Abstract
Aspirin-exacerbated respiratory disease (AERD) is a late onset condition characterized by the Samter triad (aspirin sensitivity [as well as sensitivity to any nonselective cyclooxygenase inhibitor], nasal polyps, asthma) and additional features, including eosinophilic chronic rhinosinusitis, hypereosinophilia, anosmia, frequent absence of atopy, and, intolerance to ingestion of red wine and other alcoholic beverages. The diagnosis is rare, and, because of this, it is also often missed by physicians. However, it is highly overexpressed in patients with severe asthma (and severe chronic rhinosinusitis with nasal polyps), which makes its recognition essential. For this review, we considered mechanisms involved in the pathogenesis of this disease and discussed the clinical symptoms of AERD. We also discussed the role of aspirin desensitization in the treatment of AERD. Also, we considered medications (e.g, leukotriene modifiers) and surgical interventions that have a role in the treatment of AERD.
Keywords: Aspirin-exacerbated respiratory disease, aspirin sensitivity, aspirin desensitization, leukotriene modifiers, surgical intervention
Aspirin-exacerbated respiratory disease (AERD) was originally defined in 1922 as discussed by Stevenson and Szczeklik1 and Widal et al.2 However, clues regarding its existence were evident even earlier than this. A decade before, Gilbert3 first noted an aspirin-induced asthma attack, and, in 1902, Hirschberg4 described adverse reactions to aspirin in Germany, only 4 years after aspirin was introduced into the market. Despite the evidence, AERD was not widely recognized until 1968 when Samter and Beers5,6 described patients with nasal polyps (NP), asthma, and aspirin sensitivity, a triad of symptoms ultimately known as the Samter triad.1,5,6
It has become clear that clinicians routinely miss the diagnosis of AERD because of an insufficient index of suspicion. For example, when considering all the patients with a diagnosis of asthma, those with AERD comprise only 0.6–2.5% of this population.7 However, AERD represents 14.9% of all the patients with severe asthma and 8.7% of the patients with chronic rhinosinusitis with nasal polyps (CRSwNP).7 Furthermore, aspirin intolerance occurs in as many as 20% of patients with adult onset asthma without sinus disease.8,9 For this review, we considered the mechanisms involved in this disease and discussed the clinical symptoms of AERD. We also discussed the role of aspirin desensitization in the treatment of AERD. We considered medications that have a role in its treatment.
NATURAL HISTORY AND PREVALENCE OF DISEASE
Unlike other allergic inflammatory conditions, AERD develops in the third or fourth decade of life.1 The disease has a male predominance (2.3:1)10; when diagnosed in women, the disease is usually more severe.11 In most studies, rhinitis symptoms typically preceded asthma by 1–5 years, and these symptoms can be severe, including chronic congestion, anosmia, and NP.1,9
PATHOPHYSIOLOGY AND MECHANISMS OF DISEASE
The underlying respiratory and sinus inflammatory processes of AERD activate an intense infiltration of mast cells, basophils, and eosinophils into the mucosa that synthesize and secrete high levels of cysteinyl leukotrienes (CysLT).12,13 Mast cells also release histamine, tryptase, and prostaglandin (PG) D2, vasodilating and bronchoconstricting agents that augment the leukotriene (LT) response. Patients with AERD displayed dramatic upregulation of two essential enzymes involved in CysLT synthesis: 5-lipoxygenase (5-LO) and LTC4 synthase.14–16 This overexpression drives both the constitutive overproduction of CysLTs and the life-threatening surge that occurs with ingestion of aspirin and other nonselective cyclooxygenase (COX) inhibitors.17
In addition to their overproduction, these patients display greatly enhanced sensitivity to the CysLTs, which reflects overexpression of the CysLT receptors.18,19 As indicated by the distinct sensitivity of these patients to LTE4, AERD is likely also associated with enhanced expression of one or more of the newly described selective LTE4 receptors.20–22 Both blood and sinonasal tissue of patients with AERD have a significant number of platelets adhered to neutrophils, monocytes, and eosinophils.23 Platelets express LTC4 synthase and can use it to generate CysLTs via transcellular transfer of LTA4 derived from these 5-LO–expressing leukocytes. It is estimated that up to 70% of CysLTs produced in AERD are generated via this mechanism.23,24
CHRONIC SINUSITIS WITH NPs
Patients with AERD have evolving sinusitis that starts as mild mucosal inflammation and progresses into a severe persistent disease that often completely fills the sinus cavities with inflammatory tissue and becomes associated with NP.1 The NPs are intensely eosinophilic, and most patients with AERD have anosmia.1,25 Computed tomographies of subjects with AERD showed pansinusitis and are typically some of the worst seen in chronic sinus disease, with complete or near-complete opacification of the sinuses.26 In reflecting the progressive nature of this inflammatory process, surgery is unlikely to be curative. Even when followed by optimal medical therapy, patients with AERD typically required multiple revision surgeries in their lifetime.1,10
ASPIRIN SENSITIVITY
In patients with AERD, aspirin and other nonselective nonsteroidal anti-inflammatory drugs (NSAID) that inhibit COX-1 induce unique non–immunoglobulin E (IgE) mediated reactions that consist of attacks of rhinitis and asthma.1 COX-2 inhibitors, including celecoxib, are generally (but not always) tolerated, but there is a somewhat greater risk of reaction with less selective COX-2 inhibitors, e.g., meloxicam, especially at higher doses.5,6,27 Despite general tolerance of selective COX-2 inhibitors in patients with AERD, it is recommended that test doses be performed under strict and careful clinical purview if these drugs are required. Reactions to acetaminophen are unusual but can occur.28–32 The dogma indicates that reactions in patients with AERD are triggered by the inhibition of COX-1, which is not a biologic activity of nonaspirin salicylates and, as such, dietary salicylates are generally not thought to trigger reactions. However, results of recent research showed that a low-salicylate diet perhaps leads to a reduction in symptoms in some patients with AERD.33 Although limited by being a single-blind study, this finding indicated that, in patients who are refractory to regular management, one might consider a low salicylate diet in addition to typical treatments. Reactions to aspirin in patients with AERD do not develop until systemic concentrations of these agents achieve pharmacologically active concentrations and, as such, generally occur 30–90 minutes after a therapeutic oral dose but can be delayed up to 3 hours after ingestion. Symptoms include a spectrum of respiratory reactions, including rhinitis, flushing, congestion, laryngospasm, and asthma exacerbations.1
ASTHMA
Asthma is not always present, thus, the current preference for the term AERD rather than “aspirin intolerant asthma.” Typically, in AERD, asthma symptoms develop ∼1 to 3 years after the development of rhinitis but can occur even later or, in certain instances, never develop.11 When present, the asthma of AERD, much like the sinus disease component, is severe and difficult to treat. AERD is also linked to increased airway remodeling, which results in increased residual volume and diminished diffusing capacity.26,34
GASTROINTESTINAL AND DERMATOLOGIC REACTORS
Recently, Cahill et al.35 described a new phenotype of AERD. These patients have predominant gastrointestinal and dermatologic findings after exposure to aspirin and are frequently unable to tolerate aspirin desensitization, despite the use of a CysLT receptor antagonists. Symptoms with exposure to COX-1 inhibitors include stabbing abdominal pain, nausea, watery diarrhea, and an erythematous and pruritic macular rash that erupts on the distal extremities and palmar surfaces and not accompanied by urticaria or angioedema. These patients have higher baseline levels of CysLTs and demonstrate no suppression of prostanoid synthesis at threshold aspirin doses.35 Specifically, they were found to produce significantly more PGD2, a potent bronchoconstrictor, during the reactions in comparison with the larger AERD population that tolerated aspirin desensitization.35,36
DIAGNOSIS
Diagnosis of AERD can be determined with some degree of certainty in a patient with more than one compelling history of a reaction to aspirin or other nonselective NSAID, especially in the setting of extensive chronic rhinosinusitis, NPs, anosmia, and severe asthma. Interestingly, up to 70% of these patients report sensitivity to red wine and other alcoholic beverages, and this history may also indicate the diagnosis of AERD.37 There is no reliable in vitro diagnostic test for AERD, and, as such, in the absence of a history of aspirin or NSAID use or an ambiguous history of symptoms after exposure, the patient should be referred for aspirin challenge to receive a definitive diagnosis.1,10,38,39 Diagnostic challenges must be performed without LT modifiers (in contrast to desensitization [as described below]) because these agents can completely mask the symptoms and signs of a reaction.17,40–42 As such, this diagnostic tool must be used with caution because the expected reaction to aspirin in a patient who is allergic has the potential to induce severe bronchospasm.10
Aspirin challenge should only be performed after confirmation that the patient's forced expiratory volume in 1 second is within 10% of his or her previous best values and also is ≥60% of the predicted value, and it is imperative that the patient's asthma symptoms are well controlled at the time of challenge. Responses to aspirin can be delayed by as much as 90 minutes or more after ingestion; therefore, progressive doses should be at least this far apart, and the physician must be prepared to monitor the patient for 2–3 hours after ingestion. Oral aspirin challenge can be performed by using graduated doses of aspirin given over 2 days (Table 1).43 The dose immediately below the reacting dose for provocation challenge can be used as the starting dose for a subsequent desensitization if required.
Table 1.
Aspirin challenge

ACE = Angiotensin-converting-enzyme; FEV1 = forced expiratory volume in 1 second.
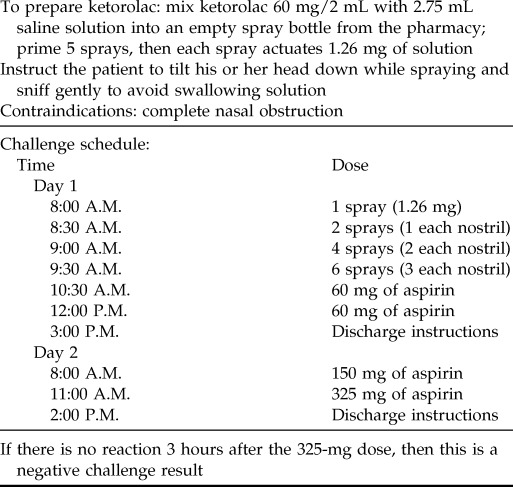
An alternative and perhaps safer approach to evaluate aspirin sensitivity is to perform diagnostic nasal ketorolac challenge. This method is not indicated for patients with severe nasal obstruction, including those with significant polyposis. To perform this procedure, liquid ketorolac is diluted into a spray container that will deliver ketorolac intranasally. Doses are administered at 30-minute intervals in a progressive fashion, as shown in Table 2. If the patient does not experience any nasal symptoms or bronchospasm with ketorolac, then oral challenges of aspirin are given at 2–3 hour intervals (Table 2).
Table 2.
Ketorolac challenge
BIOMARKERS FOR DIAGNOSIS OF AERD
Because of the inherent risks associated with diagnostic aspirin challenge in patients who may have AERD, researchers are evaluating other ways to confirm aspirin sensitivity. Several biomarkers have been evaluated, and the most promising is 24-hour urinary LTE4. Higher baseline levels of urinary LTE4 levels are typical in AERD and, in contrast to patients with aspirin-tolerant asthma, these levels dramatically increase after aspirin challenge.44,45 Follow-up studies demonstrated that ≥166 pg LTE4/mg creatinine indicates the presence of AERD with 89% specificity, whereas a ≥241 pg LTE4/mg creatinine discriminated subjects with “challenge-confirmed” aspirin sensitivity with 92% specificity.46
Exhaled nitric oxide (FeNO) levels is used as a biomarker for assessing acute bronchospasm and for supervising asthma control over time.44,47,48 A recent study discovered that giving low-dose aspirin (40 mg) and measuring FeNO levels 1 hour later produced a significant decrease from baseline in mean FeNO by 19% in patients with AERD. In the study, FeNO had a sensitivity and specificity of 90% and 100%, respectively, for identifying AERD. In contrast, patients with aspirin tolerant asthma had increased or stable FeNO levels.44 Due to the possibility of severe reactions during standard aspirin challenge, this diagnostic tool needs to be further investigated because of the potential for a more-convenient diagnosis.
TREATMENT
Symptomatic control of both the upper and lower airway is essential in preventing asthma exacerbations and airway remodeling, and in improving overall quality of life. Treatment of AERD begins with avoidance of aspirin and NSAIDs.1 To diminish nasal inflammation and cease polyp formation, intranasal steroids can be beneficial.1,49 This may prove more effective after a 2- to 3-week course of systemic corticosteroids, which will aid in shrinking NPs and will at least temporarily reestablish sinus drainage and accessibility of nasally administered agents into the sinuses. As with any patient with asthma, lower airway symptoms require treatment with high-dose inhaled corticosteroids, with or without add-on long-term controllers. Unfortunately, a subset of patients with AERD will gradually require continuous systemic corticosteroids. In one study, 95 of 300 patients (32%) with AERD were found to use systemic corticosteroids on a daily basis, whereas 134 (45%) were found to need short courses.1,10 As systemic steroids become regularly used in patients with AERD, the risk for significant adverse effects increases.
LT MODIFIERS
As discussed in Pathophysiology and Mechanisms of Disease, AERD is uniquely defined by the excessive production of CysLTs. These mediators drive the severity of the disease, as demonstrated by the ability of LT modifiers, such as zafirlukast or montelukast, to improve lung function, decrease rescue bronchodilator use, reduce symptoms, and improve quality of life.1,50 Furthermore, CysLTs are particularly important in driving the bronchospastic response to aspirin and other COX inhibitors, and the concomitant use of these agents greatly attenuates these reactions.51–53 Inhibition of 5-LO through the use of zileuton also improves symptoms in AERD.1 In fact, this medication seems to be uniquely capable of improving upper respiratory symptoms in the disease.54 A study by Dahlen et al.50,55 showed significant improvement in smell, rhinorrhea, and congestion with the administration of zileuton. Although these medications improve symptoms in an additive manner, they seldom “stand alone” in the treatment of AERD.
ASPIRIN DESENSITIZATION
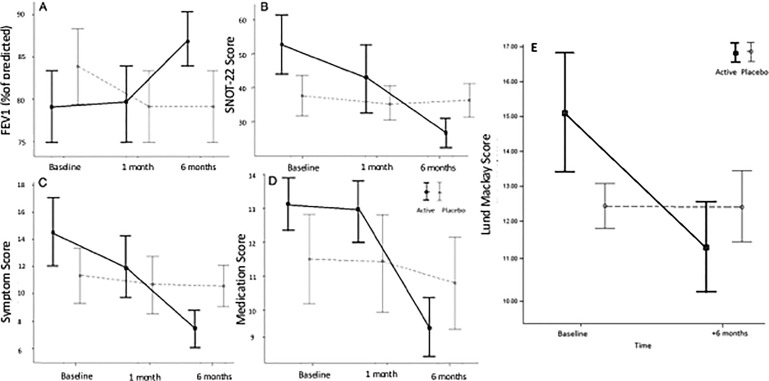
After confirming that the patient, indeed, is aspirin allergic, one should proceed with aspirin desensitization, given the usual inadequate control of AERD achieved with standard medical and surgical therapies. Aspirin desensitization in these patients improves asthma and sinus symptoms as well as slows NP regrowth after surgery, which thus increases the time to surgical revision.56–59 Most importantly, aspirin desensitization can decrease oral steroid use.60 Twice daily aspirin, 650 mg, not only improved nasal symptoms but also improved lung function, decreased the need for medications, improved quality of life, and improved sinus computed tomography scores in a randomized, double-blind, placebo-controlled study done by Esmaeilzadeh et al.57 (Fig. 1).
Figure 1.
Double-blind placebo-controlled trial of aspirin desensitization in patients with aspirin-exacerbated respiratory disease (AERD). Analysis of the data supported significant improvements in forced expiratory volume in 1 second (FEV1) (A), 22-item Sino-Nasal Outcome Test (SNOT-22) scores (B), symptom scores (C), and medication scores (D). (E) Further, there is evidence of improved computed tomography scores at 6 months (Lund-Mackay) (from Ref. 57) (republished with permission).
Ideally, aspirin desensitization should be performed after functional endoscopic sinus surgery (FESS). After FESS, aspirin desensitization has the most benefit compared with desensitization alone with regard to improved sense of smell, decreased nasal blockage, and decreased 22-item Sino-Nasal Outcome Test scores soon after surgery (a set of 22 nasal- and sinus-related symptoms graded from absent to severe).56 This reflects the recognition that long-term aspirin desensitization does a better job in slowing the regrowth of hyperplastic sinus tissue and polyps rather than in inducing regression of established disease. In addition, because the infiltrating mast cells, basophils, and eosinophils, along with their adherent platelets, are a prominent source of the surge in CysLTs released after aspirin exposure, the severity of the reaction and extent of bronchospasm produced during aspirin desensitization will be attenuated by surgical removal of these tissues before performing desensitization. Also, many surgeons prefer performing surgery in patients who are not on daily aspirin, given the associated bleeding risk. As such, if desensitization does not produce a profound clinical result, then the daily aspirin will have to be discontinued before surgery can be performed, which necessitates a second desensitization procedure in short order. The optimal time to desensitize to aspirin is 2–4 weeks after nasal polypectomy before polypoid changes have had a chance to reemerge.
As with the diagnostic challenge procedure, physicians should confirm stable asthma with a forced expiratory volume in 1 second that is within 10% of historical bests and ≥60% of predicted. The use of LT modifiers is essential to minimize the risk of bronchospasm associated with this procedure, in contrast to diagnostic challenge in which use of LT modifiers can mask the ability to recognize sensitization. In addition to LT receptor antagonists, many physicians also recommend premedicating with zileuton before and throughout the desensitization. Although they do not prevent reactions to aspirin, oral steroids administered before and throughout the procedure should also be considered to optimize baseline respiratory status. Antihistamines are generally avoided because, by blocking LTs and providing steroids, it should be possible to prevent or at least greatly ameliorate all lower respiratory symptoms and thereby still allow histamine-associated upper respiratory symptoms to be evident.40,61 The presence of these upper respiratory symptoms will give the clinician more confidence regarding the diagnosis of AERD (if a diagnostic challenge was not first performed). A typical desensitization protocol is provided in Table 3. The standard starting dose of aspirin after desensitization is 650 mg twice daily, although the beneficial dose can be anywhere between 325 and 1350 mg daily.60,62
Table 3.
Aspirin desensitization
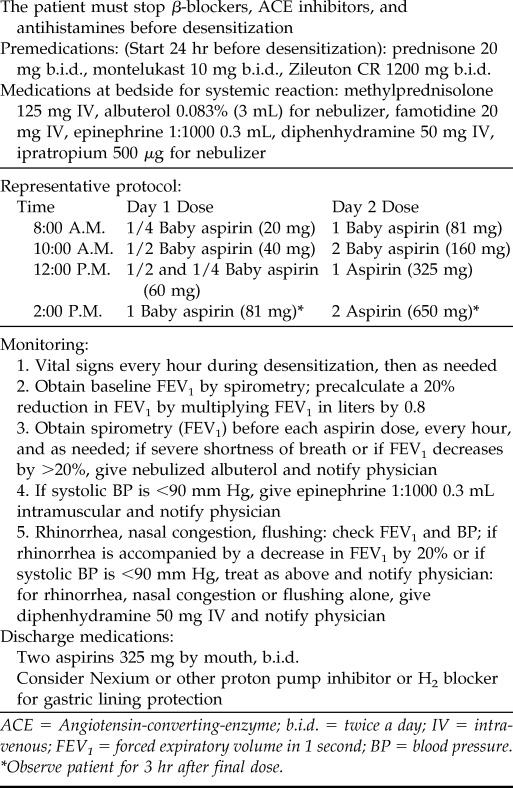
ACE = Angiotensin-converting-enzyme; b.i.d. = twice a day; IV = intravenous; FEV1 = forced expiratory volume in 1 second; BP = blood pressure.
*Observe patient for 3 hr after final dose.
Aspirin desensitization does not produce tolerance. This is important to recognize because patients who are desensitized need to remain on aspirin indefinitely to retain their desensitized state. If the drug is stopped for longer than 48 hours, then the patient should not resume taking aspirin because he or she is at risk for a reaction; therefore, the patient should contact his or her physician for a repeated desensitization. It is important to keep in mind that adverse effects to aspirin desensitization are fairly common. In fact, results of studies showed that adverse effects can occur in almost a fourth of all patients who have this procedure.59 Therefore, there is a high rate of patients who will stop taking aspirin in the long term due to noncompliance or treatment complications such as urticaria; tinnitus; and gastrointestinal symptoms, including gastric ulcers.63,64
SURGERY
Before desensitization, patients with AERD have often undergone multiple surgical interventions to remove polyps and to address the hyperplastic sinus tissue. In his original description of the “triad,” Samter reported that surgery alone is ineffective in AERD.5 Subsequently, in 1996, Stevenson et al.65 described long-term outcomes for AERD treatment and confirmed that surgery alone is not curative. Mendelsohn et al.66 reported similar poor outcomes regarding nasal polyposis with management that included surgery in isolation. In this study, 37% of patients required a revision surgery at 5 years and a staggering 89% required a revision surgery at 10 years.66 However, surgical management is an essential adjunct to management of the upper airway component of this disease.67 As discussed, aspirin desensitization is primarily effective in slowing the recurrence of hyperplastic sinus tissue and NPs, and is unlikely to regress established disease.
Aspirin desensitization in conjunction with FESS reduced the need for revision polypectomy from an average of once every 3 years, before aspirin desensitization, to once every 10 years during long-term therapy with aspirin.68,69 Insofar as nasal sinus irrigation, often with concomitant corticosteroids, can be an essential component of medical management of CRSwNPs, surgery is often essential to allow access of the irrigant into the sinus cavities, and, again, the hyperplastic sinus tissue is a “source” for the surge in CysLTs that occurs with the desensitization procedure (a “leukotrienectomy” of sorts), so removing the tissue first allows for a more agreeable desensitization.65 In summary, AERD cannot be cured with surgery, nor can it be properly medically managed without surgery. The ideal management for a patient with AERD remains a comanagement approach between allergy/immunology and otolaryngology specialists to provide personalized and optimal care.
ON THE HORIZON
There are no current studies that specifically used monoclonal antibodies for AERD; however, analysis of data of these medications' proven efficacy in patients with asthma and allergy and in patients with asthma and without allergy indicates potential efficacy in AERD. Omalizumab is an anti-IgE monoclonal antibody that is beneficial to patients with moderate-to-severe allergic asthma and in some nonallergic conditions (e.g., chronic idiopathic urticaria and nonallergic asthma).70,71 In a small study, omalizumab was given to 21 patients with AERD in whom it lowered the urinary concentrations of LTE4 and PGD2, which indicated inhibition of mast cell and eosinophil activation. In the same study, asthma exacerbations and hospitalizations were decreased, and symptom improvement was reported.72 In another study that used omalizumab to treat CRSwNP, significant improvements in symptoms and NP size were observed, and, although not specifically analyzed for the presence of AERD, this condition was likely present in some of the subjects.73 The obvious difficulty for clinicians in prescribing omalizumab for AERD remains that many patients with AERD have no evidence of specific IgE sensitization, which makes it nearly impossible to obtain approval when considering current insurance guidelines for this medication. The problem is amplified because many patients with AERD may have high levels of total IgE, yet no evidence of specific allergen sensitization, which makes it all the more frustrating for clinicians.74
Mepolizumab is an anti–interleukin (IL) 5 antibody that prevents eosinophil hematopoiesis and promotes eosinophil apoptosis, which thereby leads to rapid and profound reductions of blood and tissue eosinophil numbers.75 In patients with CRSwNP, mepolizumab induced a reduction in NP size and symptom improvement.76 Again, although not specifically analyzed for the presence of AERD, AERD was likely present in some of these patients. A competing anti–IL-5 antibody, reslizumab, was studied in patients with asthma and demonstrated significant amelioration of asthma symptoms and improvement in quality of life primarily in the subset of patients who also had NPs, which perhaps indicates an impact in the subset of patients with AERD.77,78 Benralizumab is an antibody that binds to the IL-5 receptor and inhibits engagement by IL-5 and, in contrast to the anti-IL-5 antibodies, will also cause eosinophil, as well as basophil, destruction through antibody-dependent cell-mediated cytotoxicity.79,80 As with the anti-IL-5 antibodies, benralizumab is effective in patients with eosinophilic asthma, yet, again, specific efficacy in those with AERD was not addressed.80,81 Given the particularly robust expression of eosinophils, patients with AERD seem to have a phenotype in which intervention with IL-5 targeting therapies is particularly inviting, but specific studies are needed to confirm efficacy in this population.
Other drugs that are being developed for asthma, including those that target IL-4, IL-13, and thymic stromal lymphopoeitin, also encourage investigation into the AERD phenotype.82 For example, dupilumab, an anti–IL-4R antibody that blocks activity of both IL-4 and IL-13 was recently demonstrated to have clinical efficacy in a pilot study in patients with CRSwNP.83,84 In addition to its more general role in driving T-helper type 2 inflammation, TSLP is suspected in driving mast cell overproduction of PGD2 and, as such, may play a role in the constitutive overexpression and rapid release of PGD2 during aspirin-induced reactions.85 The PGD2 receptor CRTH2 (CD294) is expressed on T-helper type 2 cells, innate lymphoid type 2 cells, eosinophils, and basophils, and promotes their recruitment and survival. Most importantly, PGD2 directly stimulates cytokine release from these cells.86–88 These antagonists are undergoing clinical development in asthma; however, they have yet to be evaluated in AERD.85
CONCLUSION
AERD typically presents in adulthood and is clinically characterized by severe chronic rhinosinusitis, nasal polyposis, severe persistent asthma, and sensitivity to aspirin and other NSAIDs. Exposure to these agents results in a reaction that can include rhinitis, laryngospasm, and asthma exacerbations. Diagnosis is essential both to prevent potentially life-threatening reactions to these agents and also to define patients who may uniquely respond to AERD-specific therapies. Carrying out challenge with graduated aspirin dosing is the standard diagnostic modality for aspirin sensitivity, although graded nasal provocation testing with ketorolac may be equally sensitive and pose much-less risk. In patients in whom medical management of their CRSwNP or asthma failed, as is typical in this disease, and, with strong clinical suspicion based on a compelling history of symptoms that develop after more than one exposure or, alternatively, when challenge testing is positive, aspirin desensitization can prove extremely beneficial.
FESS and polypectomy ideally should be performed by an experienced otolaryngologist before desensitization. In addition to aspirin desensitization, LT modifiers and, in particular, the 5-LO inhibitors can also improve symptoms. Many biologics in development show promise in eosinophilic airway disease, and, although not specifically studied in AERD, given the profound intensity of eosinophilia in this disorder, it is likely that this phenotype defines a cohort in whom these agents should prove especially effective. Omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, anti-TSLP, and the PGD2 (CRTH2) antagonists are all potential pharmacologic options that welcome further investigation in this disorder.
Footnotes
Presented at the North American Rhinology and Allergy Conference, St Thomas, VI, January 15, 2016
Supported by National Institutes of Health (NIH), National Center for Advancing Translational Sciences grants KL2TR000063 and ULL1TR000039; NIH and National Institute of Allergy and Infectious Diseases grants P20GM103625, 1K08AI121345–01A1, RO1 AI1057438, UO1 AI100799, and R56 AI120055
L Borish has received grants and honorarium from TEVA and Genetics. The remaining authors have no conflicts of interest pertaining to this article
REFERENCES
- 1. Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol 118:773–786; quiz 787–788, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Widal F, Abrami P, Lermoyez J. First complete description of the aspirin idiosyncrasy-asthma-nasal polyposis syndrome (plus urticaria)—1922 (with a note on aspirin desensitization). By F. Widal, P. Abrami, J. Lermoyez. J Asthma 24:297–300, 1987. [PubMed] [Google Scholar]
- 3. Gilbert GB. Unusual idiosyncrasy to aspirin. JAMA 56:1262–1263, 1911. [Google Scholar]
- 4. Hirschberg. Anaphylactoid reaction to aspirin (1902). Allergy Proc 11:249–250; discussion 251–252, 1990. [PubMed] [Google Scholar]
- 5. Samter M, Beers RF., Jr Concerning the nature of intolerance to aspirin. J Allergy 40:281–293, 1967. [DOI] [PubMed] [Google Scholar]
- 6. Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med 68:975–983, 1968. [DOI] [PubMed] [Google Scholar]
- 7. Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol 135:676–681.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax 55(suppl. 2):S42–S44, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J 16:432–436, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 89:474–478, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Marquette CH, Saulnier F, Leroy O, et al. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am Rev Respir Dis 146:76–81, 1992. [DOI] [PubMed] [Google Scholar]
- 12. Perez-Novo CA, Watelet JB, Claeys C, et al. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 115:1189–1196, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Steinke JW, Bradley D, Arango P, et al. Cysteinyl leukotriene expression in chronic hyperplastic sinusitis–nasal polyposis: Importance to eosinophilia and asthma. J Allergy Clin Immunol 111:342–349, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Adamjee J, Suh YJ, Park HS, et al. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol 209:392–399, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Cowburn AS, Sladek K, Soja J, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest 101:834–846, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aksu K, Kurt E, Alatas O, Gulbas Z. Effect of aspirin desensitization on T-cell cytokines and plasma lipoxins in aspirin-exacerbated respiratory disease. Allergy Asthma Proc 35:148–155, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis 143:1025–1029, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Corrigan C, Mallett K, Ying S, et al. Expression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J Allergy Clin Immunol 115:316–322, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Sousa AR, Parikh A, Scadding G, et al. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med 347:1493–1499, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A 105:16695–16700, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun 337:281–288, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med 206:2543–2555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease–New prime suspects. N Engl J Med 374:484–488, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Laidlaw TM, Kidder MS, Bhattacharyya N, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 119:3790–3798, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stevenson DD, Simon RR, Zuraw BL. Sensitivity to Aspirin and NSAIDs. Philadelphia: Mosby, 2003. [Google Scholar]
- 26. Mascia K, Borish L, Patrie J, et al. Chronic hyperplastic eosinophilic sinusitis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 94:652–657, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Vanselow NA, Smith JR. Bronchial asthma induced by indomethacin. Ann Intern Med 66:568–572, 1967. [DOI] [PubMed] [Google Scholar]
- 28. Knowles SR, Drucker AM, Weber EA, Shear NH. Management options for patients with aspirin and nonsteroidal antiinflammatory drug sensitivity. Ann Pharmacother 41:1191–1200, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Kowalski ML, Makowska J. Use of nonsteroidal anti-inflammatory drugs in patients with aspirin hypersensitivity: Safety of cyclo-oxygenase-2 inhibitors. Treat Respir Med 5:399–406, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Settipane RA, Stevenson DD. Cross sensitivity with acetaminophen in aspirin-sensitive subjects with asthma. J Allergy Clin Immunol 84:26–33, 1989. [DOI] [PubMed] [Google Scholar]
- 31. Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum 46:2201–2206, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Kim YJ, Lim KH, Kim MY, et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol Res 6:156–162, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sommer DD, Rotenberg BW, Sowerby LJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: The low-salicylate diet: A multicenter randomized control crossover trial. Int Forum Allergy Rhinol 6:385–391, 2016. [DOI] [PubMed] [Google Scholar]
- 34. ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol 109:621–626, 2002. [DOI] [PubMed] [Google Scholar]
- 35. Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): A dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 135:245–252, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song WL, Stubbe J, Ricciotti E, et al. Niacin and biosynthesis of PGD(2) by platelet COX-1 in mice and humans. J Clin Invest 122:1459–1468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardet JC, White AA, Barrett NA, et al. Alcohol-induced respiratory symptoms are common in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract 2:208–213, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brune K, Hinz B. The discovery and development of antiinflammatory drugs. Arthritis Rheum 50:2391–2399, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Gamboa P, Sanz ML, Caballero MR, et al. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy 34:1448–1457, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy 32:1491–1496, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Dahlen B, Kumlin M, Margolskee DJ, et al. The leukotriene-receptor antagonist MK-0679 blocks airway obstruction induced by inhaled lysine-aspirin in aspirin-sensitive asthmatics. Eur Respir J 6:1018–1026, 1993. [PubMed] [Google Scholar]
- 42. Pauls JD, Simon RA, Daffern PJ, Stevenson DD. Lack of effect of the 5-lipoxygenase inhibitor zileuton in blocking oral aspirin challenges in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol 85:40–45, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Lee RU, Stevenson DD. Aspirin-exacerbated respiratory disease: Evaluation and management. Allergy Asthma Immunol Res 3:3–10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jerschow E, Ren Z, Hudes G, et al. Utility of low-dose oral aspirin challenges for diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 116:321–328.e1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Juergens UR, Christiansen SC, Stevenson DD, Zuraw BL. Inhibition of monocyte leukotriene B4 production after aspirin desensitization. J Allergy Clin Immunol 96:148–156, 1995. [DOI] [PubMed] [Google Scholar]
- 46. Divekar R, Hagan J, Rank M, et al. Diagnostic utility of urinary LTE4 in asthma, allergic rhinitis, chronic rhinosinusitis, nasal polyps, and aspirin sensitivity. J Allergy Clin Immunol Pract 4:665–670, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184:602–615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haccuria A, Michils A, Michiels S, Van Muylem A. Exhaled nitric oxide: A biomarker integrating both lung function and airway inflammation changes. J Allergy Clin Immunol 134:554–559, 2014. [DOI] [PubMed] [Google Scholar]
- 49. Mastalerz L, Milewski M, Duplaga M, et al. Intranasal fluticasone propionate for chronic eosinophilic rhinitis in patients with aspirin-induced asthma. Allergy 52:895–900, 1997. [DOI] [PubMed] [Google Scholar]
- 50. Dahlen B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med 157:1187–1194, 1998. [DOI] [PubMed] [Google Scholar]
- 51. Liu T, Garofalo D, Feng C, et al. Platelet-driven leukotriene C4-mediated airway inflammation in mice is aspirin-sensitive and depends on T prostanoid receptors. J Immunol 194:5061–5068, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu T, Kanaoka Y, Barrett NA, et al. Aspirin-exacerbated respiratory disease involves a cysteinyl leukotriene-driven IL-33-mediated mast cell activation pathway. J Immunol 195:3537–3545, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stevenson DD, Zuraw BL. Pathogenesis of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol 24:169–188, 2003. [DOI] [PubMed] [Google Scholar]
- 54. Steinke JW, Kennedy JL. Leukotriene inhibitors in sinusitis. Curr Infect Dis Rep 14:147–154, 2012. [DOI] [PubMed] [Google Scholar]
- 55. Dahlen SE, Malmstrom K, Nizankowska E, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 165:9–14, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Cho KS, Soudry E, Psaltis AJ, et al. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngol Head Neck Surg 151:575–581, 2014. [DOI] [PubMed] [Google Scholar]
- 57. Esmaeilzadeh H, Nabavi M, Aryan Z, et al. Aspirin desensitization for patients with aspirin-exacerbated respiratory disease: A randomized double-blind placebo-controlled trial. Clin Immunol 160:349–357, 2015. [DOI] [PubMed] [Google Scholar]
- 58. Swierczynska-Krepa M, Sanak M, Bochenek G, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: A double-blind study. J Allergy Clin Immunol 134:883–890, 2014. [DOI] [PubMed] [Google Scholar]
- 59. Xu JJ, Sowerby L, Rotenberg BW. Aspirin desensitization for aspirin-exacerbated respiratory disease (Samter's Triad): A systematic review of the literature. Int Forum Allergy Rhinol 3:915–920, 2013. [DOI] [PubMed] [Google Scholar]
- 60. Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 111:180–186, 2003. [DOI] [PubMed] [Google Scholar]
- 61. White AA, Stevenson DD, Simon RA. The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 95:330–335, 2005. [DOI] [PubMed] [Google Scholar]
- 62. Stevenson DD, Pleskow WW, Simon RA, et al. Aspirin-sensitive rhinosinusitis asthma: A double-blind crossover study of treatment with aspirin. J Allergy Clin Immunol 73:500–507, 1984. [DOI] [PubMed] [Google Scholar]
- 63. Forer B, Kivity S, Sade J, Landsberg R. Aspirin desensitization for ASA triad patients: Prospective study of the rhinologist's perspective. Rhinology 49:95–99, 2011. [DOI] [PubMed] [Google Scholar]
- 64. Sweet JM, Stevenson DD, Simon RA, Mathison DA. Long-term effects of aspirin desensitization–treatment for aspirin-sensitive rhinosinusitis-asthma. J Allergy Clin Immunol 85(pt. 1):59–65, 1990. [DOI] [PubMed] [Google Scholar]
- 65. Stevenson DD, Hankammer MA, Mathison DA, et al. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: Long-term outcomes. J Allergy Clin Immunol 98:751–758, 1996. [DOI] [PubMed] [Google Scholar]
- 66. Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: A recurrence analysis. Ann Otol Rhinol Laryngol 120:162–166, 2011. [DOI] [PubMed] [Google Scholar]
- 67. Yip J, Yao CM, Lee JM. State of the art: A systematic review of the surgical management of aspirin exacerbated respiratory disease. Am J Rhinol Allergy 28:493–501, 2014. [DOI] [PubMed] [Google Scholar]
- 68. Gosepath J, Schaefer D, Amedee RG, Mann WJ. Individual monitoring of aspirin desensitization. Arch Otolaryngol Head Neck Surg 127:316–321, 2001. [DOI] [PubMed] [Google Scholar]
- 69. Rozsasi A, Polzehl D, Deutschle T, et al. Long-term treatment with aspirin desensitization: A prospective clinical trial comparing 100 and 300 mg aspirin daily. Allergy 63:1228–1234, 2008. [DOI] [PubMed] [Google Scholar]
- 70. Menzella F, Piro R, Facciolongo N, et al. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol 7:9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Llano LP, Vennera Mdel C, Alvarez FJ, et al. Effects of omalizumab in non-atopic asthma: Results from a Spanish multicenter registry. J Asthma 50:296–301, 2013. [DOI] [PubMed] [Google Scholar]
- 72. Hayashi H, Mitsui C, Nakatani E, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha,11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 137:1585–1587.e4, 2016. [DOI] [PubMed] [Google Scholar]
- 73. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 131:110–116.e1, 2013. [DOI] [PubMed] [Google Scholar]
- 74. Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy 28:287–289, 2014. [DOI] [PubMed] [Google Scholar]
- 75. Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood 73:1504–1512, 1989. [PubMed] [Google Scholar]
- 76. Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 128:989–995.e1–8, 2011. [DOI] [PubMed] [Google Scholar]
- 77. Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: A randomized, placebo-controlled study. Am J Respir Crit Care Med 184:1125–1132, 2011. [DOI] [PubMed] [Google Scholar]
- 78. Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 118:1133–1141, 2006. [DOI] [PubMed] [Google Scholar]
- 79. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 125:1344–1353.e2, 2010. [DOI] [PubMed] [Google Scholar]
- 80. Varricchi G, Bagnasco D, Borriello F, et al. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: Evidence and unmet needs. Curr Opin Allergy Clin Immunol 16:186–200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 132:1086–1096.e5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 370:2102–2110, 2014. [DOI] [PubMed] [Google Scholar]
- 83. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: A randomized clinical trial. JAMA 315:469–479, 2016. [DOI] [PubMed] [Google Scholar]
- 84. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388:31–44, 2016. [DOI] [PubMed] [Google Scholar]
- 85. Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 137:1566–1576.e5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy 45:394–403, 2015. [DOI] [PubMed] [Google Scholar]
- 87. Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12:1055–1062, 2011. [DOI] [PubMed] [Google Scholar]
- 88. Takeshita K, Yamasaki T, Nagao K, et al. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol 16:947–959, 2004. [DOI] [PubMed] [Google Scholar]