Abstract
Background:
Topical antibiotics, delivered optimally as high-volume culture-directed sinus irrigations, are being increasingly used for recalcitrant chronic rhinosinusitis (CRS). Their impact on subjective and objective outcome measures, however, is still unclear.
Objective:
To assess if the use of topical antibiotics in recalcitrant CRS is associated with improved 20-Item Sino-Nasal Outcome Test and Lund-Kennedy endoscopic scores, and to determine the negative posttreatment culture “control” rate.
Methods:
Patients were included in the study if they met diagnostic criteria for CRS, received high-volume topical antibiotic sinus irrigations twice daily for 1 month, between December 2009 and May 2015, and had undergone endoscopic sinus surgery. The primary outcome was the 20-Item Sino-Nasal Outcome Test score. Secondary outcomes were the Lund-Kennedy endoscopic score and a negative posttreatment culture “control” rate. Paired t-tests were used to compare pre- and posttreatment scores. Patients with cystic fibrosis were analyzed separately.
Results:
Of the 58 patients included, 47% had nasal polyposis, 57% had asthma, 16% had aspirin sensitivity, and 55% had environmental allergies. The median Lund-Mackay computed tomography score was 11 (interquartile range, 6–16), and the median time to follow-up was 8 weeks (interquartile range, 6–10 weeks). The 20-Item Sino-Nasal Outcome Test scores improved from pre- to posttreatment period, although this was not significant mean 1.5 [confidence interval {CI} 1.3, 1.7] to mean 1.3 [CI 1.1, 1.6]; p = 0.16). Lund-Kennedy endoscopic scores, however, significantly improved from pre- to posttreatment (mean 4.9 [CI 4.3, 5.6] to mean 4.1 [CI 3.5, 4.7]; p = 0.05). Of the 47 patients with complete culture data, 72% had negative posttreatment culture results, defined as “controlled.” Only one patient discontinued treatment, related to discomfort from irrigations.
Conclusion:
In patients with recalcitrant CRS, the use of topical antibiotics trended toward improvement in symptom severity and significantly improved endoscopic appearance. Furthermore, 72% had negative posttreatment culture results, meaning microbiological “control.” The results of this study support the use of high-volume culture-directed topical antibiotics, and, in the future, more rigorous prospective studies are warranted.
Keywords: Culture-directed, topical antibiotic, high-volume, sinus, irrigation, chronic rhinosinusitis, recalcitrant, 20-Item Sino-Nasal Outcome Test, Lund-Kennedy, culture
Sinusitis is a common clinical entity that affects ∼14% of adults, with a subset of these patients developing chronic rhinosinusitis (CRS).1 Endoscopic sinus surgery (ESS) has been shown to significantly improve quality of life outcomes and is indicated in patients with CRS for whom medical therapy failed.2 Some patients with CRS, however, have persistent infection despite both medical therapy and ESS.3,4 Topical antibiotic therapy is a promising option for these patients, allowing the administration of a higher concentration of antibiotic directly to the site of infection. It also has the benefit of reducing the potential for adverse systemic effects attributed to systemic antibiotic therapy.2,5
The existing literature has a lack of variability in study design, patient characteristics, and delivery methods used, and has small sample sizes. As a result, for even routine CRS, recommendations have varied regarding the use of topical antibiotic therapy. A 2013 evidence-based review highlighted three randomized controlled trials and one systematic review, and recommended against topical antibiotics for nebulizer and spray delivery in routine CRS, and had no recommendation for other delivery methods, e.g., high-volume irrigation.5 The three randomized controlled trials compared topical antibiotic therapy to placebo and demonstrated no clinical benefit.6–8 Although all the patients had had ESS, all three randomized controlled trials used delivery methods (intranasal spray or nebulizer) other than high-volume irrigation and were underpowered.6–8 The systematic review identified 14 studies of heterogeneous study design that looked at both topical antibiotics and antifungals in CRS and concluded that topical antibiotic therapy may be beneficial.9 Another 2013 evidence-based review included an additional five studies and recommended against topical antibiotic therapy for all delivery methods in routine CRS, which is consistent with the updated 2014 evidence-based review recommendation.2,10,11
The role of topical antibiotic therapy in nonroutine medically and surgically recalcitrant CRS is even more unclear. This subset of patients with CRS and who had ESS has few other therapeutic options and stands to benefit significantly if topical antibiotic therapy proves to be effective. Recently, post-ESS status, high-volume irrigation delivery method, and culture-directed therapy have been recognized as factors that optimize the success of topical antibiotic therapy and should be incorporated into study protocols.2–4 Thus far, only a few studies focused on patients with medically and surgically recalcitrant CRS and incorporated these factors. Evaluating, specifically, topical mupirocin in patients with Staphylococcus aureus, they demonstrated significant benefit in comparison with saline solution but were limited by small sample sizes.3,4,12,13
This study sought to add to the existing literature by focusing on nonroutine CRS in a larger sample, by incorporating the above-mentioned factors in the study protocol, and by evaluating a broader range of topical antibiotics. The purpose of this study was to assess if the use of high-volume, culture-directed, topical antibiotic sinus irrigations are associated with improved quality of life, endoscopic, and culture outcomes in patients with medically and surgically recalcitrant CRS.
METHODS
Patient Selection
This retrospective case series was approved by our local institutional review board. Patients were considered for inclusion if they met diagnostic criteria for CRS (as defined by the American Academy of Otolaryngology—Head and Neck Surgery 2007 clinical practice guidelines1), received high-volume topical antibiotic sinus irrigations between February 2009 and May 2015, and had undergone ESS with the standard technique with the extent of surgery based on clinical and radiographic evidence of sinus disease. The type of topical antibiotic prescribed was based on culture and susceptibility results and on patient factors (e.g., allergies, comorbidities), and included tobramycin (100 mg/100 mL), vancomycin (200 mg/100 mL), levofloxacin (100 mg/100 mL), mupirocin (15 mg/100 mL), gentamicin (80 mg/100 mL), ceftriaxone (200 mg/100 mL), and ceftazidime (600 mg/100 mL). The patients irrigated each nostril with 50 mL of one or more of the antibiotics twice daily for 30 days. Patients were excluded if they had an unspecified topical antibiotic start date, were lost to follow-up, or had a skull base malignancy.
Data Collection
Descriptive characteristics were recorded: demographics (age and sex), relevant comorbidities (nasal polyposis, asthma, aspirin sensitivity, environmental allergies, cystic fibrosis [CF], and tobacco use), the Lund-Mackay CT score (for the scan closest to the treatment period), the time since ESS, multiple previous ESS, concurrent therapies (systemic antibiotics, systemic steroids, and topical steroids), time to follow-up, and type of topical antibiotic(s) used. Pretreatment outcome data were collected from the closest clinic visit before starting the topical antibiotic treatment. Posttreatment outcome data were collected from the closest clinic visit after completing 1 month of the topical antibiotic treatment.
The primary outcome was the 20-Item Sino-Nasal Outcome Test (SNOT-20), which consisted of 20 items, each scored from 0–5; the total score was recorded as the average of all the items, 0–5). A secondary outcome was the Lund-Kennedy endoscopic score (10 items, each scored from 0–2; the total score was recorded as the sum of all the items, 0–20). Another secondary outcome was a negative posttreatment culture “control” rate. A posttreatment culture result was defined as “negative” if the pathogen targeted on pretreatment culture, in other words, within the treatment's microbiologic profile, was absent. Cultures were collected with 30° endoscopic visualization by using a sterile alligator forceps and culture swab (Becton, Dickinson and Company, Franklin Lakes, NJ) or Xomed Sinus Secretion Collector (Medtronic-Xomed, Jacksonville, FL).
Statistical Analysis
Statistical analyses were performed by using Stata/IC 13.1 software (StataCorp LP, College Station, TX). Patients without CF and patients with CF were analyzed separately. Distribution and summary statistics were evaluated for descriptive characteristics and negative posttreatment culture results. Median and interquartile ranges are presented unless otherwise specified. Data were examined for normality before hypothesis testing. Comparisons between pre- and posttreatment SNOT-20 and Lund-Kennedy endoscopic scores were performed using a paired t-test. Mean and 95% confidence intervals are presented. A p value of <0.05 was considered significant.
RESULTS
Descriptive Characteristics
Eighty-three patients met inclusion criteria, and 14 patients were excluded (4 had an unspecified topical antibiotic start date, 8 were lost to follow-up, and 2 had a skull base malignancy). Descriptive data for patients without CF (n = 58) and patients with CF (n = 11) are depicted in Table 1 and represent characteristics consistent with patients with challenging CRS.
Table 1.
Baseline patient demographics and clinical data
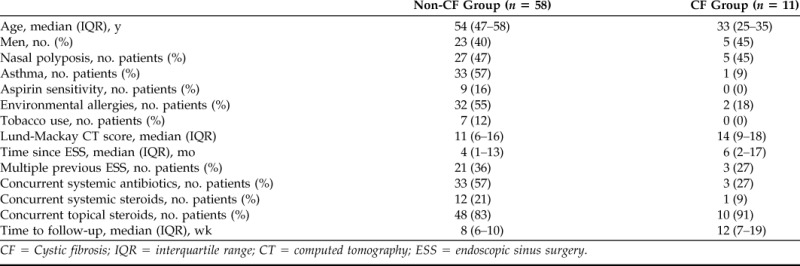
CF = Cystic fibrosis; IQR = interquartile range; CT = computed tomography; ESS = endoscopic sinus surgery.
Of the patients with pretreatment culture results data, 54% (37/68; non-CF 53% [30/57] and CF 63% [7/11]) were positive for S. aureus and 38% (26/68; non-CF 32% [18/57] and CF 73% [8/11]) were positive for Pseudomonas aeruginosa. Additional pathogens identified on culture were Streptococcal species (22% [15/68]; non-CF 26% [15/57] and CF 0% [0/11]), other gram-positive species (1% [1/68]; non-CF 2% [1/57] and CF 0% [0/11]), and other gram-negative species (26% [18/68]; non-CF 26% [15/57] and CF 27% [3/11]). Types of topical antibiotics prescribed were tobramycin (30% [21/69]; non-CF 28% [16/58] and CF 45% [5/11]), vancomycin (28% [19/69]; non-CF 28% [16/58] and CF 27% [3/11]), levofloxacin (6% [4/69]; non-CF 7% [4/58] and CF 0% [0/11]), mupirocin (33% [23/69]; non-CF 36% [21/58] and CF 18% [2/11]), gentamicin (6% [4/69]; non-CF 5% [3/58] and CF 9% [1/11]), and cephalosporin (4% [3/69]; non-CF 3% [2/58] and CF 9% [1/11]). Multiple topical antibiotics were used in 7% of the patients (5/69; non-CF 7% [4/58] and CF 9% [1/11]).
Outcome Measures
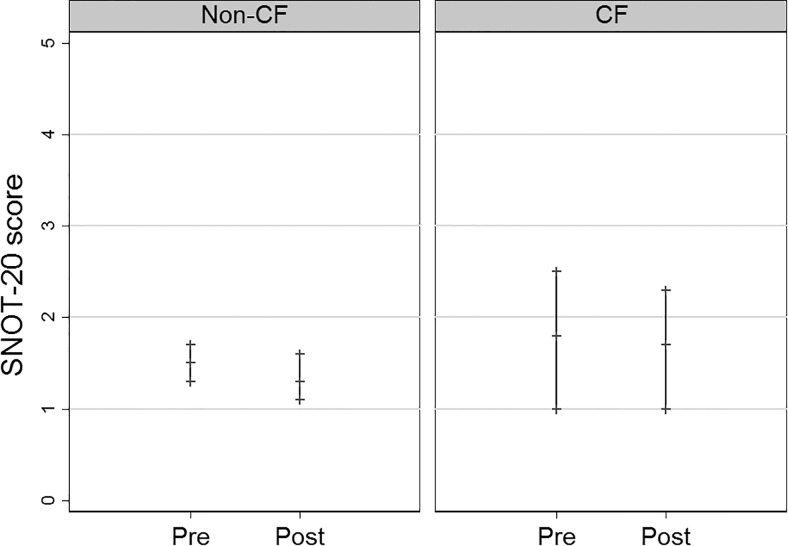
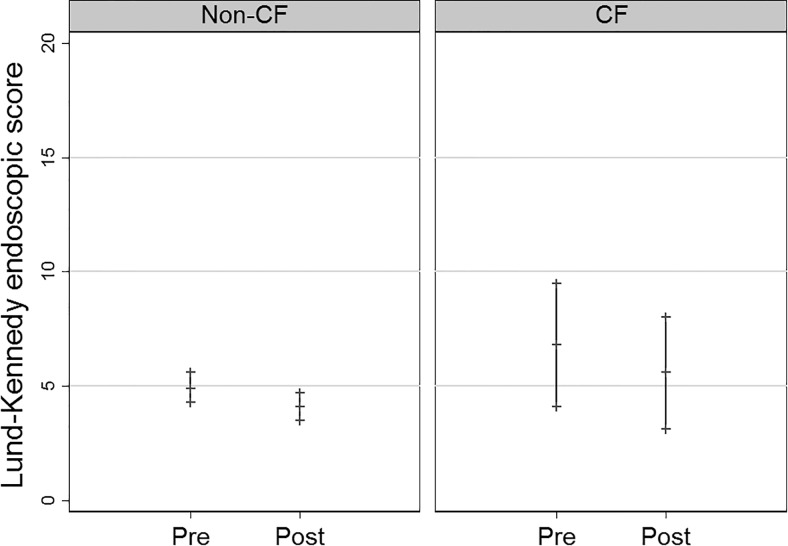
For the non-CF group, the SNOT-20 scores improved from pretreatment (mean 1.5 [confidence interval {CI} 1.3, 1.7] to posttreatment mean 1.3 [CI 1.1, 1.6]), but this difference was not significant (p = 0.16) (Fig. 1). There were two patients who had missing posttreatment SNOT-20 scores and were excluded. Lund-Kennedy endoscopic scores improved from pretreatment (4.9 [4.3, 5.6]) to posttreatment (4.1 [3.5, 4.7]), and this difference was significant (p = 0.05) (Fig. 2). Of the patients with complete culture data, 72% (34/47) had negative posttreatment culture results for the targeted pathogen, defined as “controlled.” There were 11 patients who lacked either pre- or posttreatment culture data, and they were excluded.
Figure 1.
Pre- and posttreatment SNOT-20 scores mean and 95% confidence intervals for the non-CF (p = 0.16) and CF (p = 0.68) groups. SNOT-20 = 20-Item Sino-Nasal Outcome Test; CF = cystic fibrosis.
Figure 2.
Pre- and posttreatment Lund-Kennedy endoscopic score mean and 95% confidence intervals for the non-CF (p = 0.05) and CF (p = 0.16) groups. CF = cystic fibrosis.
For the CF group, SNOT-20 scores were similar from pretreatment (mean 1.8 [CI 1.0, 2.5] to posttreatment mean 1.7 [CI 1.0, 2.3]) (p = 0.68) (Fig. 1). Lund-Kennedy endoscopic scores improved from pretreatment (6.8 [4.1, 9.5]) to posttreatment (5.6 [3.1, 8.0]), but this difference was not significant (p = 0.16) (Fig. 2). There were two patients who had missing posttreatment Lund-Kennedy endoscopic scores, and they were excluded. Of the patients with complete culture results data, 29% (2/7) had negative posttreatment culture results for the targeted pathogen defined as “controlled.” There were four patients who lacked either pre- or posttreatment culture results data and were excluded.
Adverse Effects
One patient discontinued treatment early due to discomfort from combination tobramycin and mupirocin irrigations. There were no adverse effects of bronchospasm, serum toxicity, nephrotoxicity, or ototoxicity noted.
DISCUSSION
In this study, for the non-CF group, high-volume topical antibiotic sinus irrigations resulted in an improvement trend in symptom severity as assessed by the SNOT-20 quality of life index. There also was a significant improvement in endoscopic appearance as assessed by the Lund-Kennedy endoscopic score. In addition, there was a negative posttreatment culture results achieved in 72% of patients, meaning clinical microbiological “control.” For the CF group, the results were less promising. Symptom severity was similar from the pre- to posttreatment period, and, although there was an improvement trend in endoscopic appearance, only 29% of the patients achieved a negative posttreatment culture result. No serious adverse effects were noted. The results of this study supported the use of culture-directed, high-volume topical antibiotic sinus irrigations in patients with medically and surgically recalcitrant CRS. Topical antibiotics may be less beneficial in patients with CF.
Our study was unique in that it focused on the recalcitrant CRS population, those patients who continued to have persistent infections despite maximal medical therapy with systemic antibiotics and steroids, and those who had ESS with surgically opened sinonasal passages. These patients are challenging to treat because few therapeutic options exist. Recent evidence-based reviews recommended against topical antibiotic therapy in routine CRS cases, but there is a paucity of literature regarding the use of topical antibiotic therapy for patients with challenging CRS.2,5,10,11 Our study provided data to help address this gap in the literature. Furthermore, although a high-volume irrigation delivery method, post-ESS status, and culture-directed therapy are well-accepted factors that optimize the success of topical antibiotic therapy, there are few studies that evaluated the effect of topical antibiotic therapy in this context, and the studies that were done were limited by small sample sizes and were restricted to S. aureus–positive CRS.2,3 In this study, all the patients had ESS and received high-volume irrigations, with all but one patient who had culture results available to guide the choice of topical antibiotic. In addition, this study had a relatively large sample size in comparison with previous studies and evaluated established subjective and objective outcome measures. In our experience, a lack of insurance coverage for compounded medications and monetary expense limit the use of topical antibiotic therapy.
Our study, however, had several limitations. First, it was a retrospective case series, and thus lacked a control group, which made it difficult to attribute the improvement in outcome measures specifically to topical antibiotic therapy. The impact of adjuvant therapy, including systemic antibiotics, systemic steroids, and topical steroids, can contribute to a successful outcome in patients with CRS. This study design, given its retrospective nature, also was limited due to heterogeneity in patient selection, demographics, relevant comorbidities, concurrent therapies, interval follow-up, and type of topical antibiotic, and, thus, there is the potential for confounding. There also was subject and clinician nonblinding, which can further confound the SNOT-20 and Lund-Kennedy endoscopic measures, respectively.
Second, whether the improvements observed in the outcome measures were clinically significant was unclear. Although there was a decrease of 0.2 in the SNOT-20 scores from the pre- to posttreatment period, this was not statistically significant and was still less than the decrease of 0.8 that is considered clinically significant.14 The clinical significance threshold for Lund-Kennedy endoscopic scores and a negative posttreatment culture “control” rate is unknown.15 In addition, the definition of culture negativity used in this study was based on the absence of the targeted pathogen but is imperfect in that it does not account for the subsequent appearance of other bacterial species that may contribute to the pathogenesis of CRS.16 Regardless, the results that showed improvements in these outcomes were noteworthy. Even more important, topical therapy was used in this clinical practice only for patients with recalcitrant CRS for whom traditional systemic antibiotic and steroid therapies failed.
This study may have been underpowered to detect clinically significant improvements. In addition, the results of this study may not be generalizable to the medically and surgically recalcitrant CRS population as a whole. As a tertiary rhinology practice, there are fairly large percentages of patients with relevant comorbidities, including nasal polyposis, asthma, aspirin sensitivity, environmental allergies, CF, current smoking, or the triad that compromises aspirin-exacerbated respiratory disease, which may not be representative of patients in a community practice. The advantage of this, however, is that this study may have better truly captured the patient with most recalcitrant CRS and that the more modest benefits observed in this population may be even greater in a less comorbid population.
CONCLUSION
The results of this study gave support to the use of high-volume culture-directed topical antibiotic therapy in the recalcitrant CRS population, in which this therapeutic option arguably has the potential for high impact, given the lack of other available options. Future, more rigorous, prospective studies that include a control group and incorporate randomization are needed to definitively evaluate the impact of culture-directed high-volume topical antibiotic therapy in patients with medically and surgically recalcitrant CRS.
ACKNOWLEDGMENTS
We thank Carolyn Bea, our research coordinator, for her work in obtaining institutional review board approval for this study. V.S. Lee has received support through the National Institutes of Health T32 DC000018 Research Training Grant.
Footnotes
Presented as a podium at the American Academy of Otolaryngology—Head and Neck Surgery Foundation annual meeting, Dallas, Texas, September 27–30, 2015
Funded by NIH T32 DC000018 Research Training grant
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg 137(suppl.):S1–S31, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Orlandi RR, Smith TL, Marple BF, et al. Update on evidence-based reviews with recommendations in adult chronic rhinosinusitis. Int Forum Allergy Rhinol 4(suppl. 1):S1–S15, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Jervis-Bardy J, Boase S, Psaltis A, et al. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope 122:2148–2153, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Jervis-Bardy J, Wormald PJ. Microbiological outcomes following mupirocin nasal washes for symptomatic, Staphylococcus aureus-positive chronic rhinosinusitis following endoscopic sinus surgery. Int Forum Allergy Rhinol 2:111–115, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: An evidence-based review with recommendations. Int Forum Allergy Rhinol 3:281–298, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Sykes DA, Wilson R, Chan KL, et al. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis. A controlled study. Lancet II:359–360, 1986. [DOI] [PubMed] [Google Scholar]
- 7. Desrosiers MY, Salas-Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large-particle nebulizer: Results of a controlled trial. Otolaryngol Head Neck Surg 125:265–269, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Videler WJ, van Drunen CM, Reitsma JB, Fokkens WJ. Nebulized bacitracin/colimycin: A treatment option in recalcitrant chronic rhinosinusitis with Staphylococcus aureus? A double-blind, randomized, placebo-controlled, cross-over pilot study. Rhinology 46:92–98, 2008. [PubMed] [Google Scholar]
- 9. Lim M, Citardi MJ, Leong JL. Topical antimicrobials in the management of chronic rhinosinusitis: A systematic review. Am J Rhinol 22:381–389, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Soler ZM, Oyer SL, Kern RC, et al. Antimicrobials and chronic rhinosinusitis with or without polyposis in adults: An evidenced-based review with recommendations. Int Forum Allergy Rhinol 3:31–47, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Lee JT, Chiu AG. Topical anti-infective sinonasal irrigations: Update and literature review. Am J Rhinol Allergy 28:29–38, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Solares CA, Batra PS, Hall GS, Citardi MJ. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am J Otolaryngol 27:161–165, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Uren B, Psaltis A, Wormald PJ. Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope 118:1677–1680, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126:41–47, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg 117(pt 2):S35–S40, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Mahdavinia M, Keshavarzian A, Tobin MC, et al. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin Exp Allergy 46:21–41, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]