Abstract
Purpose. To evaluate the long-term efficacy and safety of the iStent inject device (Glaukos Corporation, Laguna Hills, CA) combined with phacoemulsification in patients with coexistent cataract and open-angle glaucoma or ocular hypertension (OHT). Methods. A prospective, uncontrolled, nonrandomized, interventional case series study was conducted in patients with both mild or moderate open-angle glaucoma or OHT and cataract. Patients underwent cataract surgery along with the implant of two iStent inject devices. Outcome measures were intraocular pressure (IOP), topical hypotensive medications required, and best-corrected visual acuity (BCVA). Results. 20 patients were enrolled. Mean follow-up was 47.4 ± 18.46 months. Mean baseline IOP was 19.95 ± 3.71 mmHg with medication and 26 ± 3.11 mmHg after washout. Mean end-follow-up IOP was 16.25 ± 1.99 mmHg, representing an IOP decrease of 36.92%, 9.74 ± 3.14 mmHg (P < 0.001), from baseline washout IOP. The mean number of medications was significantly reduced from 1.3 ± 0.66 to 0.75 ± 0.79 (P = 0.017). 45% of patients were medication-free by the end of follow-up. Mean logMAR BCVA improved significantly from 0.42 ± 0.16 to 0.18 ± 0.16 (P < 0.001). No complications of surgery were observed. Conclusion. The iStent inject device combined with cataract surgery served to significantly reduce both IOP and medication use in the long term in patients with coexistent open-angle glaucoma or ocular hypertension (OHT) and cataract.
1. Introduction
Glaucoma is the leading cause of irreversible blindness worldwide and is estimated to currently affect more than 60 million people [1]. Elevated intraocular pressure (IOP) is the main risk factor for the onset and advance of glaucoma. In effect, reducing IOP is the accepted consensus strategy to delay or even avoid the development of glaucoma and to manage its progression [2]. Thus, the ideal treatment for glaucoma should offer continuous IOP management along with a favourable safety profile.
Filtration surgery is usually performed in moderate or advanced cases but is linked to complications [3] including visual loss, bleb leak, inflammation, hypotony, and endophthalmitis [4]. Microinvasive glaucoma surgery tries to preserve conjunctival tissue of scarring through an ab interno microincisional approach, with efficacy, high safety profile, and fast recovery in mild-to-moderate glaucoma [5]. The iStent Trabecular Micro-Bypass (Glaukos Corporation, Laguna Hills, CA, USA) bypasses the trabecular meshwork (TM), which is the major source of resistance to outflow in open-angle glaucoma. iStent has been successfully employed to increase outflow in human anterior segments in vitro [6] and in glaucoma patients [7].
The first-generation iStent, Model GTS-100, is a titanium L-shaped stent that has proved safe and effective in lowering IOP in patients with mild-to-moderate glaucoma [7–10], even in the long term [11]. This device has also been described to achieve an IOP reduction to less than 15 mmHg if two or more iStent devices are implanted during cataract surgery [12].
A second-generation new microscale stent recently developed, the iStent inject Trabecular Micro-Bypass Model GTS-400, also increased outflow facility in cultured human anterior eye segments [13]. So far, three case series have revealed the safety and efficacy of the iStent inject device [14–16]. Another randomized study compared outcomes of two iStent inject devices versus two ocular hypotensive agents [17], showing that the use of iStent inject device is at least as effective as two medications. However, the follow-up of these studies was no longer than 12 months.
The purpose of our study was to assess the long-term efficacy and safety of implanting of two iStent inject Model GTS-400 stents combined with phacoemulsification in patients with coexistent cataract and open-angle glaucoma or ocular hypertension (OHT). This study is a continuation of our initial work [14], in which we reported outcomes one year after iStent inject device placement inserted via first-generation prototype G2-0 injectors.
2. Methods
Twenty patients with cataract and primary open-angle and pseudoexfoliative glaucoma or OHT were enrolled for this prospective, noncomparative, uncontrolled, nonrandomized, intervention study.
The study protocol adhered to the tenets of the Declaration of Helsinki and Spanish legislation and was approved by our Institutional Review Board. Before recruitment, written legally binding informed consent for Glaukos iStent inject implantation and cataract surgery was obtained from each patient.
Detailed subject inclusion criteria and surgical technique were previously described [14]. The main inclusion criteria were a previous diagnosis of mild-to-moderate open-angle glaucoma (including pseudoexfoliative glaucoma) or OHT and an IOP of 14–30 mmHg as measured at the last two consecutive visits if receiving ocular hypotensive medications, of 22–30 mmHg if not, or of 22–32 mmHg after hypotensive drops washout.
Before surgery, all the patients receiving ocular hypotensive medications were instructed to discontinue their use to obtain baseline IOP measurements. All surgical procedures were performed by two of the authors (JMMC and JGF), both with similar experience in MIGS surgery. Surgical technique is similar to that described for the GTS-100 device [13, 14]. After phacoemulsification and IOL placement, two GTS-400 iStent devices were implanted through the clear corneal incision (approximately 2.85 mm) placed for phacoemulsification. First-generation prototype G2-0 injectors were used to deliver the stents. Antiglaucoma topical therapy was introduced postoperatively if the desired target IOP range, as judged by the investigator, was not achieved.
Postoperative visits were scheduled for 1 day, 1 week, 1, 3, and 6 months, and 1, 2, 3, 4, 5, and 6 years after surgery. Every visit included slit-lamp biomicroscopy, applanation tonometer (Clement Clarke Perkins MK2 Tonometer) IOP measurement, number of glaucoma medications, and BCVA. Preoperatively and one month, three months, and every year postoperatively, the nasal angle was examined by gonioscopy.
2.1. Data Analysis
Efficacy outcome measures were IOP and topical ocular hypotensive medications used pre- and postoperatively. Successful treatment was defined as an IOP reduction ≥ 20% regardless of medication. We also defined “complete success” as an IOP ≤ 18 mmHg without postoperative medication and “qualified success” as an IOP ≤ 18 mmHg with medication [18]. These outcomes were determined by proportional analysis. Kaplan-Meier graphs were constructed to estimate surgical success according to those success criteria.
Failure rate included those patients who did not meet success criteria, required further glaucoma surgery, or had severe visual loss secondary to surgery itself.
Safety outcome measures were complications and logMAR BCVA.
For a descriptive statistical analysis, we used Excel 2007 (Microsoft Corp.) with SPSS software (version 15.0, SPSS Inc.). Results are provided as the mean ± standard deviation (SD).
The Kolmogorov-Smirnov test was used to check the normal distribution of data. A paired-sample t-test was used to compare outcomes in the study group. Significance was set at P ≤ 0.05.
3. Results
Combined surgery was uneventful in all the participating patients and the iStent was successfully implanted in all eyes, although three patients received just one iStent for logistic reasons. Postoperatively, gonioscopy revealed the presence of only one iStent in a further four patients (20%). So, in seven patients (35%), only one iStent was confirmed as functional. Both groups were comparable in baseline IOP and meds data, because no significant differences were obtained at that time point.
3.1. Patient Demographics
Twenty Caucasian subjects (11 women) were enrolled in the study, eight of whom had primary open-angle glaucoma, eight had OHT, and four had pseudoexfoliative glaucoma.
Mean follow-up was 47.4 ± 18.46 months (range 12–72 months) and median follow-up was 60 months. Eleven of the patients completed at least five years of follow-up, and two of them were followed six years (Table 1). Nine patients did not complete five years visit, due to logistic reasons (n = 4) and mortality (n = 5).
Table 1.
Postoperative follow-up of 20 patients (n: number of patients).
| Surgery | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | |
|---|---|---|---|---|---|---|---|
| n | 20 | 20 | 19 | 15 | 12 | 11 | 2 |
3.2. IOP Results
Mean IOP with medication at baseline was 19.95 ± 3.71 mmHg and after washout was 26 ± 3.11 mmHg (Figure 1). At the end of follow-up, mean IOP was 16.25 ± 1.99 mmHg. The IOP decrease from preoperative washout IOP was 9.74 ± 3.14 mmHg, representing a significant decrease of 36.92% (P < 0.001). The mean IOP drop relative to preoperative IOP with medication was 3.7 ± 3.7 mmHg, representing a 16.49% decrease (P < 0.001). Figure 2 shows preoperative IOP and final IOP without and with medication in each patient via scatter plot (Figure 2).
Figure 1.
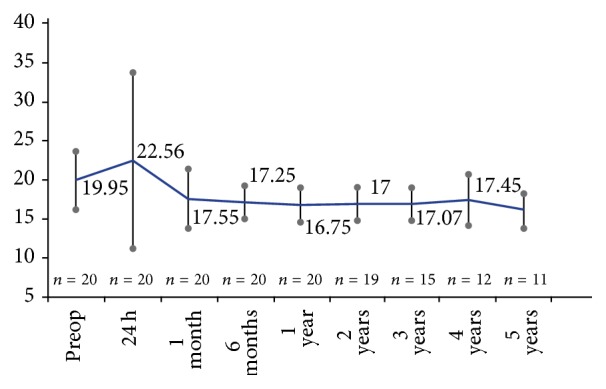
Mean intraocular pressure (IOP) (±standard error of the mean) recorded at each follow-up visit (baseline IOP is medicated IOP).
Figure 2.
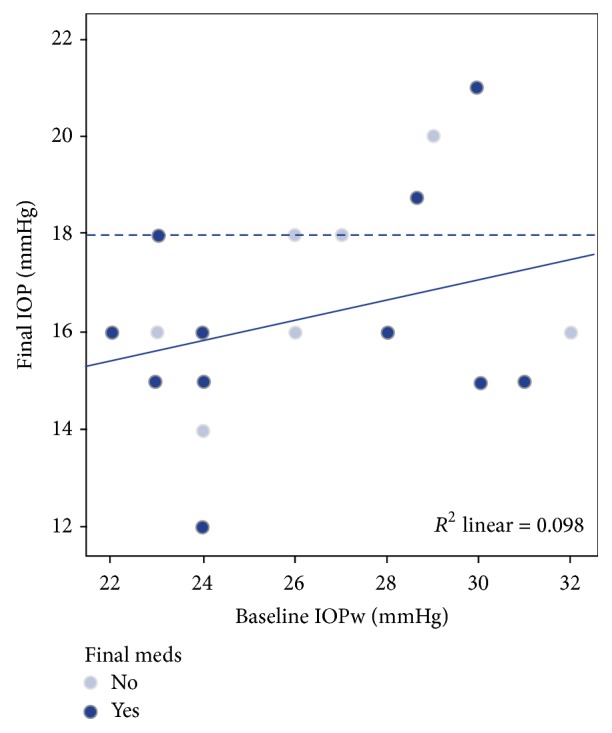
Scatter plot representing preoperative intraocular pressure (IOP) after washout and postoperative IOP without and with meds. Continuous line represents regression line (R 2 = 0.098). Discontinuous line represents IOP of 18 mmHg, defined as success in the study.
In the 11 patients completing at least five years of follow-up, mean IOP with medication at baseline was 20.36 ± 4.57 mmHg and after washout was 25.7 ± 3.06 mmHg. End-follow-up mean IOP was 16.18 ± 2.27 mmHg. Reductions in IOP from preoperative washout IOP and from preoperative IOP with meds (9.5 ± 3.1 mmHg and 4.18 ± 4.62 mmHg; 36.56% and 17.52%; P < 0.001 and P = 0.013, resp.) recorded in this subset of 11 eyes were similar to the reduction observed for the entire set of 20 eyes.
Subanalysis of IOP results depending of the number of functional iStent devices showed no significant differences in final IOP decrease between one and two functional iStent devices (P = 0.425). However, the group with two iStent devices had an IOP decrease of 10.42 ± 3.6 mmHg versus 8.57 ± 1.81 mmHg in the one functioning iStent group.
Three eyes (15%) showed transient IOP elevation to above 30 mmHg one day postoperatively. We previously attributed this to retained viscoelastic and observed its resolution by the one-week visit [14]. There were no additional reports of IOP elevation during the remaining course of long-term follow-up.
3.3. Antiglaucoma Medications
The majority of subjects reduced their medication burden during the course of follow-up. Before surgery, the mean number of glaucoma medications was 1.3 ± 0.66 (Figure 3). At the end of follow-up, the mean number of medications decreased to 0.75 ± 0.79, representing a significant mean reduction in glaucoma medications of 0.5 ± 0.89 (P = 0.017). Table 2 shows the proportion and number of patients receiving no, one, or two medications at baseline and at the final of follow-up.
Figure 3.
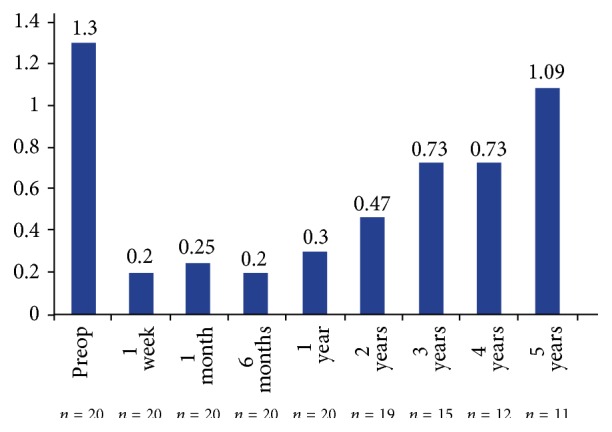
Use of glaucoma medications at each follow-up visit.
Table 2.
Number of antiglaucoma medications used at baseline and final follow-up.
| Follow-up time | None drops | One drop | Two drops |
|---|---|---|---|
| Baseline | 2 (10%) | 10 (50%) | 8 (40%) |
| Final | 9 (45%) | 7 (35%) | 4 (20%) |
At one year, 15 patients (75%) required no topical therapy, while just one subject (5%) needed two antiglaucoma medications. At the 3-year visit, seven of the 13 patients (53.9%) who completed the three years of follow-up were using one or two medications, while six patients were medication-free (46.2%). At five years, three patients (27.3%) were medication-free; four patients (36.4%) were taking one medication, while four patients (36.4%) were using two antiglaucoma drops. In the 11 eyes with 5-year follow-up data, the number of subjects on two medications translated to a nonsignificant glaucoma medication reduction in that group of patients.
The mean number of medications administered preoperatively in the patients who completed five years of follow-up was 1.18 ± 0.75. At the end of follow-up, this figure fell to 1.09 ± 0.83 medications, representing a mean reduction of 0.09 ± 0.94 (P = 0.756). At five years, three of the 11 patients (27.3%) were free of medication.
Subanalysis of medication use results depending of the number of functional iStent devices showed no significant differences in the final mean number of glaucoma medications (P = 0.255). However, the group with two iStent devices had a mean reduction of 0.77 ± 0.93 meds versus 0.14 ± 0.9 meds in the one functional iStent group. Besides, the percentage of eyes not requiring antiglaucomatous medications at the end of follow-up was higher in the group with two functioning iStent devices (61.5%) than in the group with one functioning iStent (14.3%).
3.4. Success Rate
At three years of follow-up, 33% of patients achieved an unmedicated IOP reduction ≥ 20% versus baseline unmedicated IOP, and 86% experienced an IOP reduction ≥ 20% regardless of medication. At five years, the percentage of patients showing an unmedicated IOP reduction ≥ 20% versus baseline washout IOP was 20%, while 100% experienced an IOP reduction ≥ 20% regardless of medication. At this follow-up time, seven out of 11 patients (63.63%) showed an IOP ≤ 16 mmHg regardless of medication.
The cumulative probability of success defined as an IOP reduction ≥ 20% using Kaplan-Meier survival analysis was 72.4% at two years and 64.4% at three years (Figure 4).
Figure 4.
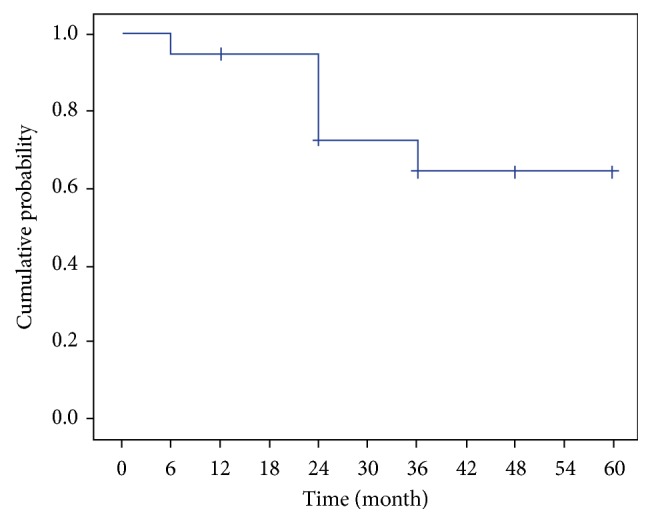
Kaplan-Meier plot of the cumulative probability of success defined as an IOP reduction ≥ 20% regardless of medication.
Complete success at the end of follow-up was recorded in eight patients (40%) and qualified success in 10 of our 20 patients (50%). Similar rates were obtained for the subset of patients completing five years of follow-up, though the complete success rate was slightly lower and the qualified success higher (Table 3). The cumulative probability of complete and qualified success using Kaplan-Meier survival analysis at two years was 10% and 35%, respectively (Figures 5 and 6).
Table 3.
Success rates recorded during follow-up (w/o: without).
| Follow-up time | IOP reduction ≥ 20% | Complete success | Qualified success | |
|---|---|---|---|---|
| w/o meds | with or w/o meds | |||
| 1 month | 63% | 84% | 55% | 10% |
| 3 months | 84% | 100% | 65% | 15% |
| 6 months | 73% | 94% | 50% | 15% |
| 1 year | 73% | 100% | 50% | 20% |
| 2 years | 25% | 62% | 35% | 41% |
| 3 years | 33% | 86% | 31% | 46% |
| 4 years | 30% | 80% | 28% | 36% |
| 5 years | 20% | 100% | 28% | 64% |
Figure 5.
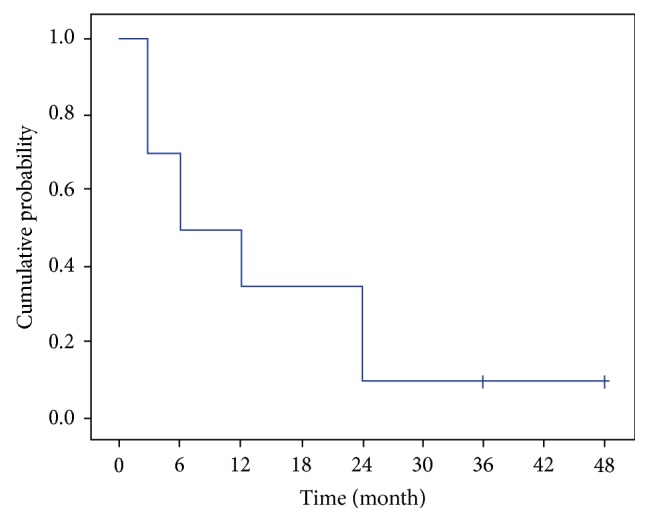
Kaplan-Meier plot of the cumulative probability of complete success, defined as an IOP ≤ 18 mmHg without postoperative medication.
Figure 6.
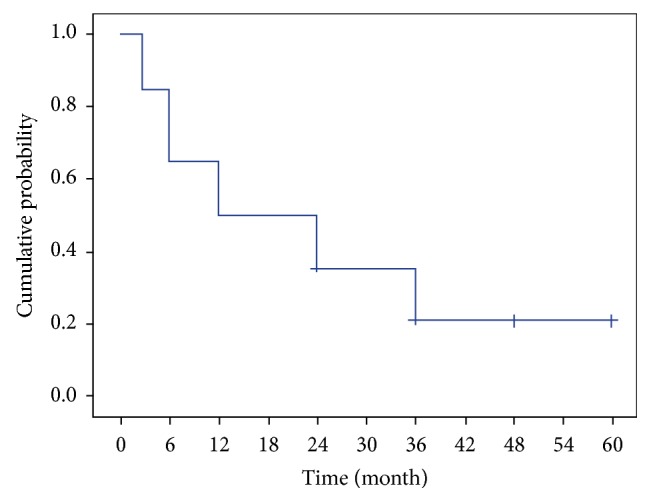
Kaplan-Meier plot of the cumulative probability of “qualified success,” defined as an IOP ≤ 18 mmHg with medication.
By the end of follow-up, 15 of the 20 subjects (75%) showed an IOP ≤ 16 mmHg regardless of medication, including six patients of the 20 (30%) requiring no medication.
No patient needed any further glaucoma surgery over the follow-up period.
3.5. Safety Outcomes
No visual acuity loss was recorded; logMAR BCVA significantly improved from a mean of 0.42 ± 0.16 preoperatively to 0.18 ± 0.16 at the end of follow-up (P < 0.001).
No adverse events related to iStent inject implantation were reported. During the long-term follow-up, five patients suffered an eye condition unrelated to iStent inject implantation. Three of these patients required Nd:YAG capsulotomy due to posterior capsule opacification (PCO). One patient (who had OHT and high myopia) suffered retinal detachment 40 months after the initial surgery, with excellent visual recovery after pars plana vitrectomy. The last of these patients developed epiretinal membrane three years after the initial procedure. However, no surgery was required due to the patients' lack of symptoms and good visual acuity.
4. Discussion
In this series of 20 patients, the implant of two iStent inject devices using first-generation prototype G2-0 injectors combined with phacoemulsification led to a significant IOP reduction (P < 0.001) and a significant decrease in the number of antiglaucoma medications required (P = 0.017) after a mean follow-up of almost four years. The subset of 11 patients completing five years of follow-up showed a similar significant IOP decrease (P < 0.001) along with a nonsignificant decrease in the number of antiglaucoma medications required (P = 0.756).
In both the full set of 20 eyes and the subset of 11 eyes, the IOP reduction from medicated preoperative IOP was approximately 4 mmHg. This decrease is higher than the approximate 2 mmHg decrease reported after cataract surgery alone [19].
IOP reduction in this small patient cohort was not as pronounced as that reported in the larger studies that have addressed iStent inject implantation (Table 4) as a single procedure [15–17]. In all these studies, two iStent inject devices were implanted per eye. This difference in outcome may be attributed to shorter follow-up times. Our study outcomes may have been affected by the fact that the patients in this trial were the first at our centre to receive an iStent inject device such that surgeons were at the start of their learning curve. Further, we used first-generation prototype G2-0 injectors in all 20 patients in this series although a second-generation injector (G2-M-IS) has since been introduced. This new model is able to hold two stents so that the clinician can insert two devices while entering the eye only once. This improved injector design reduces the number of surgical steps and thus raises the chances of more reliable surgery and improved outcomes. Further, in our prior study, in a large proportion of patients (7/20), only one functional stent was observed on postsurgery gonioscopy [14]. It is likely that these patients would have shown a greater IOP reduction if two stents had been confirmed as functioning, based on in vitro results indicating that that a second stent achieved a further increase in outflow facility [4]. In effect, Belovay et al. [12] observed the improved ability of multiple GTS-100 iStent devices to reduce IOP to below 15 mmHg and reduce topical ocular hypotensive medications. In fact, our subgroup analysis, although not significant, showed higher decrease in IOP and medication use at the end of the follow-up in the group of two functioning iStent devices. These differences could be significant with higher sample size.
Table 4.
Outcome variables reported in the studies that have addressed the efficacy of iStent GTS-400 placement. The data provided for the present study are end-follow-up data for the 20 eyes enrolled and 5-year results for 11 eyes.
| Source | n | Combined phaco | Follow-up (months) | Mean IOP reduction (mmHg) from preop | IOP ≤ 18 mmHg w/o meds |
Mean reduction in meds |
|---|---|---|---|---|---|---|
| Arriola-Villalobos et al. [14]∗ | 20 | Yes | 12 | 3.2 ± 3.75 | 50% | 1 ± 0.79 |
| Voskanyan et al. [15] | 92 | No | 12 | 22.1 ± 3.3 versus 15.7 ± 3.7† | 66% | NA |
| Klamann et al. [16] | 17 | No | 6 | 21.19 ± 2.56 versus 14.19 ± 1.38† | NA | 2.19 ± 0.91 versus 0.88 ± 0.62† |
| Fea et al. [17] | 94 | No | 12 | 8.1 ± 2.6 | 92.6% | NA |
| Present study∗ | 20 | Yes | 47.4 ± 18.46 | 3.7 ± 3.7 | 40% | 0.6 ± 0.88 |
| 11 | 60 | 4.18 ± 4.62 | 28% | 0.09 ± 0.94 |
Preoperative IOP refers to medicated IOP. (n: number of patients; w/o: without; †data not available, mean preoperative versus mean postoperative data; ∗same set of patients; NA: not available.)
In our patients, IOP was stable during follow-up (Figure 1). The number of medications used increased over time after the one-year visit (Figure 3). A slight increase in the number of medications beyond one year of follow-up was also reported in the long-term study in which one iStent GTS-100 was implanted during phacoemulsification [11]. Notwithstanding, a discrete reduction was produced in the number of antiglaucoma drops at five years, including three patients (27.3%) who were medication-free. A recent report has shown that the placement of two iStent GTS-400 devices as a sole procedure is at least as effective as two medications [17]. Reducing or eliminating the use of antiglaucoma drops is highly desirable because the chronic use of antiglaucoma drugs may lead to ocular surface damage and conjunctival inflammation [20] or even reduce the success of subsequent trabeculectomy [21].
Success in our study defined as an IOP reduction ≥ 20% regardless of medication was achieved in all the patients at the end of follow-up. At that time point, an IOP reduction ≥ 20% with no medications was achieved in nine out of 20 patients, to give a success rate of 45%. Beyond the one-year visit, success rates fell (Table 2), consistent with the increased use of antiglaucoma medications. We hypothesize that ultrastructural changes in the TM or Schlemm's canal could be responsible for this increase in medications required. Our complete success rate at one year (50%) was lower than the other reported rates for two GTS-400 devices at one year. Fea et al. [17] reported a 92.6% of complete success, while Voskanyan et al. [15] reported 66%. Probable reasons for this discrepancy have been analysed above.
Our findings indicated a highly acceptable safety profile, with no adverse events or long-term complications related to stent implant. No subjects experienced hypotony, endophthalmitis, or sight-threatening complications associated with more invasive surgery procedures. Mean visual acuity was significantly improved at each follow-up visit. There was a mild decrease in mean visual acuity beyond the one-year visit, attributed mainly to PCO.
Our study has several limitations. The number of patients was low and, being a long-term study, several patients were lost to follow-up such that only slightly more than half of the patients completed 60 months of follow-up. Being uncontrolled, we could not determine the individual effects of phacoemulsification or iStent placement on IOP and the number of hypotensive drugs required. The cohort examined was a heterogeneous group of subjects, with mild-moderate primary open-angle or pseudoexfoliative glaucoma along with OHT patients. Moreover, as reported above, these patients were the first to receive the iStent GTS-400 at our centre, meaning that, despite experience with the GTS-100 model, the surgeons were at an early learning stage with the use of the new model, which could affect outcomes. Finally, in seven patients (35%), only one iStent was confirmed as functional [14]. This potential shortcoming could also affect the IOP and medication use results [7, 12], although nonsignificant differences were found.
5. Conclusions
To the best of our knowledge, this is the first study to assess combined GTS-400 iStent implantation and phacoemulsification surgery over a follow-up period longer than one year. Significant reductions were achieved in IOP and number of medications required after more than 47 months of follow-up. At the end of follow-up, 45% of the patients were medication-free and all patients showed good visual outcomes with no serious adverse events recorded. Our findings suggest that iStent GTS-400 placement added to phacoemulsification could be a long-term safe and effective treatment option alternative for patients with both cataract and mild-moderate open-angle glaucoma or OHT. These findings, nevertheless, require confirmation in randomized controlled studies conducted in large patient cohorts.
Acknowledgments
Glaukos Corporation provided study devices and payment of the article processing charges.
Competing Interests
The authors have no commercial or proprietary interest in any of the products or companies mentioned in this article. Julian Garcia-Feijoo is a consultant for the Glaukos Corporation.
References
- 1.Quigley H., Broman A. T. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leske M. C., Heijl A., Hyman L., Bengtsson B., Komaroff E. Factors for progression and glaucoma treatment: the Early Manifest Glaucoma Trial. Current Opinion in Ophthalmology. 2004;15(2):102–106. doi: 10.1097/00055735-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Jampel H. D., Musch D. C., Gillespie B. W., Lichter P. R., Wright M. M., Guire K. E. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS) American Journal of Ophthalmology. 2005;140(1):16–22. doi: 10.1016/j.ajo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Ang G. S., Varga Z., Shaarawy T. Postoperative infection in penetrating versus non-penetrating glaucoma surgery. British Journal of Ophthalmology. 2010;94(12):1571–1576. doi: 10.1136/bjo.2009.163923. [DOI] [PubMed] [Google Scholar]
- 5.Saheb H., Ahmed I. I. K. Micro-invasive glaucoma surgery: current perspectives and future directions. Current Opinion in Ophthalmology. 2012;23(2):96–104. doi: 10.1097/icu.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 6.Bahler C. K., Smedley G. T., Zhou J., Johnson D. H. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. American Journal of Ophthalmology. 2004;138(6):988–994. doi: 10.1016/j.ajo.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Barrientos Y., García-Feijoo J., Martínez-de-la-Casa J. M., Pablo L. E., Fernandez-Perez C., Sanchez J. G. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Investigative Ophthalmology and Visual Science. 2010;51(7):3327–3332. doi: 10.1167/iovs.09-3972. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel D., Wetzel W., Neuhann T., et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. European Journal of Ophthalmology. 2009;19(3):393–399. doi: 10.1177/112067210901900311. [DOI] [PubMed] [Google Scholar]
- 9.Fea A. M. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. Randomized double-masked clinical trial. Journal of Cataract and Refractive Surgery. 2010;36(3):407–412. doi: 10.1016/j.jcrs.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Samuelson T. W., Katz L. J., Wells J. M., Duh Y.-J., Giamporcaro J. E. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Arriola-Villalobos P., Martínez-de-la-Casa J. M., Díaz-Valle D., Fernández-Pérez C., García-ánchez J., García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. British Journal of Ophthalmology. 2012;96(5):645–649. doi: 10.1136/bjophthalmol-2011-300218. [DOI] [PubMed] [Google Scholar]
- 12.Belovay G. W., Naqi A., Chan B. J., Rateb M., Ahmed I. I. K. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. Journal of cataract and refractive surgery. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Bahler C. K., Hann C. R., Fjield T., Haffner D., Heitzmann H., Fautsch M. P. Second-generation trabecular meshwork bypass stent (istent inject) increases outflow facility in cultured human anterior segments. American Journal of Ophthalmology. 2012;153(6):1206–1213. doi: 10.1016/j.ajo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Arriola-Villalobos P., Martínez-de-la-Casa J. M., Díaz-Valle D., et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. British Journal of Ophthalmology. 2013;97(10):1250–1255. doi: 10.1136/bjophthalmol-2012-302394. [DOI] [PubMed] [Google Scholar]
- 15.Voskanyan L., García-Feijoó J., Belda J. I., Fea A., Jünemann A., Baudouin C. Prospective, unmasked evaluation of the iStent® Inject system for open-angle glaucoma: synergy trial. Advances in Therapy. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klamann M. K. J., Gonnermann J., Pahlitzsch M., et al. iStent inject in phakic open angle glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology. 2015;253(6):941–947. doi: 10.1007/s00417-015-3014-2. [DOI] [PubMed] [Google Scholar]
- 17.Fea A. M., Belda J. I., Rekas M., et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clinical Ophthalmology. 2014;8:875–882. doi: 10.2147/opth.s59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer D. K., Barton K., Grehn F., et al. Consensus on definitions of success. In: Shaarawy T. M., Sherwood M. B., Grehn F., editors. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Amsterdam, The Netherlands: World Glaucoma Association. Kugler; 2009. pp. 15–24. [Google Scholar]
- 19.Shrivastava A., Singh K. The effect of cataract extraction on intraocular pressure. Current Opinion in Ophthalmology. 2010;21(2):118–122. doi: 10.1097/icu.0b013e3283360ac3. [DOI] [PubMed] [Google Scholar]
- 20.Baudouin C., Pisella P.-J., Fillacier K., et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi: 10.1016/s0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 21.Broadway D. C., Grierson I., O'Brien C., Hitchings R. A. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Archives of Ophthalmology. 1994;112(11):1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]


