Abstract
Objective:
Acute otitis media (AOM) causes an inflammatory response in the middle ear. We assessed differences in innate immune responses involved in bacterial defense at onset of AOM in children who were stringently defined as otitis prone (sOP) and children not otitis prone (NOP).
Study Design:
Innate immune genes analysis from middle ear fluid (MEF) samples of children.
Methods:
Genes of toll-like receptors (TLR), nod-like and retinoic acid-inducible gene-I-like receptors, downstream effectors important for inflammation and apoptosis, including cytokines and chemokines, were studied from MEF samples by using a real-time polymerase chain reaction array. Protein levels of differentially regulated genes were measured by Luminex.
Results:
Gene expression in MEF among children who were sOP was significantly different in upregulation of interleukin 8, secretory leukocyte peptidase inhibitor, and chemokine (C-C motif) ligand 3, and in downregulation of interferon regulatory factor 7 and its related signaling molecules interferon alpha, Toll-like receptor adaptor molecule 2, chemokine (C-C motif) ligand 5, and mitogen-activated protein kinase 8 compared with children who were NOP. Differences in innate gene regulation were similar when AOM was caused by Streptococcus pneumoniae or nontypeable Haemophilus influenzae.
Conclusion:
Innate-immune response genes are differentially regulated in children who were sOP compared with children with NOP.
Keywords: Acute otitis media, middle ear fluid, otitis-prone, innate-immune response
Children who experience recurrent episodes of acute otitis media (AOM) become classified as otitis prone (OP)1,2 after they experience three AOM events within a 6-month time span or four AOM events within 12 months.3 Previously, we published data that showed children who were OP and stringently defined as OP (sOP) based on tympanocentesis confirmation of all clinical diagnoses had lower mucosal and serum antibody responses against Streptococcus pneumoniae and nontypeable Haemophilus influenzae antigens.4–7 We also found fewer B-cell memory and CD4 T-cell memory responses after AOM infections in children who were sOP.8–11
The innate immune system plays a fundamental role in the prevention of AOM. Our group studied the innate immune responses in the blood and nasopharynx at the onset of AOM in children who were sOP compared with children who were not otitis prone (NOP).9,12–14 Differential expression of innate immunity genes and the mechanisms that regulate innate immunity in chronic rhinosinusitis have also been described previously.15,16 Here, to our knowledge, for the first time, we assessed differences in innate immunity in the middle ear of children who were sOP and children who were NOP. Real-time polymerase chain reaction (PCR) array was used to study the expression of different genes that mainly involve human antibacterial defense. The array included genes involved in bacterial-activated pattern recognition receptor (PRR) signal transduction, encoding downstream effectors important for inflammation and apoptosis, and encoding immune cell–expressed antimicrobial peptides. Such gene expression profiles provide insights into the molecular and cellular changes that occur during AOM and may provide avenues to novel therapeutic treatments or novel biomarkers for disease diagnosis.
METHODS
Study Population
Our group is in the ninth year of a prospective, longitudinal study of the immunologic dysfunction in children who are OP, funded by the National Institutes of Deafness and Communication Disorders (R0108671). Subjects included in these experiments were children who experienced an AOM episode. Children were enrolled from a middle-class, suburban, sociodemographic population in Rochester, New York. The details of the study design were previously described.17 The study was approved by the Rochester General Hospital institutional review board. Children received all doses of a pneumococcal conjugate vaccine according to the U.S. schedule with either pneumococcal conjugate vaccine 7 or pneumococcal conjugate vaccine 13, depending on the date of enrollment. A diagnosis of AOM was performed by validated otoscopists (J.C., M.P.) and was based on signs of inflammation (bulging) of the tympanic membrane and presence of middle ear fluid (MEF) documented by tympanocentesis. Written informed consent was obtained from parents of the children at study entry. Tympanocentesis was performed for all AOM episodes. Children who were sOP were defined as those who experienced three or more episodes of AOM within 6 months or four or more episodes within a year. Each MEF was cultured for otopathogens by using standard culture techniques. MEF sampling procedures, microbiology, and organism identification have been previously described.18–20 Analysis of data reported here are from 24 children who were aged matched at 12 ± 1 months old into two groups; children who were sOP (n = 12) and children who were NOP (n = 12). Subject selection included only those with either S. pneumoniae (n = 12) or nontypeable H. influenzae (n = 12) culture positive MEFs.
Isolation of RNA from MEF Samples
MEF samples varied in quantity from 50 to 250 μL, and the entire sample was added to 500 μL of phosphate buffered saline solution (pH 7.4). After culturing a small portion of MEF, the remainder was centrifuged for 10 minutes at 4°C; 1 mL of TRIzol reagent (Sigma-Aldrich, St. Louis, MO) was added to the MEF pellet after removal of the supernatant, mixed by pipetting, and left at room temperature for 5 minutes. TRIzol-containing MEF samples were stored at −80°C for further processing of DNA and RNA isolation. RNA isolation was performed as described previously.21 To remove traces of DNA, a DNA digestion step was performed by using deoxyribonuclease I enzyme from Qiagen (Hilden, Germany). After RNA isolation, ribonuclease inhibitor was added to each sample to avoid degradation. The quantity of RNA was determined by Nanodrop (Thermo Scientific, Wilmington, DE). Criteria for inclusion in downstream reverse transcriptase (RT-PCR) applications was based on an optical density of wavelength 260/280 of >2.0.
Complementary DNA Synthesis and Real-Time PCR Array
First-strand complementary DNA (cDNA) was generated for each RNA sample isolated from MEF by using a RT2 first-strand kit (Qiagen) by following the manufacturer's instructions. An RT2 Profiler PCR array was performed in 96-well plates to measure expression of 84 genes involved in antibacterial responses (catalog PAHS-148Z; Qiagen). The array included genes involved in bacterial-activated pattern recognition receptors (toll-like receptors [TLR], nod-like and retinoic acid-inducible gene-I-like receptors) for signal transduction as well as genes encoding downstream effectors important for inflammation and apoptosis, including cytokines and chemokines, and genes encoding immune cell-expressed antimicrobial peptides). Per experiment, a set of five housekeeping genes was included to normalize the data. Controls were also included in each array for genomic DNA contamination, RNA quality, and general PCR performance.
cDNA template from each MEF sample was mixed with the appropriate ready-to-use PCR master mix, aliquoted in equal volumes to each well of the same PCR array and amplification performed (10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 1 minute at 60°C), according to the manufacturer's guidelines by using a CFX96 real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA). Only genes with threshold cycle (Ct) values of <35 were considered to be detectable, and the Ct values of the genes were normalized with the average Ct value of all five housekeeping genes on the array data analysis. The analysis was performed based on the ΔΔCt method.22 The relative gene expression of different genes was calculated as ΔCt sample = (Ct sample gene) − (Ct sample average of 5 housekeeping genes). The average ΔCt, 2−ΔCt, fold change, and p value were calculated by using the software by SA Biosciences Valencia, CA.
Measurement of Chemokine Protein Levels in the MEF Supernatant
Based on RT-PCR data, we elected to measure interleukin (IL) 8, chemokine (C-C motif) ligand (CCL) 2 (monocyte chemoattractant protein-1), CCL5 (RANTES), and interferon (IFN) α protein levels in which we found higher expression for IL-8, no difference for CCL2, and low expression for CCL5 and IFN-α chemokines in the children who were sOP. Measurement of selected chemokines in the MEF supernatant was performed with a Bio-Plex assay kit (Bio-Rad) per the manufacturer's instructions. MEF supernatants stored at −80°C were thawed at room temperature. Undiluted samples were incubated with antibody conjugated magnetic beads for 1 hour at room temperature and then biotin-labeled cytokine-detection antibodies were added, followed by incubation at room temperature for 30 minutes. After incubation, phycoerythrin-conjugated streptavidin antibodies were added and incubated for 30 minutes. The samples were run per the manufacturer's instructions on a Bio-Plex 200 system with Luminex xMAP technology (Bio-Rad). IFN-α levels were detected in separate experiments with the MEF according to Bio-plex assay instructions. To correct for differential dilution effects that occurred during the MEF sample collection, total protein concentrations were determined by using the Pierce BCA Protein assay kit (ThermoFisher Scientific, Waltham, MA) according to the manufacturer's protocol, and data were normalized with total protein concentrations. Chemokine protein levels obtained from the Luminex readout were then corrected according to the total protein concentration in the sample. The results were expressed as a ratio of the chemokine protein level to the total protein level in the same sample as described previously.13
Statistical Analysis
Statistical analyses for RT2 PCR array data was performed according to the manufacturer's instructions (SA Biosciences). Changes in gene expression were more than twofold and p < 0.05 were considered significant. Graph Pad Prism Software version 6.04 (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis of Luminex data. A two-tailed Student's t-test was used for comparisons between the groups, and p < 0.05 was considered significant. Demographic comparisons between the two groups were performed with Fisher's exact test.
RESULTS
Child Population
Twenty-four children (median age, ∼12 months old) with AOM were included in this study. Detailed demographic information about the children, including culture results, is presented in Table 1. The sOP population included 72% boys compared with 40% boys in the NOP population, but this difference was not significant (p = 0.214).
Table 1.
Demographic information of children

sOP = Stringently defined otitis prone; NOP = not otitis prone; SD = standard deviation; MEF = middle ear fluid.
Innate Immune Response Difference in Children Who Were sOP and Children Who Were NOP
Most of the 84 genes analyzed had similar expression in the two study groups. The genes that showed significant differences are listed in Table 2. Three genes differed significantly in upregulation. IL-8 plays a major role in neutrophil recruitment in otitis media,23 and expression was 4.4-fold higher in the middle ears of children who were sOP. CCL3 protein is involved in the acute inflammatory state in the recruitment and activation of neutrophils, and expression was 2.1-fold higher in children who were sOP. Secretory leukocyte protease inhibitor (SLPI) is considered to be the predominant neutrophil elastase inhibitor in secretions. It was 3.2-fold higher in children who were sOP. Six genes were significantly different in downregulation. IFN regulatory factor 7 (IRF7), which is a key transcriptional regulator of type 1 INF-dependent immune responses, showed 2.72-fold downregulation. The corresponding genes, IFN-α and Z-DNA binding protein 1 (ZBP1) (Z-DNA–dependent activator of IFN-regulatory factors), were 3.79- and 2.43-fold downregulated, respectively, in children who were sOP. The CCL5 gene, which is chemotactic for T cells, eosinophils, and basophils, and plays an active role in recruiting leukocytes into inflammatory sites, was 2.3-fold downregulated in children who were sOP. Similarly, mitogen-activated protein kinase 8 (MAPK8) and Toll-like receptor adaptor molecule 2 (TICAM2) genes were 2.20- and 2.31-fold downregulated in children who were sOP compared with children who were NOP.
Table 2.
Fold regulation of genes that shows significant difference in children who were sOP (n = 12) compared with age-matched children who were NOP (n = 12)
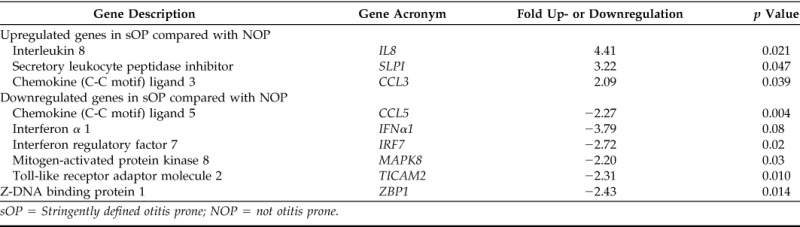
sOP = Stringently defined otitis prone; NOP = not otitis prone.
Innate gene expression might vary according to the causative otopathogen; therefore, we compared gene expression in relation to the presence of either S. pneumoniae (Fig. 1 A) or nontypeable H. influenzae (Fig. 1 B) in children who were sOP and in children who were NOP. Some genes, e.g., IL-8, TICAM2, and ZBP1 (zinc binding proteins) showed a similar trend of differential expression in relation to both S. pneumoniae and nontypeable H. influenzae (Fig. 1) However, pathogen-specific innate immune response differences in children who were sOP versus children who were NOP were identified as shown in Fig. 1, including -3.6-fold downregulation of the Caspase Recruitment Domain Family (CARD) 6 gene and 1.8-fold upregulation of heat shock proteins (HSP90AA1) in children who were sOP with AOM caused by S. pneumoniae. CARD6 plays a role in immune defense,24 the HSP90AA1 protein is an inducible molecular chaperone and aids in the proper folding of specific target proteins by use of an adenylpyrophosphatase activity that is modulated by co-chaperones.
Figure 1.
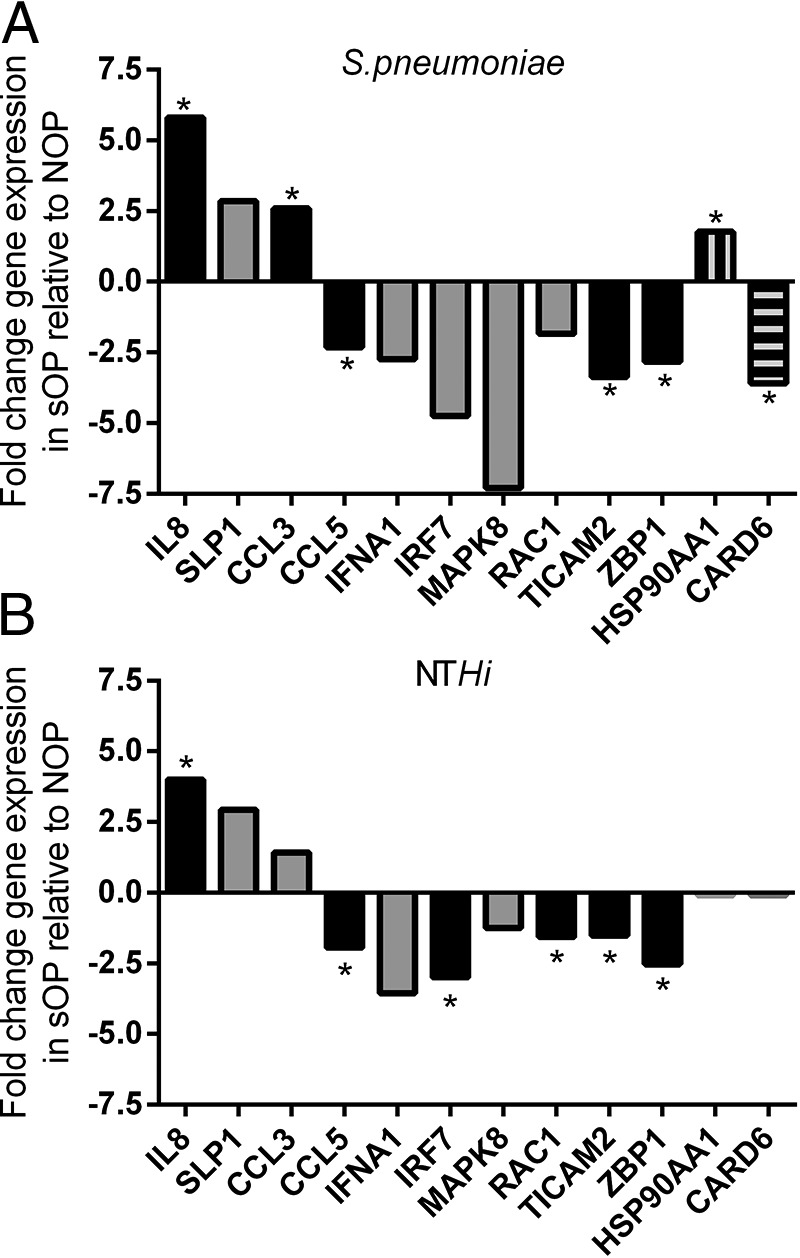
Comparison of innate immune response in the middle ear fluid (MEF) in relation to the presence of Streptococcus pneumoniae (Spn) or nontypeable Haemophilus influenzae (NTHi) of children who with stringently defined otitis prone (sOP) versus children who were not otitis prone (NOP) in which more than twofold differences were observed. Black bar shows similar trend in gene expression in relation to S. pneumoniae and NTHi. Gray bar represent no statistical significance was found and solid box bar represents genes that showed expression in S. pneumoniae only. *Significant difference (p < 0.05) was found in children who were sOP compared with children who were NOP.
Protein Analysis
We analyzed chemokine protein levels in the MEF of four proteins (IL8, CCL5, IFN-α, and CCL2) to determine whether messenger RNA differences observed between the two groups correspond to protein levels. IL-8 protein levels were significantly higher in children who were sOP, which corresponded to the higher levels of gene expression (Fig. 2 A). In contrast, protein levels of CCL5 were not reflective of gene expression differences (Fig. 2 B). IFN-α protein levels were significantly lower in children who were sOP (Fig. 2 C), which corresponded to lower levels of gene expression. CCL2 protein levels corresponded to gene expression levels in that they were not significantly different in children who were sOP versus children who were NOP.
Figure 2.
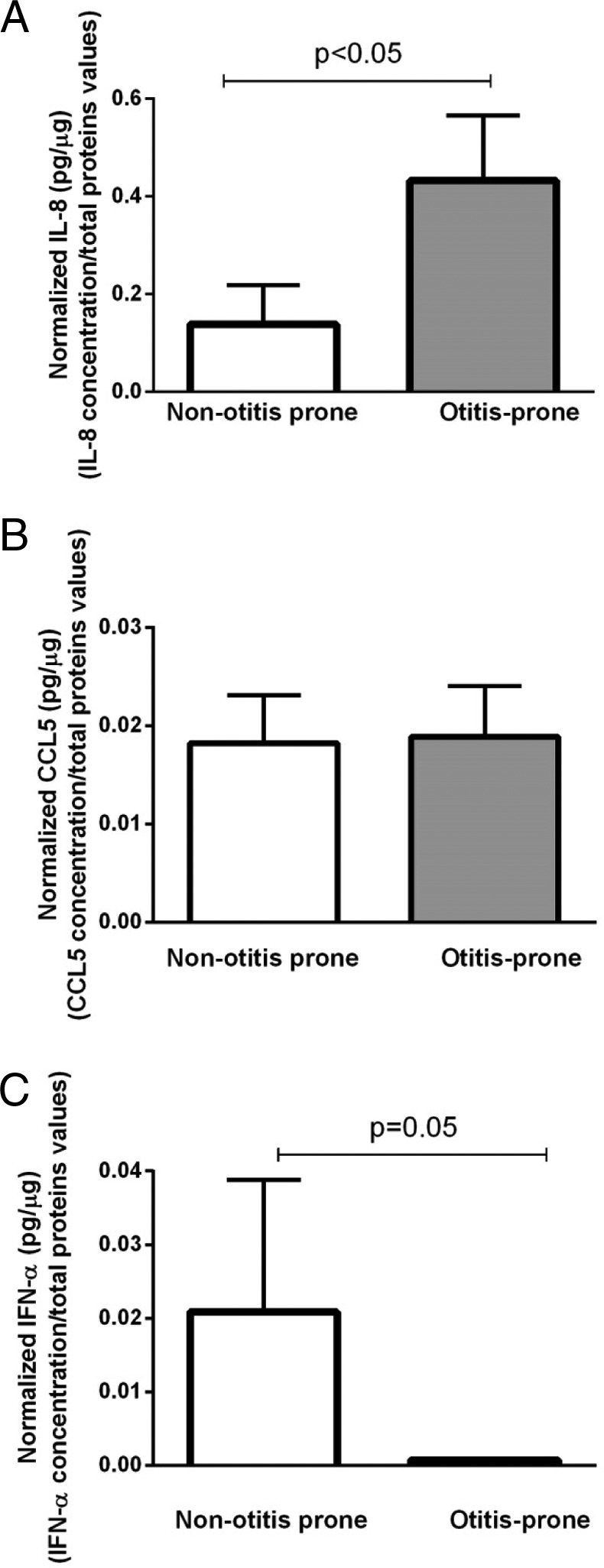
Comparison of (A) interleukin (IL) 8, (B) chemokine (C-C motif) ligand 5 (CCL5), and (C) interferon (IFN-α) at the proteomic levels in the middle ear fluid (MEF) of children with stringently defined otitis prone (sOP) versus children who were not otitis prone (NOP). Data were normalized with total proteins concentrations. Data shown in the figure represent the mean ± SE.
DISCUSSION
Here, to our knowledge for the first time, we assessed the innate response in the middle ear in children who were sOP with recurrent AOM infections compared with children who were NOP. We used a real-time PCR array that focused on inflammatory cytokines whose role in AOM has been studied extensively21,25–27 but not previously in an sOP population. We found differential expression in the two populations of children for important genes that relate to host defense. IL-8 showed higher expression in children who were sOP. A previous study showed that overstimulation and dysfunction of recruited neutrophils within the lung result in release of a number of proinflammatory molecules and proteases, which results in damage of lung tissue.28 This is likely the case in children who were sOP and who experience AOM as well.
IL-8 levels are increased by oxidant stress, which thereby causes recruitment of inflammatory cells and induces a further increase in oxidant stress mediators, which makes it a key cytokine involved in localized inflammation.29 Observing higher levels of IL-8 messenger RNA as well as higher levels of IL-8 protein in children who were sOP indicated that these children with excessive neutrophil recruitment and localized middle ear mucosa damage due to greater inflammation than occurs in children who were NOP. Elevated IL-8 gene expression was similar whether S. pneumoniae or nontypeable H. influenzae caused AOM. In a previous study, we found lower expression levels of IL-8 in the nasopharynx in children who were sOP.12 We speculated that the difference is attributable to the time of measurement. The measurement of expression of IL-8 in the nasopharynx was after 5–7 days of upper respiratory infection (URI), whereas measurement of IL-8 in MEF was on day 1 of clinical illness. The role and requirement for IL-8 in chemotaxis for neutrophil recruitment during recurrent infections have been described in other studies that involved other disease conditions.30,31
We found fourfold higher levels of SLPI gene expression in children who were sOP. SLPI is generally expressed at mucosal surfaces and plays a role in the defense against the destruction of epithelial tissues by neutrophil elastase.32 Its higher expression is consistent with the need to control neutrophil elastase damage as well as to indicate that this antineutrophil elastase defense mechanism is not impaired in children who were sOP. In addition, SLPI exerts an impact on mucin gene expression, which is known to be an important regulator in otitis media.33 Previous studies on lung pathology showed that increased levels of SLPI in nasal secretions and bronchoalveolar fluids may indicate inflammatory lung conditions.32,34
Almost always AOM is universally preceded by viral upper respiratory infections (URI).35,36 Immune responses to control viral URI are critical to prevent the progression to AOM. We observed a significantly lower expression of IRF7 in children who were sOP. We also observed lower IFN-α expression in children who were sOP. Lower IRF7 signaling is consistent with our observations that children who were sOP were more prone to viral URIs and consequent bacterial AOM. Howie et al.37 compared the association of viral URIs and IFN production and found the presence of IFN in most MEF specimens from which virus was absent and bacteria were isolated. Pitkaranta et al.38,39 indicated that leukocyte cultures of children with recurrent respiratory tract infections and otitis media with effusion produce less IFN than those of healthy children. Similarly, lower levels of ZBP1, MAPK8, and TICAM2 in MEF samples of children with sOP was consistent with the observed downregulation of the entire IRF7-dependent signaling pathway. TICAM2 was shown to be required for IRF7 activation.40
We found a 2.5-fold lower production of CCL5 (RANTES) expression in children who were sOP. CCL5 is a chemoattractant for T cells, eosinophils, and basophils. We did not detect differences in protein levels of CCL5 in sOP compared with children who were NOP. This may be due to issues of assay sensitivity or sample size and will require further study. Because we previously showed differences in the transcriptome from peripheral blood mononuclear cells when young children experience AOM caused by S. pneumoniae versus nontypeable H. influenzae.41,42, we sought to determine if differences in the innate immune response gene expression occurred in MEF of children who were sOP versus children who were NOP. We found that most of the genes showed a similar pattern, consistent with our previous study21 However, a few genes showed pathogen-specific differences, including CARD downregulation in children who were sOP caused by S. pneumoniae.
Our study had some limitations. Differences in fluid volumes of MEF and dilutions of the samples could affect the levels of detection of messenger RNA and proteins. We sought to mitigate this by normalizing the data with total protein concentrations in MEF. To minimize variability attributable to other aspects of using human samples, we selected samples from children of similar age, race, and URI time points during an AOM event, and restricted to two major otopathogens, nontypeable H. influenzae and S. pneumoniae. Our results could be attributed to concurrent viral URI, bacterial colonization, or a combination of the two as well as AOM when the samples were collected. Previous studies that used a conventional culture method showed that 15% of the cases of AOM have bacteria and virus together in MEF and that 5% of MEFs contain viruses alone36,43; by using molecular diagnostics assays, bacteria and virus together have been detected in up to 66% of AOM cases,43–47 therefore, the gene expression difference shown in this study could be attributed to viral co-infection in some of the children. Because IL-8 and IRF7 gene pathways are also affected by viral infection,48,49 it is not possible to determine if the noted gene expression differences are an epiphenomenon secondary to viral infection or represent a primary host immune response defect.
CONCLUSION
Our group previously identified adaptive and innate immunologic deficiencies in blood associated with the propensity to recurrent AOM infections in children who were sOP.6,7,8–11,14,50 Our previous work at the mucosal level in the nasopharynx12,13,50,51 identified dysfunction in multiple immune cells that play a key role in immunologic control of bacterial and viral infections in children who were sOP. The overall findings by our group to date are depicted in Fig. 3. As our group continues to study children who are sOP, we anticipate defining additional clinically significant aberrations in their immune system. Here, we showed the innate immune response of children who were sOP differed from that of children who were NOP when measurements were made from MEF. The defect in innate response occurring in the middle ear likely contributes to otitis proneness.
Figure 3.
Overall immune responses found in the different compartments (blood, nasopharynx, and middle ear) of children with stringently defined otitis prone (sOP).
ACKNOWLEDGMENTS
The authors thank all of the children and parents who participated in this study. We thank the staff of Legacy Pediatrics for securing samples.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
Funded by the Hearing Health Foundation (R. Kaur) and National Institutes of Health National Institutes of Deafness and Communication Disorders RO1 08671 (M. Pichichero)
REFERENCES
- 1. Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr 160:407–413, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Berman S, Murphy JR. Persistent and recurrent otitis media. A review of the “otitis-prone” condition. Prim Care 11:407–417, 1984. [PubMed] [Google Scholar]
- 3. Alho OP, Koivu M, Sorri M. What is an ‘otitis-prone’ child? Int J Pediatr Otorhinolaryngol 21:201–209, 1991. [DOI] [PubMed] [Google Scholar]
- 4. Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone children have immunologic deficiencies in naturally acquired nasopharyngeal mucosal antibody response after Streptococcus pneumoniae colonization. Pediatr Infect Dis J 35:54–60, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol 65:439–447, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine 29:1023–1028, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J 30:645–650, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basha S, Pichichero ME. Poor memory B cell generation contributes to non-protective responses to DTaP vaccine antigens in otitis-prone children. Clin Exp Immunol 182:314–322, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma SK, Pichichero ME. Cellular immune response in young children accounts for recurrent acute otitis media. Curr Allergy Asthma Rep 13:495–500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis 205:1225–1229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis 204:645–653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Med Microbiol Immunol 202:295–302, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verhoeven D, Pichichero ME. Divergent mucosal and systemic responses in children in response to acute otitis media. Clin Exp Immunol 178:94–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Surendran N, Nicolosi T, Kaur R, Pichichero ME. Peripheral blood antigen presenting cell responses in otitis-prone and non-otitis-prone infants. Innate Immun 22:63–71, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Lee RJ, Cohen NA. Sinonasal solitary chemosensory cells “taste” the upper respiratory environment to regulate innate immunity. Am J Rhinol Allergy 28:366–373, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Detwiller KY, Smith TL, Alt JA, et al. Differential expression of innate immunity genes in chronic rhinosinusitis. Am J Rhinol Allergy 28:374–377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedel V, Zilora S, Bogaard D, et al. Five-year prospective study of paediatric acute otitis media in Rochester, NY: Modelling analysis of the risk of pneumococcal colonization in the nasopharynx and infection. Epidemiol Infect 142:2186–2194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 29:304–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaur R, Adlowitz DG, Casey JR, et al. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J 29:741–745, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaur R, Chang A, Xu Q, et al. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J Med Microbiol 60:1841–1848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaur R, Casey J, Pichichero M. Cytokine, chemokine, and Toll-like receptor expression in middle ear fluids of children with acute otitis media. Laryngoscope 125:E39–E44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Johnson M, Leonard G, Kreutzer DL. Murine model of interleukin-8-induced otitis media. Laryngoscope 107:1405–1408, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Dufner A, Duncan GS, Wakeham A, et al. CARD6 is interferon inducible but not involved in nucleotide-binding oligomerization domain protein signaling leading to NF-kappaB activation. Mol Cell Biol 28:1541–1552, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ophir D, Hahn T, Schattner A, et al. Tumor necrosis factor in middle ear effusions. Arch Otolaryngol Head Neck Surg 114:1256–1258, 1988. [DOI] [PubMed] [Google Scholar]
- 26. Yellon RF, Leonard G, Marucha PT, et al. Characterization of cytokines present in middle ear effusions. Laryngoscope 101:165–169, 1991. [DOI] [PubMed] [Google Scholar]
- 27. Juhn SK, Tolan CT, Garvis WJ, et al. The levels of IL-1 beta in human middle ear effusions. Acta Otolaryngol Suppl 493:37–42, 1992. [PubMed] [Google Scholar]
- 28. Reeves EP, Williamson M, O'Neill SJ, et al. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med 183:1517–1523, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: Evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94:1878–1889, 1999. [PubMed] [Google Scholar]
- 30. Nassif PS, Simpson SQ, Izzo AA, Nicklaus PJ. Interleukin-8 concentration predicts the neutrophil count in middle ear effusion. Laryngoscope 107:1223–1227, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Skovbjerg S, Roos K, Nowrouzian F, et al. High cytokine levels in perforated acute otitis media exudates containing live bacteria. Clin Microbiol Infect 16:1382–1388, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weldon S, Taggart CC. Innate host defense functions of secretory leucoprotease inhibitor. Exp Lung Res 33:485–491, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Kerschner JE, Horsey E, Ahmed A, et al. Gene expression differences in infected and noninfected middle ear complementary DNA libraries. Arch Otolaryngol Head Neck Surg 135:33–39, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greene C, Taggart C, Lowe G, et al. Local impairment of anti-neutrophil elastase capacity in community-acquired pneumonia. J Infect Dis 188:769–776, 2003. [DOI] [PubMed] [Google Scholar]
- 35. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics 137:pii: e20153555, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nokso-Koivisto J, Marom T, Chonmaitree T. Importance of viruses in acute otitis media. Curr Opin Pediatr 27:110–115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howie VM, Pollard RB, Baron S. Presence of interferon during bacterial otitis media. J Infect Dis 152:428–429, 1985. [DOI] [PubMed] [Google Scholar]
- 38. Pitkaranta A, Hovi T, Karma P. Interferon production by leukocytes in children with otitis media with effusion. Int J Pediatr Otorhinolaryngol 34:25–33, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pitkaranta A, Karma P, Hovi T. Deficiency in interferon production by leukocytes from children with recurrent respiratory infections. Clin Diagn Virol 1:101–108, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stack J, Doyle SL, Connolly DJ, et al. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J Immunol 193:6090–6102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu K, Chen L, Kaur R, Pichichero ME. Transcriptome signature in young children with acute otitis media due to non-typeable Haemophilus influenzae. Int Immunol 25:353–361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu K, Chen L, Kaur R, Pichichero M. Transcriptome signature in young children with acute otitis media due to Streptococcus pneumoniae. Microbes Infect 14:600–609, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear: Acute otitis media pathogen or bystander? Pediatr Infect Dis J 31:325–330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beder LB, Hotomi M, Ogami M, et al. Clinical and microbiological impact of human bocavirus on children with acute otitis media. Eur J Pediatr 168:1365–1372, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Pitkaranta A, Virolainen A, Jero J, et al. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics 102:291–295, 1998. [DOI] [PubMed] [Google Scholar]
- 46. Chonmaitree T, Henrickson KJ. Detection of respiratory viruses in the middle ear fluids of children with acute otitis media by multiplex reverse transcription: Polymerase chain reaction assay. Pediatr Infect Dis J 19:258–260, 2000. [DOI] [PubMed] [Google Scholar]
- 47. Chonmaitree T. Viral and bacterial interaction in acute otitis media. Pediatric Infect Dis J 19(suppl.):S24–S30, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Friedland JS. Chemokines in viral disease. Res Virol 147:131–138, 1996. [DOI] [PubMed] [Google Scholar]
- 49. Taylor KE, Mossman KL. Recent advances in understanding viral evasion of type I interferon. Immunology 138:190–197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verhoeven D, Xu Q, Pichichero ME. Differential impact of respiratory syncytial virus and parainfluenza virus on the frequency of acute otitis media is explained by lower adaptive and innate immune responses in otitis-prone children. Clin Infect Dis 59:376–383, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu Q, Casey JR, Pichichero ME. Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal Immunol 8:1110–1117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]



