Abstract
Background:
Volumetric analysis of the olfactory cleft by using computed tomography has been associated with olfaction in patients with chronic rhinosinusitis (CRS). However, existing studies have not comprehensively measured olfaction, and it thus remains unknown whether correlations differ across specific dimensions of odor perception.
Objective:
To use comprehensive measures of patient-reported and objective olfaction to evaluate the relationship between volumetric olfactory cleft opacification and olfaction.
Methods:
Olfaction in patients with CRS was evaluated by using “Sniffin' Sticks” tests and a modified version of the Questionnaire of Olfactory Disorders. Olfactory cleft opacification was quantified by using two- and three-dimensional, computerized volumetric analysis. Correlations between olfactory metrics and olfactory cleft opacification were then calculated.
Results:
The overall CRS cohort included 26 patients without nasal polyposis (CRSsNP) (68.4%) and 12 patients with nasal polyposis (CRSwNP) (31.6%). Across the entire cohort, total olfactory cleft opacification was 82.8%, with greater opacification in the CRSwNP subgroup compared with CRSsNP (92.3 versus 78.4%, p < 0.001). The percent total volume opacification correlated with the total Sniffin' Sticks score (r = −0.568, p < 0.001) as well as individual threshold, discrimination, and identification scores (p < 0.001 for all). Within the CRSwNP subgroup, threshold (r = −0.616, p = 0.033) and identification (r = −0.647, p = 0.023) remained highly correlated with total volume opacification. In patients with CRSsNP, the threshold correlated with total volume scores (r = −0.457, p = 0.019), with weaker and nonsignificant correlations for discrimination and identification. Correlations between total volume opacification and the Questionnaire of Olfactory Disorders were qualitatively similar to objective olfactory findings in both CRSwNP (r = −0.566, p = 0.070) and CRSsNP (r = −0.310, p = 0.141) subgroups, although neither reached significance. When examined by two-dimensional planes, the percent opacification of the anterior plane had the strongest correlations with objective olfaction.
Conclusion:
Olfactory cleft opacification correlated with objective measures of olfaction in patients with CRS, which correlated with threshold values in patients with CRSsNP and all dimensions of olfaction in those with CRSwNP.
Keywords: Sinusitis, computed tomography, olfaction, threshold, discrimination, identification, sniffin sticks, questionnaire of olfactory disorders, opacification, olfactory cleft
Chronic rhinosinusitis (CRS) is a common cause of olfactory loss, with 28–84% of patients experiencing a decreased sense of smell.1,2 Impaired olfaction in CRS is thought to result from several mechanisms. A conductive etiology may be present if odorants are unable to reach the olfactory cleft because of a physical barrier created by polyps, mucosal edema, or nasal discharge.2 A sensorineural etiology may occur if direct inflammation of the olfactory cleft neuroepithelium impedes the propagation of sensory signals to higher-order cortical structures.2 In both instances, one might see perceptible inflammation in the olfactory cleft on endoscopy or imaging. A diminished sense of smell has been widely linked to decreased quality of life (QOL) and major depressive disorder.3 However, attempts to classify patients with olfactory dysfunction to guide treatment decision-making and disease prognostication remain limited.
Attempts to objectively quantify the inflammation in CRS and identify associations with olfactory dysfunction have revolved around computed tomography (CT) staging. However, the most commonly used CT scoring method, the Lund-Mackay scoring system, focuses solely on the paranasal sinuses and does not consider disease in the olfactory cleft.4–6 This may explain why previous studies reported weak or moderate correlations between CT scores and olfaction.7–10 Certainly, one can imagine a scenario with extensive sinus disease yet a clear olfactory cleft or the contrary scenario with central nasal cavity polyps that are blocking the olfactory cleft but with relatively clear sinuses.
Recent studies have begun to analyze the association between olfactory cleft opacification on CT and olfactory dysfunction.4,11–14 Initial studies used semiquantitative olfactory cleft grading systems and/or only examined limited aspects of olfaction. Results of these studies indicated that opacification of the olfactory cleft was a better predictor of olfactory loss than opacification of the sinuses proper. Recently, we extended these findings by using computerized volumetric assessment of olfactory cleft opacification. This initial study demonstrated a significant correlation between olfactory cleft opacification and olfaction in patients with polyps, but no such correlation was seen in patients without polyps. One limitation of this study was reliance on the 40-item Smell Identification Test, which only examines one aspect of olfaction, identification without assessment of threshold or discrimination.6
A comprehensive assessment of olfactory function requires measurement of odor threshold, discrimination, and identification. Threshold, discrimination, and identification are each distinct dimensions of odor perception that involve separate cortical structures and neural pathways.15 To date, studies that examined olfactory cleft opacification have not comprehensively measured olfaction, and, thus, it remains unknown whether correlations differ across specific dimensions of odor perception. The objectives of this study were (1) to evaluate the relationship between volumetric assessment of olfactory cleft opacification and comprehensive measures of olfaction, and (2) to validate initial results of volumetric grading by using a separate and distinct patient population.
METHODS
Study Population
Adults (≥18 years) who met the diagnostic criteria for CRS established by the 2012 European Position Paper on Rhinosinusitis and Nasal Polyps16 were recruited from a rhinology clinic at the Medical University of South Carolina (Charleston, SC). Patients were excluded if they had sinus surgery within the past 6 months, were on systemic corticosteroids or immunotherapy within the past month, or had a preexisting condition that caused systemic inflammation. Demographic information and history of comorbidities, such as asthma, aspirin intolerance, allergies, chronic obstructive pulmonary disease, depression, fibromyalgia, immunodeficiency, autoimmune diseases, and diabetes, were collected by using standardized questionnaires. The institutional review board at the Medical University of South Carolina approved this study, and all the subjects provided written informed consent.
Measures of CRS Severity
All the patients had a CT obtained as part of the standard of care before the enrollment date. The enrolling physician scored sinonasal opacification by using the Lund-Mackay staging system. Nasal endoscopy was used to categorize each patient as having CRS with polyps (CRSwNP) or CRS without polyps (CRSsNP) and, in addition, was scored by using the semiquantitative Lund-Mackay staging system. Study patients completed the 22-item Sino-Nasal Outcome Test, which requires patients to rate 22 symptom-related items on a scale that ranges from 0 (no problem) to 5 (problem is as bad as it can be).17 Higher scores correlate with greater impact of disease.
Olfactory Evaluation
Objective Olfaction.
Objective olfactory testing was performed by using Sniffin' Sticks (Burghardt, Wedel, Germany).18 Testing was performed by a trained clinical research coordinator (K.S.) blinded to other clinical data. The testing battery performed included odor threshold, odor discrimination, and odor identification. The odor threshold test was performed by using dilutions of n-butanol in a single-staircase, triple-forced choice procedure. The odor discrimination test used triplets of pens presented in random order with two that contained the same odorant and the third contained a different odorant. The odor identification test involved 16 odorants presented at suprathreshold intensity by using multiple-choice procedures. The subjects were blindfolded to avoid visual identification of odorant-containing pens. Each of the three individual tests was scored from 0 to 16. The overall results were combined and reported as a composite threshold-discrimination-identification score (TDI), with higher scores representing better olfaction (0–48).
Olfactory QOL.
Olfactory-specific QOL was assessed by using the previously validated, short, modified version of the Questionnaire of Olfactory Disorders (QOD-NS).19 This instrument consists of 17 negative statements that are graded on a scale from 0 to 3 for a total score that ranges from 0 to 51. The QOD-NS was graded such that higher scores reflected better QOL.
Olfactory Cleft Radiologic Evaluations
Three-Dimensional Volumetric Analysis.
Volumetric analysis of CT images was performed by using the Mayo Clinic Analyze 12.0 software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). By using coronal cuts, the olfactory cleft was considered to be a three-dimensional space that started at the anterior-most olfactory filament (the anterior plane of the middle turbinate) and ended just anterior to the face of the sphenoid sinus.19,20 The lateral boundary of the olfactory cleft was the attachment of the middle and/or superior turbinates. The roof of the olfactory cleft was the cribriform plate, and the floor was an imaginary line drawn 1 cm inferior to the cribriform. Segmentation was done manually by authors (P.K. and Z.M.S.) blinded to other study data, including olfactory function. We previously showed this technique to have high interrater and intra-rater reliability.6 To assess the olfactory cleft, the range of −1024 to −500 Hounsfield units was used for air, and values above this range were considered to represent opacification. The percent opacification was defined as (1 − [total volume of air/total volume of olfactory cleft]) × 100.
Two-Dimensional Cross-Sectional Analysis.
Three coronal plane sections of the olfactory cleft were demarcated: anterior, middle, and posterior (i.e., two-dimensional planes). The anterior section was the coronal plane at the anterior limit of the olfactory cleft. The middle section was the coronal plane of the posterior globe. The posterior section was the coronal plane just anterior to the face of the sphenoid. Quantitative analysis was performed by using Analyze 12.0 software to determine the exact percentage of opacification for each of the demarcated coronal olfactory cleft sections.
Statistical Analysis
Data analysis was performed by using SPSS 23.0 (IBM Corporation, Armonk, NY). Demographic information, comorbidities, quantitative assessments of olfaction, disease severity, and QOL were investigated by using descriptive statistics. Continuous variables were assessed for normality by using the Shapiro-Wilk test and were compared between polyp and nonpolyp groups by using independent t-tests or Mann-Whitney U tests. Categorical variables were compared by using the χ2 or Fisher's exact test. The Pearson or Spearman correlations, depending on linearity, were used to characterize associations between olfaction and opacification; p values of <0.05 were considered statistically significant.
RESULTS
Study Population
There were a total of 38 patients with CRS in the overall cohort, of whom, 26 (68.4%) had CRSsNP and 12 (31.6%) had CRSwNP. Baseline demographic characteristics and medical comorbidities are described in Table 1. The mean (standard deviation [SD]) total Sniffin' Sticks test score for the cohort was 22.8 (9.7) (range, 7.0–38.5). The mean (SD) of additional measures of disease severity are illustrated in Table 2. The average total volume (SD) opacification of the olfactory cleft was 82.8% (12.6) and ranged from 56.5 to 100%. Opacification was significantly higher in CRSwNP than in CRSsNP (92.3 versus 78.4%; p < 0.001). Mean cross-sectional opacification (SD) of the anterior, middle, and posterior cross sections was 77.0% (13.9), 81.2% (13.1), and 83.3% (13.5), respectively. These results, in addition to subgroup analysis, are summarized in Table 3.
Table 1.
Baseline demographic characteristics of study population
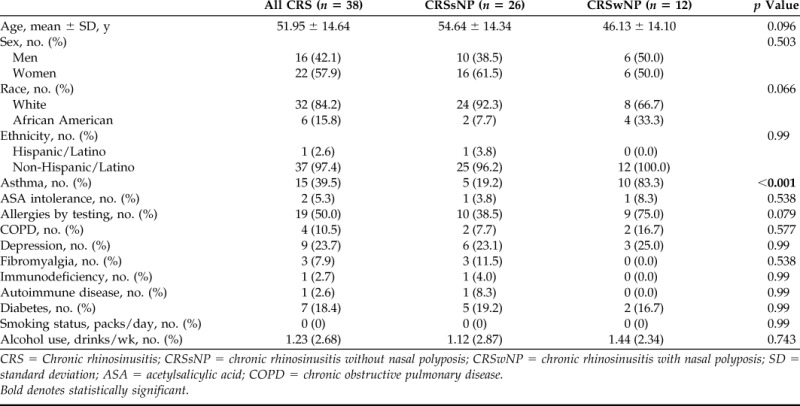
CRS = Chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyposis; CRSwNP = chronic rhinosinusitis with nasal polyposis; SD = standard deviation; ASA = acetylsalicylic acid; COPD = chronic obstructive pulmonary disease.
Bold denotes statistically significant.
Table 2.
Olfactory test scores of the study population
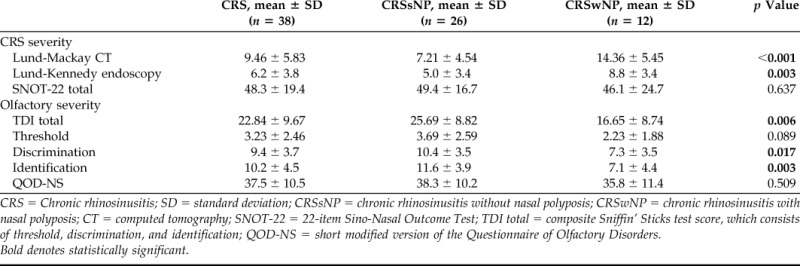
CRS = Chronic rhinosinusitis; SD = standard deviation; CRSsNP = chronic rhinosinusitis without nasal polyposis; CRSwNP = chronic rhinosinusitis with nasal polyposis; CT = computed tomography; SNOT-22 = 22-item Sino-Nasal Outcome Test; TDI total = composite Sniffin' Sticks test score, which consists of threshold, discrimination, and identification; QOD-NS = short modified version of the Questionnaire of Olfactory Disorders.
Bold denotes statistically significant.
Table 3.
Percent opacification: Total volume and cross-sectional area
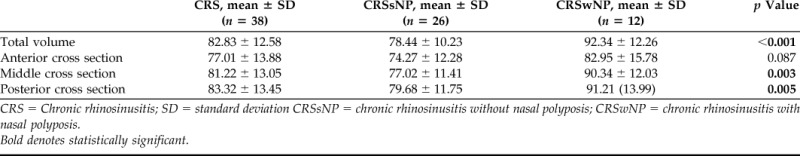
CRS = Chronic rhinosinusitis; SD = standard deviation CRSsNP = chronic rhinosinusitis without nasal polyposis; CRSwNP = chronic rhinosinusitis with nasal polyposis.
Bold denotes statistically significant.
Volumetric Analysis and Olfaction: Overall CRS cohort
In the overall cohort, the percent total volume opacification significantly correlated with objective olfaction, including TDI total scores and individual threshold, discrimination, and identification scores (Table 4). Thus, the higher the percent opacification of the entire olfactory cleft, the worse olfactory function. When specific cross sections of the olfactory cleft were examined, each region highly correlated with objective olfaction, with the strongest correlations seen in the anterior and posterior sections. The percentage of the total volume opacification seemed to also correlate with olfactory-specific QOL as measured by the QOD-NS (r = −0.297, p = 0.084), although this association was weaker and did not quite reach significance.
Table 4.
Correlation of opacification and olfaction in all the patients with CRS (n = 38)
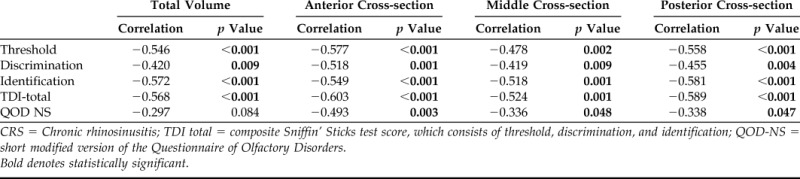
CRS = Chronic rhinosinusitis; TDI total = composite Sniffin' Sticks test score, which consists of threshold, discrimination, and identification; QOD-NS = short modified version of the Questionnaire of Olfactory Disorders.
Bold denotes statistically significant.
Volumetric Analysis and Olfaction: Subgroup Analysis
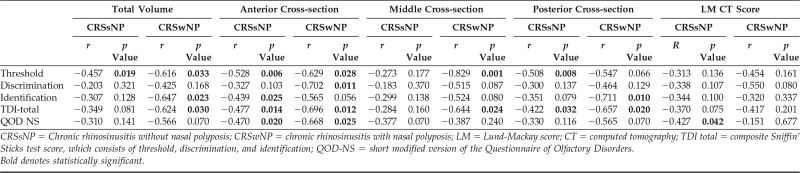
The correlation between olfactory cleft opacification and olfaction was then examined across subgroups defined by polyp status (Table 5). The total volume remained highly correlated to TDI total scores in patients with CRSwNP (r = −0.624, p = 0.030) but seemed weaker in patients with CRSsNP and no longer reached significance (r = −0.349, p = 0.081). When examining dimensions of olfaction, threshold (r = −0.616, p = 0.033) and identification (r = −0.647, p = 0.023) remained highly correlated with total volume opacification of the olfactory cleft in patients with CRSwNP. In patients with CRSsNP, the threshold significantly correlated with total volume scores (r = −0.457, p = 0.019), with weaker and nonsignificant correlations for discrimination and identification scores. Correlations between total volume opacification and QOD-NS were qualitatively similar to objective olfactory findings in both CRSwNP (r = −0.566, p = 0.070) and CRSsNP (r = −0.310, p = 0.141) subgroups, although neither reached significance. Correlations between Lund-Mackay CT scores and objective olfaction were not significant for any measure.
Table 5.
Correlation of opacification and olfaction in CRSsNP (n = 26) and CRSwNP (n = 12)
CRSsNP = Chronic rhinosinusitis without nasal polyposis; CRSwNP = chronic rhinosinusitis with nasal polyposis; LM = Lund-Mackay score; CT = computed tomography; TDI total = composite Sniffin' Sticks test score, which consists of threshold, discrimination, and identification; QOD-NS = short modified version of the Questionnaire of Olfactory Disorders.
Bold denotes statistically significant.
When specific cross sections of the olfactory cleft were examined by polyp status, findings in the CRSwNP group closely mirrored overall total volume findings (Table 5). However, in the CRSsNP group, correlations in the anterior and posterior cross sections were actually more robust than the middle cross section or overall total volume. For the anterior cross section in patients with CRSsNP, percent opacification correlated with TDI total scores (−0.477, p = 0.014) as well as threshold (r = −0.528, p = 0.006) and identification (r = −0.439, p = 0.025). In the posterior cross section, percent opacification correlated with TDI total scores (r = −0.422, p = 0.032) and threshold (r = −0.508, p = 0.008). Opacification of the anterior cross section also correlated with QOD-NS in the overall group (r = −0.493, p = 0.003) as well as CRSsNP (r = −0.470, p = 0.020) and CRSwNP (r = −0.668, p = 0.025) subgroups (Tables 4 and 5).
DISCUSSION
The current study added to the overall body of research that supports the use of olfactory cleft opacification scores to predict olfactory loss in patients with CRS. We previously found a strong correlation between the 40-item Smell Identification Test and total volume opacification in CRSwNP but were unable to demonstrate an association in CRSsNP. The results of the current study corroborated these findings by using olfactory identification as measured by the Sniffin' Sticks test in a completely separate and distinct patient population. However, olfactory cleft opacification was found to significantly correlate with threshold values in CRSsNP, which indicated that it had informative value in the CRSsNP population as well.
Olfactory threshold is a test of basic acuity and measures the minimum stimulus strength needed to detect odors.20 Conversely, identification testing involves presentation of odorants at suprathreshold values. When comparing CRSsNP and CRSwNP subgroups, a few things were readily apparent. Overall, the subjects with CRSwNP have worse total TDI scores, which are driven mainly by decreased identification and discrimination compared with patients with CRSsNP. The threshold seemed to be decreased in both groups, although the difference between the groups was not as extreme and failed to reach significance. Based on these findings, it was not surprising that correlations between olfactory cleft opacification and olfaction were only seen for threshold scores in patients with CRSsNP because this was the olfactory dimension most impacted in that subgroup and would thus have the highest power. These findings indicated that relying on a single olfactory dimension has the potential of overlooking existing relationships, even though individual olfactory dimensions are usually correlated statistically. Future studies might explore why differences existed across olfactory domains between patients with CRSsNP and patients with CRSwNP.
Although the calculation of total volume opacification provides the most complete assessment of olfactory cleft opacification, it is laborious and would be difficult to implement in a clinical setting. However, analyzing cross sections of a sinus CT is much more feasible for clinical use or large-scale research studies. When comparing cross sections, the anterior plane seemed to provide the most information across both subgroups, equivalent or perhaps even superior to total-volume opacification. Opacification of the posterior plane also provided good correlations, whereas the middle plane had notably weaker correlations in patients with CRSsNP. For clinical use or future large-scale studies, consideration could be given to only assessing the anterior plane or perhaps both anterior and posterior planes because these are most predictive and likely impact anterograde and retrograde movement of odorants, respectively.
To our knowledge, this was the first study to query whether olfactory cleft opacification also impacts olfactory-specific QOL. When it comes to sinus-specific QOL, the severity of patient-reported QOL does not always correlate with objective measures of disease severity on imaging. Within this patient cohort, those with greater olfactory cleft opacification reported worse olfactory-specific QOL as measured by the QOD-NS, although not all correlations were statistically significant. The fact that olfactory cleft opacification seemed to correlate with both objective and patient-reported olfactory outcomes indicated that it was a clinically relevant metric. Again, the percent opacification of the anterior cross section had the strongest correlations to QOD-NS in both CRSsNP and CRSwNP populations.
The main strengths of this study were the comprehensive evaluation of olfaction, including both objective and patient-reported metrics, as well as quantitative CT scoring. There were several important limitations worth considering. The study population was derived solely from a tertiary medical center, including many patients with severe olfactory dysfunction. Findings, therefore, may not be generalizable to all patients with CRS, and future studies should aim to include patients with milder forms of disease. Overall, the study had a relatively small sample that limited power available for some analyses. Focus on anterior-posterior cross sections would make larger scale studies more feasible in the future, particularly if olfactory cleft opacification is used as one of several measures to predict olfaction. Also, the process of calculating the percent opacification of the olfactory cleft is subject to anatomic variation. For example, patients with curved middle turbinates or naturally wide bony septums could make it difficult to determine the precise dimensions of the olfactory cleft, and this could alter the opacification values and introduce unnecessary noise, which weakens correlations to olfaction. Imaging analytic techniques could potentially be refined in the future to further enhance the predictive ability of olfactory cleft opacification.
CONCLUSION
The percentage of olfactory cleft opacification correlated with objective measures of olfaction and olfactory-specific QOL in patients with CRS. Significant correlations were seen for all dimensions of olfaction in the patients with CRSwNP, whereas threshold values correlated most strongly in patients with CRSsNP. The percent opacification of the anterior cross section seemed to offer the most predictive information in both CRSsNP and CRSwNP subgroups.
Footnotes
Z.M. Soler is supported for this investigation by a grant from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (P.I., Z.M. Soler; R03 DC013651–01); is a consultant for Olympus, which is not affiliated with this manuscript. R.J. Schlosser is supported by grants from OptiNose and IntersectENT, neither are associated with this manuscript; is also a consultant for Olympus, Meda, and Arrinex, which are not affiliated with this study. The remaining authors have no conflicts of interest pertaining to this article
REFERENCES
- 1. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life–an updated review. Chem Senses 39:185–194, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Thompson CF, Kern RC, Conley DB. Olfaction in endoscopic sinus and skull base surgery. Otolaryngol Clin North Am 48:795–804, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Rudmik L, Smith TL. Quality of life in patients with chronic rhinosinusitis. Curr Allergy Asthma Rep 11:247–252, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Chang H, Lee HJ, Mo JH, et al. Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg 135:988–992, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Pallanch JF, Yu L, Delone D, et al. Three-dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: Clinical correlations and comparison to Lund-Mackay scoring. Int Forum Allergy Rhinol 3:963–972, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soler ZM, Pallanch JF, Sansoni ER, et al. Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 5:846–854, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol 22:445–448, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy 23:139–144, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuperan AB, Lieberman SM, Jourdy DN, et al. The effect of endoscopic olfactory cleft polyp removal on olfaction. Am J Rhinol Allergy 29:309–313, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Gupta D, Gulati A, Singh I, Tekur U. Impact of endoscopic sinus surgery on olfaction and use of alternative components in odor threshold measurement. Am J Rhinol Allergy 29:e117–e120, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Vandenhende-Szymanski C, Hochet B, Chevalier D, Mortuaire G. Olfactory cleft opacity and CT score are predictive factors of smell recovery after surgery in nasal polyposis. Rhinology 53:29–34, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Kim BG, Kang JM, Shin JH, et al. Do sinus computed tomography findings predict olfactory dysfunction and its postoperative recovery in chronic rhinosinusitis patients? Am J Rhinol Allergy 29:69–76, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy 25:e90–e94, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Kim DW, Kim KJ, Kim SW, Jeon SY. Postoperative olfactory results in chronic rhinosinusitis with nasal polyposis according to wound healing status. Clin Exp Otorhinolaryngol 6:146–151, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gross-Isseroff R, Luca-Haimovici K, Sasson Y, et al. Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry 35:798–802, 1994. [DOI] [PubMed] [Google Scholar]
- 16. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Morley AD, Sharp HR. A review of sinonasal outcome scoring systems—Which is best? Clin Otolaryngol 31:103–109, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Kobal G, Hummel T, Sekinger B, et al. “Sniffin' sticks”: Screening of olfactory performance. Rhinology 34:222–226, 1996. [PubMed] [Google Scholar]
- 19. Simopoulos E, Katotomichelakis M, Gouveris H, et al. Olfaction-associated quality of life in chronic rhinosinusitis: Adaptation and validation of an olfaction-specific questionnaire. Laryngoscope 122:1450–1454, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Sanders RD, Gillig PM. Cranial nerve I : Olfaction. Psychiatry (Edgmont) 6:30–35, 2009. [PMC free article] [PubMed] [Google Scholar]