Our study supports the feasibility and efficacy of HDR brachytherapy as postoperative monotherapy not only for metastatic or recurrent disease, but also as adjuvant therapy for primary soft tissue sarcomas. The limitations of this study include small sample size and relatively short median follow-up. The 2-year local control rate of 89% is comparable to the studies outlined above. The overall survival rate in this study cannot be directly compared to the rates published in these randomized trials, as almost half of the patients in our study had metastatic disease at the time they presented for consideration for radiotherapy. Of the patients who were M0 at the time of treatment with HDR brachytherapy, all remain alive with a median follow-up of 20.8 months.
Other reports of treatment of small numbers of patients with adjuvant HDR brachytherapy have yielded similar conclusions to our study [14–17]. A prospective, nonrandomized study of adjuvant HDR brachytherapy alone in children reported high local control and acceptable toxicity with a median follow-up of ten years [18]. However, only 2 patients had their primary tumor location in the extremity, and rhabdomyosarcoma was the most frequent histology, making comparison to the patients in our study difficult. Another pediatric study reported on 30 children treated with adjuvant HDR brachytherapy alone with local control of 84% and overall survival of 95% with median 51 months follow-up [16]. Koizumi et al. [15] reported increased local failure with positive margins after adjuvant HDR brachytherapy. In a retrospective comparison, Alekhteyar et al. [19] found a trend for improved local control in patients with positive margins when treated with combination LDR brachytherapy and EBRT compared to LDR brachytherapy alone (90% vs. 59%, p = 0.08). In our data, 1 patient had a positive margin and 4 patients had close margins; none of these patients developed a local recurrence with adjuvant HDR brachytherapy alone. Torres et al. [20] did not find a local control benefit when recurrent tumors were resected and treated with adjuvant reirradiation compared to resection alone. Furthermore, they reported a significant increase in complications and extremity amputation after reirradiation. The majority of the patients (33 of 37) underwent reirradiation with LDR brachytherapy (45 or 50 Gy). The local control rate of 51% in this study is similar to the 5 year local recurrence free survival of 52% reported by Pearlstone et al. [21] in a retrospective review of 26 patients undergoing reirradiation with LDR brachytherapy. However, Nori et al. [22] reported a higher 5-year local control rate of 68% for recurrent extremity sarcomas treated with surgery and LDR brachytherapy, and 12.5% of patients developed complications. Similarly, a retrospective review by Catton et al. [23] report a local control rate of 36% in patients treated with surgery alone compared to a 100% local control rate for surgery plus radiation therapy. In our study, 3 patients were treated for local recurrence after having received prior radiation to the treated site. One developed subsequent local recurrence after treatment with HDR brachytherapy (marginal recurrence) and the other 2 patients have maintained local control. Two of these 3 patients experienced wound healing toxicity.
There are no reports in the literature focused solely on the use of HDR brachytherapy as monotherapy to obtain local control in patients with metastatic disease. A few studies have included small numbers of patients with metastatic disease in their cohort [23, 24]. In our study, 50% of the treated sites were in patients with metastases. This treatment modality is well-suited for patients with metastatic disease for whom the burden of treatment time and toxicity should be minimized.
Conclusions
Limited data exist to support the routine use of HDR brachytherapy as a sole adjuvant treatment for patients with soft tissue sarcomas. Some studies, including ours, have explored the role of adjuvant HDR brachytherapy alone for treatment of primary, recurrent, and metastatic tumors with encouraging results. These preliminary data support the need for future research in this area, including prospective, randomized trials. HDR brachytherapy offers advantages compared to external beam radiotherapy, but the properly selected patients to benefit from this treatment remain to be defined.
References
- 1.Pisters P, Harrison L, Leung D, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft-tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Narayana A, Cohen G, Zaider M, et al. High-dose-rate interstitial brachytherapy in recurrent and previously irradiated head and neck cancers – preliminary results. Brachytherapy. 2007;6:157–163. doi: 10.1016/j.brachy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Pohar S, Haq R, Lizhong L, et al. Adjuvant high-dose-rate and low-dose-rate brachytherapy with external beam radiation in soft tissue sarcoma: a comparison of outcomes. Brachytherapy. 2007;6:53–57. doi: 10.1016/j.brachy.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Laskar S, Bahl G, Puri A, et al. Perioperative interstitial brachytherapy for soft tissue sarcomas: prognostic factors and long-term results of 155 patients. J Surg Oncol. 2006;14:560–567. doi: 10.1245/s10434-006-9137-2. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan B, Davis A, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 6.Heller L, Ballo MT, Cormier JN, et al. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg. 2008;60:58–63. doi: 10.1097/01.sap.0000258956.66990.9a. [DOI] [PubMed] [Google Scholar]
- 7.Duman H, Evans GR, Reece G, et al. Brachytherapy: reconstructive options and the role of plastic surgery. Ann Plast Surg. 2000;45:477–480. doi: 10.1097/00000637-200045050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Davis A, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities. Prospective randomized evaluations of (1) Limb-sparing surgery plus radiation therapy compared amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–314. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 11.Chun M, Kang S, Kim B, et al. High dose rate interstitial brachytherapy in soft tissue sarcoma: Technical aspects and results. Jpn J Clin Oncol. 2001;31:279–283. doi: 10.1093/jjco/hye050. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblatt E, Mueshar N, Eidelman M, et al. Low dose-rate interstitial brachytherapy in soft tissue sarcomas. Sarcoma. 1999;3:101–105. doi: 10.1080/13577149977721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crownover R, Marks K. Adjuvant brachytherapy in the treatment of soft-tissue sarcomas. Brachytherapy. 1999;13:595–607. doi: 10.1016/s0889-8588(05)70078-2. [DOI] [PubMed] [Google Scholar]
- 14.Nag S, Martinez-Monge R, Ruymann F, et al. Innovation in the management of soft tissue sarcomas in infants and young children: high-dose-rate brachytherapy. J Clin Oncol. 1997;15:3075–3084. doi: 10.1200/JCO.1997.15.9.3075. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi M, Inoue T, Yamazaki, et al. Perioperative fractionated high-dose rate brachytherapy for malignant bone and soft tissue tumors. Int J Radiat Oncol Biol Phys. 1999;43:989–993. doi: 10.1016/s0360-3016(98)00491-x. [DOI] [PubMed] [Google Scholar]
- 16.Laskar S, Bahl G, Muckaden M, et al. Interstitial brachytherapy for childhood soft tissue sarcoma. Pediatr Blood Cancer. 2007;39:649–655. doi: 10.1002/pbc.21118. [DOI] [PubMed] [Google Scholar]
- 17.Petera J, Neumanova Rzaki H, Odrazka K, et al. Perioperative fractionated high-dose rate brachytherapy in the treatment of soft tissue sarcomas. Neoplasma. 2004;51:59–63. [PubMed] [Google Scholar]
- 18.Nag S, Tippin D, Ruymann F. Long-term morbidity in children treated with fractionated high-dose-rate brachytherapy for soft tissue sarcomas. J Pediatric Hemat/Oncol. 2003;25:448–452. doi: 10.1097/00043426-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Alekhteyar K, Leung D, Brennan M, et al. The effect of combined external beam radiotherapy and brachytherapy on local control and wound complications in patients with high-grade soft tissue sarcomas of the extremity with positive microscopic margins. Int J Radiat Oncol Biol Phys. 1996;36:321–324. doi: 10.1016/s0360-3016(96)00331-8. [DOI] [PubMed] [Google Scholar]
- 20.Torres M, Ballo M, Butler C, et al. Management of locally recurrent soft-tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124–1129. doi: 10.1016/j.ijrobp.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Pearlstone D, Janjan N, Feig B, et al. Re-resection with brachytherapy for locally recurrent soft tissue sarcoma arising in a previously radiated field. Cancer J Sci Am. 1999;5:26–33. [PubMed] [Google Scholar]
- 22.Nori D, Shupak K, Shiu M, et al. Role of brachytherapy in recurrent extremity sarcoma in patients treated with prior surgery and irradiation. Int J Radiat Oncol Biol. 1991;20:1229–1233. doi: 10.1016/0360-3016(91)90232-s. [DOI] [PubMed] [Google Scholar]
- 23.Catton C, Davis A, Bell R, et al. Soft tissue sarcoma of the extremity. Limb salvage after failure of combined conservative therapy. Radiother Oncol. 1996;41:209–214. doi: 10.1016/s0167-8140(96)01856-7. [DOI] [PubMed] [Google Scholar]
- 24.Habrand JA, Pejovic M, et al. Twenty years experience of interstitial iridium brachytherapy in the management of soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1991;20:405–410. doi: 10.1016/0360-3016(91)90049-a. [DOI] [PubMed] [Google Scholar]