Abstract
Post-translational modifications of proteins with ubiquitin (Ub) and ubiquitin-like (Ubl) modifiers, orchestrated by a cascade of specialized E1, E2 and E3 enzymes, control a staggering breadth of cellular processes. To monitor catalysis along these complex reaction pathways, we developed a cascading activity-based probe, UbDha. Akin to the native Ub, upon ATP-dependent activation by the E1, UbDha can travel downstream to the E2 (and subsequently E3) enzymes through sequential trans-thioesterifications. Unlike the native Ub, at each step along the cascade UbDha has the option to react irreversibly with active site cysteine residues of target enzymes, thus enabling their detection. We show that our cascading probe ‘hops’ and ‘traps’ catalytically active ubiquitin-modifying enzymes (but not their substrates) by a mechanism diversifiable to Ubls. Our founder methodology, amenable to structural studies, proteome-wide profiling and monitoring of enzymatic activities in living cells, presents novel and versatile tools to interrogate the Ub/Ubl cascades.
Introduction
Post-translational modifications of cellular targets with ubiquitin (Ub) or ubiquitin-like (Ubl) modules are potent regulators of protein function and thus govern a wide range of biological processes1. While the general biochemical logistics of Ub/Ubl activation, conjugation and ligation, orchestrated sequentially by the E1, E2, and E3 enzymes, are highly conserved among eukaryotes, the number and flavour of individual players in each organism’s relevant enzymatic repertoire can differ widely1. Humans are known to harbour 2 E1, ~30 E2s and ~600 E3s in the Ub conjugation cascade, and while some are highly specific for certain targets, others appear relatively promiscuous. The sheer complexity of such enzymatic networks, further inundated by ~80 specific proteases responsible for removal of these modifications1, enables highly specialized and sensitive modes of regulation, necessary to accommodate dynamic cellular events. On the flip side, deregulation of these pathways is a common feature in cancer, neurodegenerative, and inflammatory diseases. Similarly, some pathogens have evolved to perturb or exploit the host’s Ub/Ubl conjugation cascades to their advantage2. Despite their importance, development of comprehensive tools to assess the enzymology of these processes has been a long-standing roadblock in the field.
An important class of reagents used to study enzymatic activity, structure and substrate specificity within the Ub/Ubl modification system are activity-based probes (ABPs)3,4. In the last decade, we and others have developed various ABPs for deubiquitylating enzymes (DUBs) and Ubl specific proteases5–7. Among the advantages of such probes is their ability to report on DUB activities in cellular extracts and even intact cells, thus facilitating the study of these enzymes in their biological context8. Development of analogous tools for the ligation machinery has proven challenging. Unlike DUBs, which contain highly reactive cysteine (Cys) nucleophiles, ligases possess less nucleophilic active site Cys residues, rendering them more difficult to trap with electrophiles. Recently, Ubl-AMP probes were reported to selectively label cognate activating enzymes9–12. However, because ligation requires transfer of Ub/Ubl between enzymes, the challenge of monitoring reactions throughout the cascade remained. To tackle this, we developed a mechanism-based ligase probe that undergoes sequential trans-thioesterification reactions as it cascades from the E1 to the E2 and subsequently the E3 stage. In addition, at each step of the cascade our probe has the option to irreversibly trap the active site Cys residues of the enzymes in question. This methodology, implemented and characterized using the Ub-specific conjugation machinery, is further extended to the NEDD8 enzyme family, demonstrating general applicability of our probe design to Ubls.
The unique properties of our cascading probe enable direct monitoring of sequential E1, E2 and (HECT) E3 activities in a wide variety of experimental settings. Given the ATP-dependence of its reactivity against ligases, combined with its lack of transfer to substrates, our probe is well suited for proteome-wide profiling of relevant enzymatic cascades. Furthermore, upon introduction into living cells, our probe monitors enzymatic activities of interest and reports on changes in response to chemical or genetic inhibition. From the structural perspective, our stable mechanism-based trapping of catalytic Cys residues circumvents potential disadvantages incurred by traditional methods of stable E2-Ub conjugate preparation requiring active site mutagenesis13–17. Collectively, these novel features of our ABP tool present previously inaccessible avenues for targeting and monitoring enzymatic activities along the Ub/Ubl conjugation cascades, with implications for drug discovery and cell as well as structural biology of these pathways.
Results
Design and synthesis of the cascading ABP
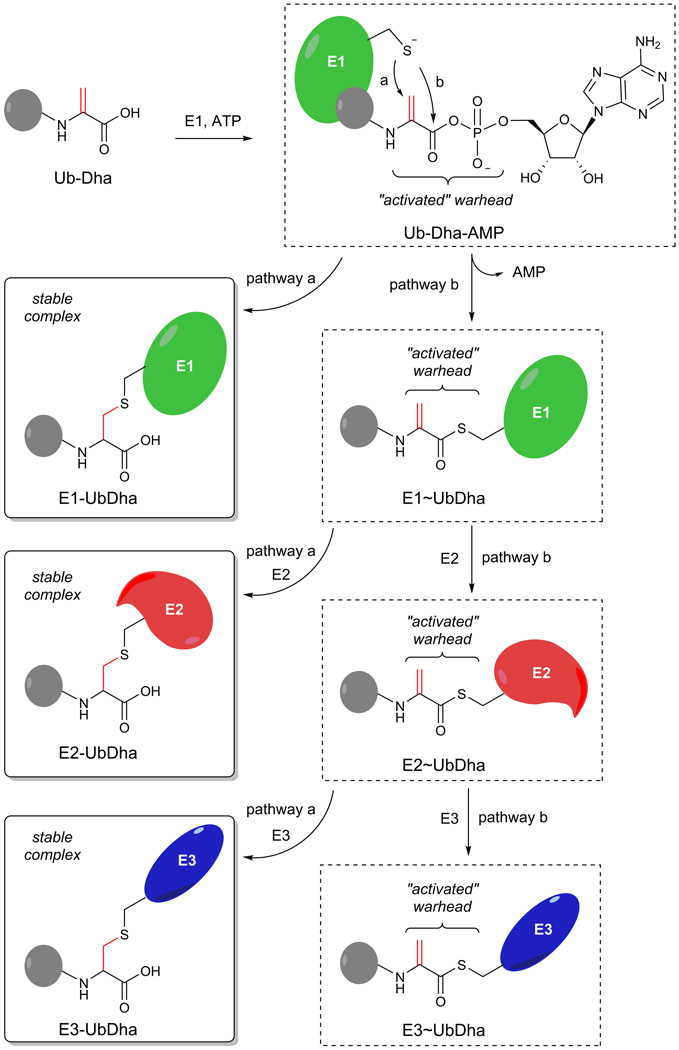
To initiate the Ub/Ubl modification cascade, the E1 activating enzyme adenylates the C-terminus of Ub at the expense of ATP, which results in a high energy E1~Ub thioester formed upon an intramolecular reaction of the intermediate adenylate with the E1 active site Cys nucleophile. Next, a trans-thioesterification reaction transfers the activated Ub to a conserved E2 Cys, thus forming an E2~Ub thioester intermediate, which subsequently enables transfer of Ub onto a substrate with the help of an E318. It is known that a Ub-G76A mutant can also be processed by the E1–E2–E3 cascade, albeit with reduced efficacy19,20. We reasoned that replacing the C-terminal alanine by a latent and electrophilic dehydroalanine (Dha) moiety, would retain recognition by the E1–E2–E3 enzymes, allowing the UbGly76Dha (UbDha) to traverse the cascade (Fig. 1). Analogous to ubiquitin activation, UbDha is activated by the E1 enzyme through the formation of an adenylate intermediate, which strongly increases the electrophilic character of the Michael acceptor (Dha moiety). The activated methylene group of the Dha moiety is now poised to either covalently trap the enzyme in an E1-UbDha thioether adduct (Fig. 1, pathway a) or follow the native ligation route resulting in an E1~UbDha thioester (pathway b). In the second scenario, UbDha is available for transfer to an E2 enzyme, during which an analogous two options of covalent thioether adduct with the probe (pathway a) or a native trans-thioesterification reaction (pathway b) arise. Lastly, similar options apply to the subsequent transfer of UbDha from the E2 to an active site cysteine-dependent E3 enzyme (i.e. from the HECT or RBR families of E3 ligases21).
Figure 1. Mechanism and synthesis of the activity based probe Ub-Dha.
In situ activation of Ub-Dha with E1 and ATP results in a mechanism based ABP for E1, E2 and Cys dependent E3 enzymes. Pathway a describes the covalent trapping of the enzyme (E-UbDha = thioether-linked adduct), while pathway b depicts the native transthioesterification processing of the probe (E~UbDha = thioester intermediate of conjugate) by the cascade.
UbDha was synthesized starting from Ub(1–75) (Supplementary results, Supplementary Fig. 1), using our previously reported linear Fmoc-based solid phase synthesis (SPPS) of Ub22, where coupling H-Cys(Bn)-OMe to the C-terminal carboxyl group of protected Ub(1–75) afforded Ub(1–75)-Cys(Bn)-OMe. This was subsequently transformed into UbDha-OMe by oxidative elimination with O-mesitylenesulfonylhydroxyl-amine (MSH)23. Finally, the methyl ester was hydrolysed, to generate the UbDha probe. We also used the recently reported 2,5-dibromohexanediamide reagent to convert a Cys into a Dha moiety (Supplementary Fig. 1; Method B)23,24. Importantly, in contrast to MSH, 2,5-dibromohexanediamide reacts with a C-terminal Cys residue and thus allows the use of recombinant Ubl G76C mutants to prepare probes.
Covalent bond formation with conjugating enzymes
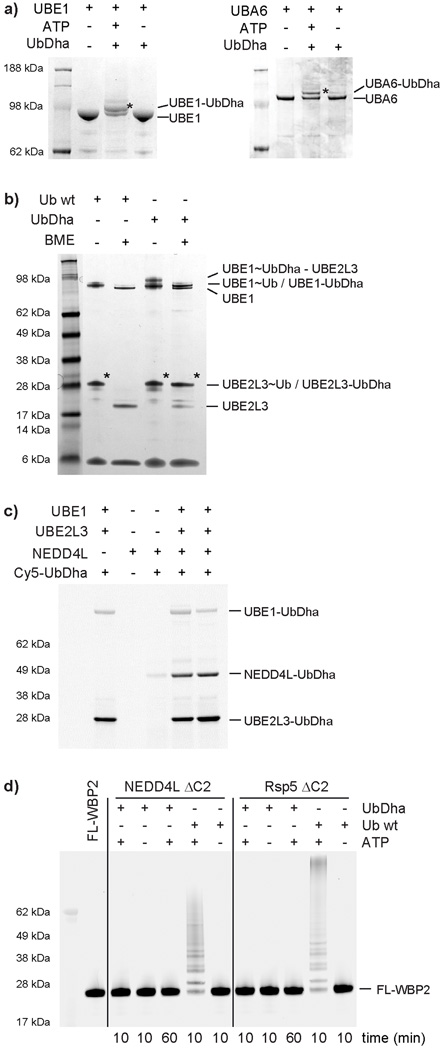
To evaluate the ability of our probe to travel the cascade, we began by subjecting the Ub-activating UBE1 enzyme to UbDha in vitro. SDS-PAGE analysis of the reaction revealed formation of an UBE1-UbDha adduct (Fig. 2a), consistent with a thioether linkage due to its stability under reducing conditions. ATP-dependence of the reaction indicated that the ligation proceeds through the adenylate intermediate (Fig. 1). Similar observations were made for UBA6 (Fig. 2a, right panel), the second Ub E1 enzyme, which also activates the Ubl modifier FAT1025. To test whether the E1-UbDha thioester can transfer UbDha to the E2 stage, we added UBE2L3 to the reaction in Fig. 2a. Whereas SDS-PAGE analysis under non-reducing conditions facilitated labeling with both native Ub and UbDha (Fig. 2b), under reducing conditions only the UbDha probe was able to form a stable adduct with the E2 enzyme. As expected, labeling was not observed in the absence of ATP (Supplementary Fig. 2). Interestingly, the double UbDha loaded UBE1 intermediate26 (Supplementary Figs. 2 and 3) observed in the absence of an E2, was sensitive to co-incubation with UBE2L3 (Supplementary Fig. 4), while adding UBE2L3 subsequent to UBE1 labeling with UbDha had no effect in this context. This may indicate a transfer of Ub from the adenylation active site to a nearby Cys in the adenylation domain of UBE1, which simply does not occur when UbDha is quickly transferred to the next step in the cascade, here transfer to E227. In addition to UBE2L3, UbDha showed labeling of 26 other ubiquitin E2s (Supplementary Fig. 5), but remained unreactive against Ubl E2s (UBE2F, UBE2I, UBE2L6 and UBE2M). UbDha was also unable to label the Ub E2 UBE2Z downstream of UBE1 due to the enzyme’s selectivity for the alternative E1, UBA625. Noncanonical catalytically inactive E2s UBE2V1 and UBE2V228 with scaffolding function also failed to react with the probe (Supplementary Fig. 5). Collectively, these results demonstrate broad utility of the UbDha probe in monitoring mechanism-based transfer of activated Ub from the E1 to a wide range of cognate E2s.
Figure 2. Covalent thioether bond formation of UbDha with conjugating enzymes.
a) ATP-dependent labeling of the E1 enzymes UBE1 (left) and UBA6 (right); b) Reactivity of UBE2L3 towards Ub and UbDha under reducing and non-reducing conditions; c) Fluorescent scan showing NEDD4L HECT labeling with the Cy5-UbDha; d) Multiple turnover ubiquitination on substrate WBP2 doesn’t occur with UbDha. Asterisks (*) indicate modified forms of UBE1, UBA6, UBE2L3 and NEDD4L. For uncut gels, see Supplementary Figure 19.
Under non-reducing conditions, (Fig. 2b, Supplementary Fig. 6) a ternary complex of E1~UbDha-E2 was revealed. Here, the acceptor E2 enzyme reacts directly with the Michael acceptor on the probe-donating E1-thioester adduct. This third pathway of probe action (Supplementary Fig. 6, right panels) was further confirmed by the stable oxyester linked E1-O~UbDha adduct (Supplementary Fig. 6).
Next, we investigated whether UbDha could be further delivered to an E3, bearing an active site Cys. The family of E3 ligases is subdivided into three major classes according to their mechanism of action21. In humans, the known thioester-forming E3s, which harbor catalytic Cys residues loaded with Ub by a mechanism analogous to the E1 and E2 enzymes, fall into the HECT (homologous to E6-AP terminus, 28 family members in humans) and RBR (RING-between-RING, 13 members in humans) classes. In contrast, the RING E3 ligases act as scaffolds between the E2~Ub thioester and the substrate protein, but do not themselves form thioesters21. We therefore examined a well-characterized HECT E3 ligase NEDD4L14. As expected, a Cy5-UbDha-NEDD4L thioether adduct was observed downstream of UBE1 and UBE2L3 (Fig. 2c). Similarly, a panel of 9 other HECT E3s also reacted with the probe (Supplementary Fig. 7). Because NEDD4L initially showed reactivity without ATP (Supplementary Fig. 7), we repeated this experiment with an active site Cys-to-Ala mutant and a mutant in which the four non-catalytic Cys residues were mutated to Ala (Supplementary Fig. 8). Labeling was clearly visible under standard assay conditions, whereas omitting either ATP, UBE1, and UBE2D2 or ATP alone resulted in virtually no labeling. Interestingly, the catalytic Cys-to-Ala mutant showed reduced but not completely abolished labeling compared to the wild-type NEDD4L. A similar observation was made for the non-catalytic 4× Cys-to-Ala mutant, suggesting that NEDD4L has at least two cysteines competent to receive an activated UbDha. Not all HECT E3s have a Cys adjacent to a noncovalent Ub-binding site. For instance, the HECT domain of Smurf2 (54% identical to NEDD4L) lacks the candidate Cys in the noncovalent Ub binding site and showed no alternative labeling of any of its six non-catalytic Cys residues with our probe (Supplementary Fig. 9).
For a native Ub, the next step in the cascade following reactivity with an E3 would result in ligation to a target substrate. To test whether our probe behaves similarly, we chose WBP229 a known substrate for the UbDha-reactive E3 HECT ligases (NEDD4L, Rsp5, WWP1 and WWP2). While incubation with Ub showed multiple turnover ubiquitination on WBP2, no ubiquitination was observed using UbDha even with prolonged reaction times (Fig. 2d and Supplementary Fig. 10). This feature makes UbDha particularly advantageous for enzymatic profiling in cellular systems, where irrespective of the presence of substrates, our activated warheads will remain on the active enzymes themselves.
Generalizing the cascading ABP methodology to Ubls
To show that our probe design is applicable beyond the Ub cascade, we synthesized the NEDD8 G76C mutant by linear Fmoc based solid phase synthesis (SPPS)30 and easily transformed the Cys into Dha by overnight incubation with 2,5-dibromohexanediamide. Following incubation of NEDD8Dha with UBA3/NAE1, SDS-PAGE analysis revealed formation of the expected UBA3-NEDD8 thioether adduct and a double NEDD8-loaded UBA3 adduct31 (Supplementary Fig. 11 and Supplementary Fig. 12). Co-incubation with UBE2M resulted in the formation of a NEDD8-UBE2M thioether adduct (Supplementary Fig. 11). As with the Ub E1 UBE1, formation of double NEDD8Dha-linked UBA3 was suppressed when UBE2M was present during the E1 labeling. Similarly, treatment with 2-mercaptoethanol had no effect on adduct formation, and labeling with NEDD8Dha failed in the absence of ATP (Supplementary Fig. 11). These experiments demonstrate that our founder cascading ABP design can be extended to other Ubl modification cascades.
Structure determination of a thioether-linked E2-Ub adduct
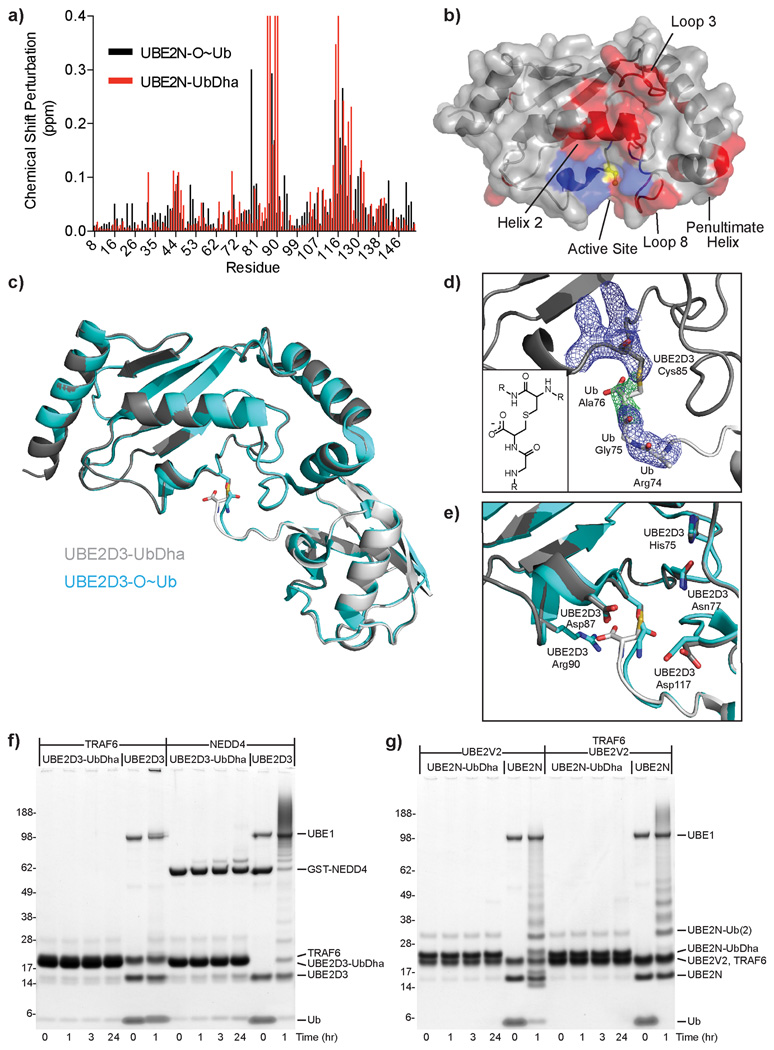
To evaluate the structural integrity of our thioether-linked adducts we performed both solution-based and crystallographic studies. Solution properties of the oxyester-linked UBE2N-O~Ub conjugate (in which the active site Cys has been mutated to Ser) have been thoroughly characterized by NMR spectroscopy and small angle X-ray scattering32, allowing us to validate the analogous thioether linkage as a suitable mimic. Analysis of chemical shift perturbations in the 1H,15N HSQC spectrum of UBE2N (Supplementary Fig. 13) arising from conjugation with Ub revealed several regions affected in both the thioether- and oxyester-linked samples (Fig. 3a; compare red to black) localized primarily to Loop 3, Helix 2, Loop 8, and the penultimate C-terminal helix (Fig. 3b, red surfaces). These perturbed regions can be attributed to the “closed” conformation of the UBE2N-UbDha thioether adduct, which is predominant in solution32 and indicates the overall behavior of the thioether-linked adduct to be similar to the oxyester linkage. Certain resonances in the UBE2N-UbDha HSQC spectrum displayed markedly different characteristics from the oxyester-linked sample (Fig. 3a), mapped to the region directly surrounding the active site (Fig. 3b, blue surfaces). Given the exquisite sensitivity of amide resonances to their local chemical environment, such differences between the two linkage types are most likely due to their differing chemical properties, and not to a larger structural change.
Figure 3. Structural studies of thioether-linked E2-Ub adducts.
a) UBE2N chemical shift perturbations upon Ub activation for oxyester (black) and thioether (red) linkages. Resonances perturbed beyond facile re-assignment were plotted using maximum perturbation value. b) Changes (from a) mapped onto the UBE2N structure (PDB 1J7D) (similarities in red; differences in blue). Active site Cys is colored yellow. c) Superposition of thioether-linked UBE2D3-UbDha conjugate (gray) over the oxyester-linked form (cyan, PDB 3UGB). d) Simulated annealing omit map of electron density surrounding the thioether linkage. 2|Fo|-|Fc| electron density (blue) contoured at 1σ, |Fo|-|Fc| density (green) contoured at 3σ. Inlay: diagram illustrating the thioether linkage. e) Overlay of E2 active site residues in thioether (gray) and oxyester (cyan) structures. Stability of the thioether-linked UBE2D3- and UBE2N-UbDHA adducts, incubated with f) RING E3 ligase TRAF6 or the HECT E3 ligase NEDD4, or g) accessory E2-variant UBE2V2, alone or in combination with the RING E3 ligase TRAF6.
While solution studies indicate normal inter-domain behavior within the thioether-linked adduct, they lack the atomic resolution of the linkage and the surrounding active site residues. To remedy this we crystallized the UBE2D3-UbDha adduct under conditions published for the oxyester-linked UBE2D3-O~Ub conjugate33. The 2.2Å UBE2D3-UbDha thioether structure was strikingly similar to the published oxyester structure (PDB 3UGB), with Cα RMSD values of 0.23 and 0.35Å for UBE2D3 and Ub, respectively (Fig. 3c, Supplementary Table 1). The only significant deviation between the two structures was found in the Ub C-terminus near the linkage itself, manifested in an RMSD of 1.13Å for Ub residues 73–76. The thioether linkage was readily revealed in the corresponding electron density (Fig. 3d), although detailed features of the omit map did suffer from high B factors in the flexible Ub C-terminus (average B-factor of 105.7 for Ub residues 75–76 compared to 49.4 for all protein). Nearby residues within the UBE2D3 active site were found to adopt nearly identical conformations, with the only exception being Arg90, which was missing from the electron density (Fig. 3e). An overlay of the oxyester and thioether structures suggests that the additional carboxylate group of the thioether linkage could displace the Arg side chain from the E2 active site cleft, although to our knowledge there is currently no known role for this residue in E2 catalysis.
Preparation of stable E2-Ub conjugates has in the past relied on the DCA method13, oxyester14–16, and isopeptide17 bonds, all necessitating mutations to the enzyme’s active site. Furthermore, oxyester-linked E2~O-Ub/ E2~O-Ubl conjugates suffer from susceptibility to hydrolysis, particularly in the presence of an active E3 ligase14–16, thereby limiting their use in structural applications and preventing any potential utility in cell-based studies. In contrast, UBE2D3-UbDha and UBE2N-UbDha thioether-linked adducts remained inert in the presence of activating factors, such as the E3 ligases TRAF6 (RING-type) or NEDD4L (HECT-type), or an accessory E2-variant UBE2V2 with or without TRAF6, respectively (Figs. 3f and 3g). As a catalytically inert mimic of native thioester-linked conjugates, the thioether-linked adduct behaved as a competitive inhibitor of the ligation machinery in single-turnover assays monitoring diUb formation by UBE2N, UBE2V2, and the E3 ligase cIAP (Supplementary Fig. 14). Combined with solution and crystallographic data, these functional assays support the utility of thioether-linked E2-UbDha adducts as stable mimics in both structural and functional studies.
UbDha probe as a proteomics tool
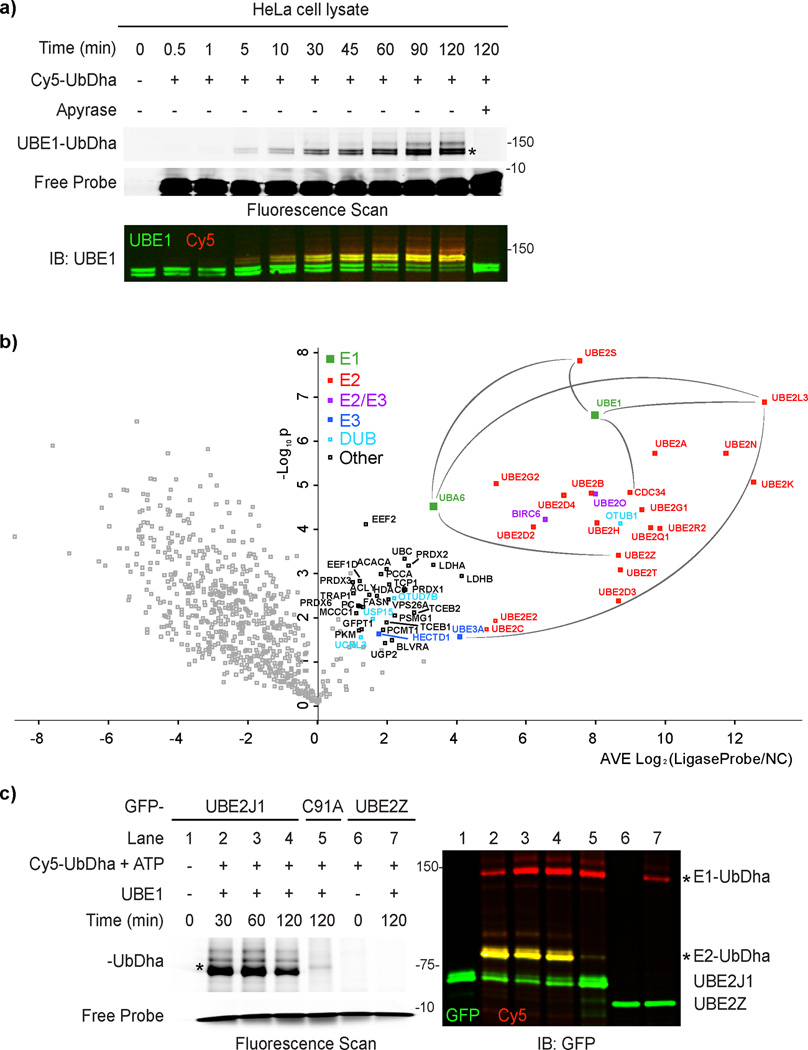
Having validated activity and structural integrity of the UbDha probe, we turned to enzymatic cascade profiling in biological samples. Incubation of cell extracts with Cy5-UbDha revealed robust labeling of both UBE1 (Fig. 4a) and UBA6 activating enzymes, reliably abrogated by apyrase treatment (Supplementary Fig. 15). Appearance of additional ATP-dependent bands was also detected on the same timescale, (Supplementary Fig. 15), supporting the in vitro data that the probe is passed downstream. To identify these proteins, we utilized the biotin-labeled probe variant for affinity-based proteomic profiling3 of human cervical cancer (HeLa, Fig. 4b) and melanoma (MelJuSo, Supplementary Fig. 16) cell extracts. We used the ATP-dependent reactivity of our probe to our advantage and performed affinity-based proteomic profiling in the presence of ATP, as compared to apyrase-mediated ATP-depletion. Mass spectrometric analysis of proteins associated with the probe in an ATP-dependent manner, retrieved both Ub E1 enzymes and numerous downstream E2 enzymes. Specifically, roughly half of known human E2 enzymes34 charged by UBE1 (as well as UBE2Z, charged specifically by UBA6) were identified with high confidence in both cell lines. Among the most enriched proteins were UBE2L3, UBE2S and UBE2K, all of which can readily accept ubiquitin from UBE1 and UBA625. Interestingly, three different members of the E2D subfamily were recovered: UBE2D2, UBE2D3 and UBE2D4, in fact the largely uncharacterized UBE2D4 was the top hit in HeLa cells (Fig. 4b). By contrast, UBE2D4 was not recovered in MelJuSo cells, exemplifying how the UbDha probe can facilitate unbiased proteome-wide comparisons of enzymatic reactivities. In addition to canonical E2s, we also detected atypical E2/E3 hybrid enzymes (UBE2O and BIRC6) as well as HECT E3s. While the E3 ligases UBE3A and HECTD1 were found in both cell lines, TRIP12 was observed only in MelJuSo cells. UBE3A prefers to accept Ub from UBE2L335, which was recovered in high abundance from both cell lines, indicating isolation of a full E1–E2–E3 cascade. Enzymes of interest, immuno-precipitated directly from cells, can be subsequently investigated using the UbDha probe in the presence of supplemented reaction components of choice. This is demonstrated by the ability of GFP-UBE2J1 but not GFP-UBE2Z to accept activated Cy5-UbDha from UBE1 (Fig. 4c). As expected, mutation of active site Cys 91 of GFP-UBE2J1 to Ala (C91A) abrogated labeling, demonstrating suitability of UbDha for mutational studies.
Figure 4. Proteome-wide activity profiling of Ub-conjugation machinery.
a) Time-course of UBE1 labeling in HeLa cell extracts with Cy5-UbDha in the absence (−) or presence (+) of ATP scavenger apyrase. b) Proteomic profiling of the Ub activation, conjugation and ligation machineries in HeLa cells. Volcano plot of pairwise comparison of proteins bound to the Biotin-UbDha probe relative to apyrase treatment (negative log10 p-value, y-axis) as a function of fold enrichment (average log2, x-axis). Confidently identified proteins (average log2 ratio >1, p < 0.05) are marked as follows: E1 (green), E2 (red), HECT E3 (blue), hybrid E2/E3 (purple) and DUBs (light blue); proteins unrelated to the Ub cycle are marked in black, and those falling below the threshold are shown in gray. Several known cascade connections are highlighted with gray lines. c) Labeling of GFP-tagged enzymes isolated from HeLa cells (UBE2J1 or it catalytic mutant C91A versus GFP-UBE2Z) with Cy5-UbDha downstream of purified UBE1. Asterisks (*) indicate modified forms of E1 and E2 enzymes. For uncut gels, see Supplementary Figure 20.
We also identified four DUBs (OTUB1, OTUD7B, UCHL3 and USP15) in pull-downs with the ligase probe (Fig. 4b and Supplemental Fig. 16). Because DUBs harbor highly reactive active site Cys residues, we assessed potential cross reactivity by incubation of the Cy5-UbDha probe with cell lysates ectopically expressing various GFP- or FLAG-tagged DUBs (Supplemental Fig. 17a) alongside the recently reported DUB-specific ABP, Cy5-UbPA30. While Cy5-UbPA readily modified all the active DUBs tested here, only incubation with excessive amounts of UbDha resulted in often marginal DUB labeling. Moreover, labeling of even highly reactive DUBs with Cy5-UbDha, such as OTUB1 and OTUB2 could be readily abolished by pretreatment with UbPA (Supplementary Fig. 17b). Of note, the DUBs recovered with UbDha in our proteomic experiment (particularly OTUB1) can interact with E2 enzymes36. Since DUB-mediated catalysis proceeds independently of ATP, recovery of these DUBs in the ATP-dependent setting can be a result of co-isolation with their active partner ligases.
Activity based protein profiling in cells
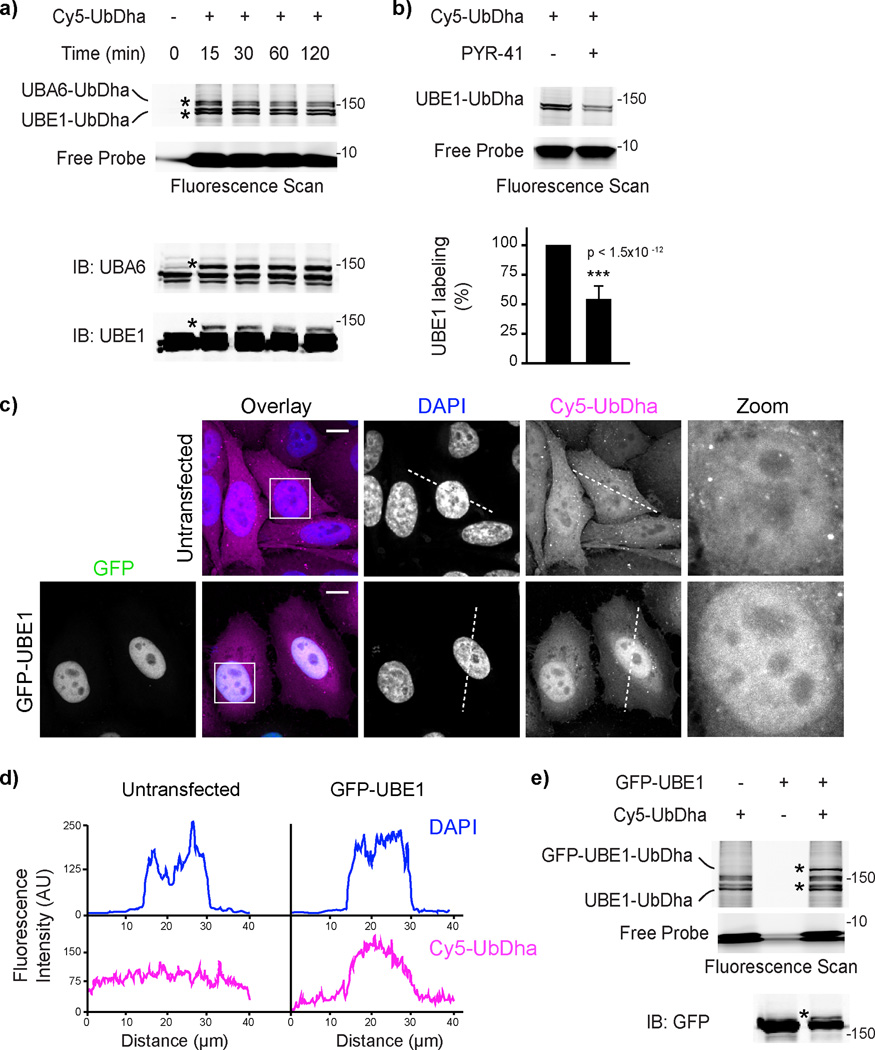
To address the efficacy of UbDha in monitoring the Ub-conjugating cascade in the cellular context, we next introduced Cy5-UbDha into HeLa cells by electroporation. In-gel fluorescence analysis followed by immunoblotting revealed speedy engagement of both human Ub activating enzymes (Fig. 5a) on a time-scale comparable to (if not faster than) that observed in lysate labeling experiments (Fig. 4a). Furthermore, treatment of cells with the UBE1 inhibitor PYR-4137 prior to introducing the probe noticeably reduced detectable UBE1 activity (Fig. 5b), indicating that the probe can be used to monitor enzymatic inhibition in living cells.
Figure 5. Activation of UbDha in vivo.
a) In vivo labeling of endogenous E1 enzymes with Cy5-UbDha. Fluorescence scanning and immunoblotting of lysates from HeLa cell electroporated with the probe and harvested at indicated time intervals following electroporation with the probe. b) In vivo labeling of UBE1 with Cy5-UbDha following UBE1 inhibitor PYR-41 (50 µM) treatment. Fluorescence scan and quantification (% labeling in the absence of PYR-41; n=3, error bars correspond to SD, with significance (p) assessed using a two-sided t-test) are shown. c) Distribution of Cy5-UbDha (magenta) in cells ectopically expressing GFP-UBE1 (green) relative to untransfected cells. Representative 3D confocal compilations of fixed cells treated as indicated are shown with DAPI (blue) overlays and nuclear insets; scale bars = 10 µm. d) Pixel traces of DAPI and Cy5-UbDha (marked with dotted lines in d) plotted as fluorescence over distance. e) Formation of the GFP-UBE1-UbDha adduct in cells. Asterisks (*) indicate modified forms of E1 enzymes. For uncut gels, see Supplementary Figure 21.
Cells harboring Cy5-UbDha were found to exhibit normal morphology, with the probe being evenly distributed throughout the nuclear and cytoplasmic space, as expected when small molecules such as ubiquitin move unrestricted across the nuclear membrane38 (Fig. 5c, top panels). Of note, in cells undergoing late stages of cell division, accumulation of Cy5-UbDha was consistently observed at the cytokinetic bridge (Supplementary Fig. 18), consistent with the site of BIRC6 activity at this time in the cell cycle39. Having detected BIRC6 in high abundance in our proteome-wide active ligase analysis (Fig. 4b), these observations suggest that our probe could be used to study spatial and/or temporal aspects of relevant enzymatic cascades. For instance, introduction of Cy5-UbDha into HeLa cells ectopically expressing predominantly nuclear GFP-UBE1 resulted in the corresponding nuclear accumulation of the probe (Figs. 5c, and 5d) relative to untransfected cells. In-gel fluorescent analysis and immunoblotting of corresponding lysates confirmed formation of a GFP-UBE1-UbDha adduct (Fig. 5e).
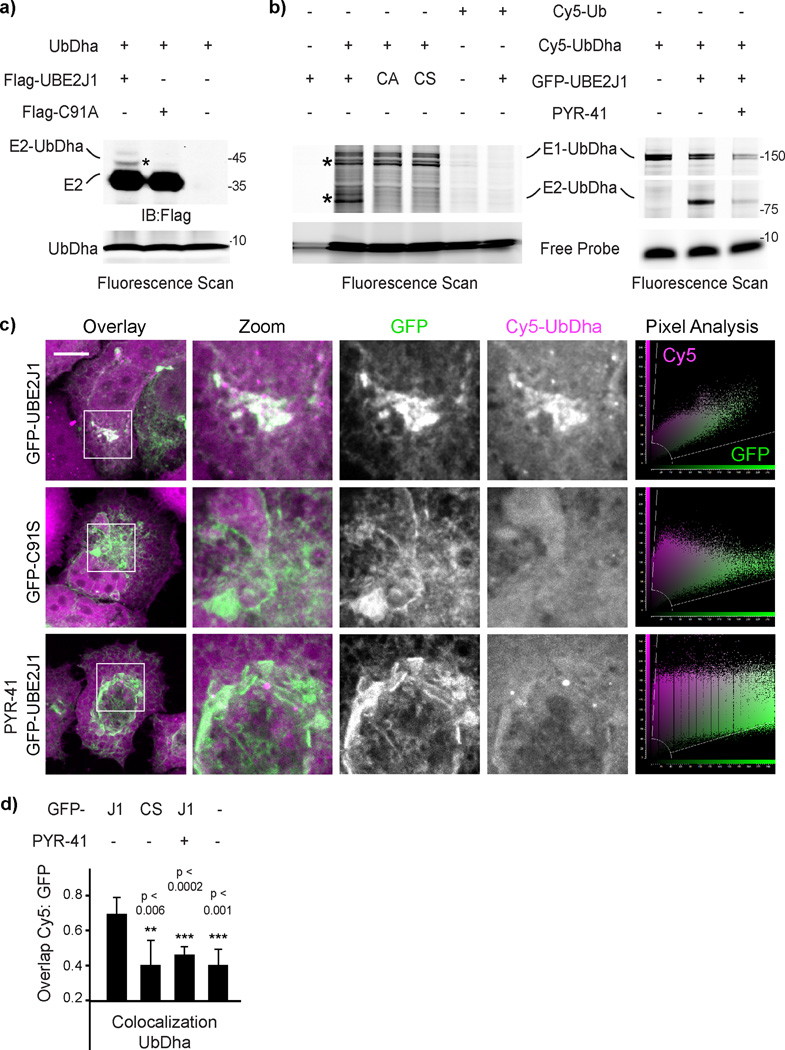
To investigate whether the probe, while inside the cell, can be passed downstream of the E1, we introduced Cy5-UbDha into cells expressing UBE2J1 or its catalytically inactive C91A or C91S mutants. Catalysis-dependent modification of the E2 with the probe was indeed observed (Figs. 6a and 6b, left panel) and found to be sensitive to inhibition of the upstream UBE1 (Fig. 6b, right panel). UBE2J1, which we isolated with UbDha from MelJuSo cell lysates (Supplementary Fig 16), localizes to the endoplasmic reticulum (ER), where it functions in ER-associated degradation (ERAD)40. In cells, Cy5-UbDha was found to readily colocalize with wild type, but not catalytically dead UBE2J1 (Figs. 6c and 6d). Importantly, such colocalization was sensitive to inhibition of the upstream E1 (Figs. 6c and 6d), indicating that imaging of intact cells harboring fluorescently labeled UbDha can report on relevant enzymatic cascades. Collectively, these experiments illustrate a wide range of utilities for UbDha in the study of ubiquitin ligase activities in cells. To the best of our knowledge, UbDha is the first probe that allows cascade-dependent profiling of ubiquitin conjugating enzymes in a physiologically relevant setting.
Figure 6. Probing in vivo E1–E2 cascade with UbDha.
a) In vivo UbDha adduct formation with Flag-UBE2J1 versus its catalytic mutant C91A by immunoblot against Flag. b) Reactivity of GFP-UBE2J1 versus its mutants C91A or C91S (left panel) or as a function of UBE1 inhibition (50 µM PYR-41, right panel) with Cy5-UbDha or -Ub electroporated into HeLa cells. Asterisks (*) indicate modified forms of E1 and E2 enzymes. c) Representative 3D confocal compilations of fixed cells of fixed cells (from c) treated as indicated. Overlays of GFP (green) with Cy5-UbDha (magenta) along with corresponding pixel plots are shown; scale bars = 10 µm. d) Colocalization (Mander’s overlap coefficient) of Cy5-UbDha with wild type (J1) or mutant (CS) GFP-UBE2J1 (n = 2, error bars correspond to SD, with significance (p) assessed using a two-sided t-test). For uncut gels, see Supplementary Figure 22.
Discussion
Given the critical roles of E1, E2 and E3 enzymes in a wide range of biological processes and their resulting emergence as drug targets41,42, there is a critical need for suitable assay reagents to study their function. The pyramidal structure of the Ub/Ubl conjugation systems, their complex cross-reactivities, and the reactive nature of the E2~Ub and E3~Ub thioester intermediates present practical challenges in dissecting interactions between Ub-loaded partner enzymes. To monitor these enzymatic cascades, we present a unique probe designed to hop from one active site to the next, leaving a detectable covalent mark at every step of the way.
Relatively inert on its own, our cascading probe (UbDha) requires ATP-dependent activation by the E1 enzyme, which increases the electrophilic character of the Dha moiety, making it suitable to follow the cascade of trans-thioesterification reactions downstream. The key conceptual advantage of Dha-based methodology lies in its unprecedented ability to choose at any point along the cascade between a native-like thioester and irreversible thioether bond formation. Indeed, we show that UbDha readily labels active site Cys residues of E1, E2 and HECT E3 enzymes. Importantly, UbDha does not get transferred to substrates. This feature endows our cascade probe with advantageous capabilities over the native Ub, particularly in complex biological settings, where enzymes are present together with their substrates. Under such circumstances, UbDha enables a direct measure of enzyme activity, rather than merely detecting consequences thereof.
Because entry of Ub(/Ubl)Dha into its cognate enzymatic cascade requires ATP, much of the background binding to the probe can be easily discriminated by eliminating ATP with apyrase. This simple feature makes UbDha suitable for activity-based profiling of Ub/Ubl cascades not only in vitro, but also in complex biological circumstances, as demonstrated by the proteome-wide analysis of Ub conjugation machineries isolated from two different cancer cell lines. The straightforward nature of the experimental setup is expected to be readily adaptable to comparative profiling of E1, E2 (and to some degree E3 enzymes) as a function of various biological perturbations (i.e. stimulation or starvation, infection, etc.). The same reagent can subsequently be used to study the effects of mutations in enzymes isolated directly from organisms of interest, as well as test for relevant factors upstream or downstream in the cascade. Standard biochemical techniques presently relied upon for such studies typically involve laborious expression and purification protocols. Furthermore, no observable reactivity in such preparations may be attributable to misfolding or lack of necessary modifications acquired in the carrier organism. Our methodology bypasses these difficulties by offering a relatively quick and easy way to assess reactivity of enzymes isolated directly from cells using simple immuno-precipitation. Then, taking cellular enzymology one step further, UbDha can be introduced into living cells to directly monitor enzymatic activities occurring in their natural context. In this way, the versatility of the UbDha cascade probe may prove invaluable in dissecting how aberrant activities of E1–E2–E3 cascades contribute to pathogenesis45,46, as well as for diagnosis and monitoring efficacy of UPS targeting therapy. Furthermore, by generating a NEDD8-based counterpart of the UbDha probe capable of labeling the NEDD8 conjugating machinery, we show our method to be diversifiable towards ubiquitin-like proteins. As such, the technology described here may be used to interrogate presently less well-defined ligation machineries of various Ubls.
In addition to cell-based applications, Ub/UblDha may prove useful in vitro, particularly for structure determination. The thioether adducts described here bypass the need for the often relied upon active site mutagenesis13–17 thus avoiding potential disturbances to catalytic properties of enzymes in question. Stability of our thioether adducts under reducing conditions, in the presence of an activating E3 ligase and in functional assays allowed us to perform NMR and X-ray crystallography studies. High degree of similarity to the published oxyester-linked structure supports their utility as stable mimics in both structural and functional studies. We foresee that UbDha may be used to expedite generation of crystal structures of E1, E2 or E3 enzymes and their complexes27. In addition, we hypothesize that the stability of our E2-UbDha adducts immobilized on affinity beads could enable proteomic profiling of cognate RING E3 enzymes, which cannot themselves be directly trapped in a mechanism-dependent manner43.
Based on the proof-of-concept studies described herein, we anticipate our cascading probe reagents to greatly facilitate future discoveries on Ub/Ubl conjugation.
Online Methods
Chemical synthesis
Detailed procedures for the synthesis of Dha-based ABPs can be found in Supplementary Note 1.
Protein expression and purification
UBE1
The UBE1 enzyme (N-terminal His-tagged) was expressed from a pET3a vector in autoinduction44 media at 37°C using E. coli BL21 (DE3). After 2–3 hours the temperature was lowered to 18°C and the bacteria were allowed to grow an additional 12h. His-tag mediated purification was performed using TALON beads (Clontech) in Buffer A containing 50 mM TRIS (pH 8.0), 150 mM NaCl and 0.25 mM TCEP, washing with 5 mM imidazole (pH 8.0) and eluting with 500 mM imidazole (pH 8.0). Further purification was performed by anion exchange using a Resource Q column (GE Healthcare) using a gradient to 1M NaCl in Buffer A, which was followed by a Superdex 200 column (GE Healthcare).
UBE1 C632S mutant
The active site cysteine in UBE1 (C632) was mutated to a serine using the protocol provided with the Quik Change Site-Directed Mutagenesis Kit (Invitrogen). The mutant was expressed and purified as described for UBE1.
6His-UBA6
A bacmid containing the verified human 6His-UBA6 cDNA sequence was purified from E. coli strain DH10Bac using the Quiagen plasmid mini kit (Quiagen) and transfected into SF21 insect cells using the TransIT 2020 transfection reagent (Mirus) leading to the production of baculovirus during three amplification steps according to standard protocols. SF21 cells were infected with the 6His-UBA6 encoding baculovirus and the 6His-UBA6 protein was purified by nickel affinity chromatography using HisTrap matrix (GE Healthcare). The function of the 6His-UBA6 protein was verified by in vitro activation assays of recombinant human ubiquitin and the recombinant human ubiquitin-like modifier FAT10.
UBE2N, UBE2V2, and UBE2D3
Full length constructs of UBE2N, UBE2V2, and UBE2D3, and NEDD4 residues 521–900, and cIAP residues 363–614 were expressed from pGEX6P-1 constructs in Rosetta 2 (DE3) pLacI cells (Novagen). TRAF6 residues 50–211 were expressed similarly from the pOPIN-K vector. 15N-labeled UBE2N was expressed in M9 minimal medium supplemented with 15N-NH4Cl. Transformed cells were cultured at 37°C to an O.D. of 0.6–0.8 and induced with 0.2mM IPTG at 18°C for 16 hours. Cells were lysed by sonication in a buffer containing 25mM Tris (pH 8.5), 200mM NaCl, and 4mM DTT. The clarified lysate was bound to glutathione resin, washed with additional lysis buffer, and the resin was incubated with GST-tagged 3C protease overnight at 4°C for cleavage of the GST fusion tag, with the exception of NEDD4 and cIAP which were eluted to retain the GST tag. Following cleavage, the released protein was washed through the resin, concentrated, and further purified by size exclusion chromatography on a Superdex 75 column (GE Healthcare) equilibrated in either 20mM Tris (pH 7.4), 100mM NaCl, 1mM DTT for reactions, or 25mM sodium phosphate (pH 7.0), 150mM NaCl for NMR spectroscopy. Mouse His-UBE1 was expressed from the pet-24 vector as above and purified by conventional Ni-NTA methods followed by anion exchange on a Resource Q column and size exclusion chromatography on a Superdex 200 column (GE Healthcare). Pure fractions of all proteins were concentrated and flash frozen for storage at −80°C.
NAE1/UBA3, HECT E3s and WBP2
Complex of GST-tagged NAE1 and UBA3 were co-expressed in E. coli RIL strain at 16°C for 16 hours. GST-tagged HECT E3s were expressed in E. coli (RIL) at 18°C overnight. GST-proteins were eluted by 10 mM glutathione followed by proteolysis (thrombin for GST-NAE1, TEV protease for all GST-HECT) at 4°C overnight and further purified by ionic exchange and size exclusion chromatography on a Superdex 200 (GE Healthcare) column.
His-MBP-tagged WBP2 was expressed in E. coli (RIL) at 20°C overnight and eluted from Ni-NTA column. TEV protease was applied to removed His-MBP tag. Full-length WBP2 was further purified by ionic exchange using fast flow Q column and size exclusion chromatography on a Superdex 200 column.
Pure proteins were concentrated, aliquoted, flash frozen by liquid nitrogen and stored at −80 °C.
E1–E2–E3 labeling assay conditions
Detailed assay conditions can be found in Supplementary Note 2.
Structure Determination
Preparation of thioether-linked 15N-UBE2N-UbDha and UBE2D3-UbDha
25 µM UBE1, 250 µM E2 (15N-UBE2N or UBE2D3), 1 mM UbDha, 5 mM ATP, and 10 mM MgCl2 were incubated at room temperature for 16 hours. Any remaining thioester linkages were reduced by the addition of 10 mM DTT at the end of the reaction. The 15N-UBE2N-UbDha adduct was purified to ~95% homogeneity by size exclusion chromatography on a Superdex 75 column (GE Healthcare) equilibrated in 25 mM sodium phosphate (pH 7.0), 150 mM NaCl. The UBE2D3-UbDha adduct was subsequently purified to >98% homogeneity by repeated cation exchange chromatography on a 6 mL Resource S column (GE Healthcare) with a 50–500 mM NaCl gradient in 50 mM Tris (pH 8.5) buffer, followed by a final size exclusion chromatography step on a Superdex 75 column (GE Healthcare) equilibrated in 25 mM HEPES, 50mM NaCl pH 7.
NMR spectroscopy 15N-UBE2N-UbDha
1H,15N BEST-TROSY experiments45 on 200 µM 15N-UBE2N or 15N-UBE2N-UbDha were acquired with optimized pulse sequences on a Bruker Avance2+ 700MHz spectrometer equipped with a cryogenic triple resonance TCl probe. Data sets were processed using Topspin (Bruker) and visualized with NMRViewJ (One Moon Scientific). Chemical shift perturbations were calculated using the equation Δδj = [(Δδj15N/5)2 + (Δδj1H)2]1/2. Perturbation values greater than 0.05 ppm were mapped onto the UBE2N crystal structure (PDB 1J7D), such that the analysis is directly comparable to previous work on the oxyester-linked UBE2N-O~Ub conjugate32.
Crystallization, data collection and structure determination UBE2D3-UbDha
The thioether-linked UBE2D3-UbDha adduct was crystallized following conditions published for the oxyester-linked form33. Purified conjugate was concentrated to 0.9 mM (23.5 mg/mL) and screened against conditions surrounding the published 200 mM tripotassium citrate, 20% PEG 3350 condition in 200nL sitting drops at a 1:1 protein:reservoir ratio. The resulting crystals were cryoprotected in LV CryoOil (MiTeGen) and vitrified prior to data collection at Diamond Light Source beamline I04-1. Data was collected at 100K with a wavelength of 0.92Å. Diffraction images were processed using XDS46 and scaled using AIMLESS47. Isolated UBE2D3 and Ub molecules from the oxyester UBE2D3-O~Ub structure (PDB 3UGB) were used as search models for molecular replacement using PHASER48. Iterative model building and refinement were performed using COOT49 and PHENIX50, respectively. The thioether linkage could be built unambiguously into the electron density. The final model contained 96.9% in favored, 2.7% in allowed, and 0.4% in outlier Ramachandran space. Data collection and refinement statistics can be found in Table 1. Structural figures were generated using PyMOL (www.pymol.org).
In vitro ubiquitination assays
Stability
The stability of thioether-linked UBE2D3-UbDha adduct was assessed by incubating 5µM purified thioether conjugate at 37°C in combination with 2µM TRAF6 or 2µM GST-NEDD4. The stability of the UBE2N-UbDha adduct was assessed similarly, but using the accessory E2 variant UBE2V2 with or without TRAF6. Samples were collected at 0, 1, 3, and 24-hour timepoints for visualization on Coomassie-stained SDS-PAGE gels. Control reactions containing 0.5µM UBE1, 5µM E2, 20µM Ub, and the relevant activating factors demonstrate the Ub chain formation activity under normal in vitro conditions.
Competitive inhibition
Single-turnover ubiquitination assays were established for UBE2N with an initial activation stage containing 10µM UBE2N, 0.5µM UBE1, 20µM Ub, 2.5mM ATP, and 5mM MgCl2 to generate native thioester linked UBE2N~Ub after 30min at 37°C. This reaction was then quenched by addition of apyrase to prevent any further formation of UBE2N~Ub. Pre-activated UBE2N~Ub was then diluted twofold into a reaction containing 5µM UBE2V2 alone or in addition to 1µM GST-cIAP, and samples were collected at 0, 15, and 30-minute timepoints for visualization of diUb product formation on Coomassie-stained SDS-PAGE gels. To test for competitive inhibition using the thioether-linked UBE2N-UbDha adduct, increasing concentrations (1.25µM, 2.5µM, 5µM, 10µM, and 20µM) of purified conjugate were added into reactions containing UBE2N~Ub, UBE2V2, and GST-cIAP as specified above, and 30-minute timepoints were collected to compare levels of diUb product formation with the control experiment.
Mammalian cell lines
HEK293T and HeLa cell lines used in this study originated from ATCC and were cultured under standard conditions in DMEM (Gibco) supplemented with 10% FCS (Sigma-Aldrich) at 37°C with 5% CO2. The previously characterized human melanoma cell line MelJuSo51 was cultured in IMDM (Gibco) supplemented with FCS. All cell lines were routinely tested for mycoplasma contamination with consistently negative outcome.
Labeling of overexpressed DUBs in cell lysates
For overexpression of epitope-tagged DUBs in HEK293T cells, previously described wild type and catalytic point mutants of USP8, OTUB1 and OTUB2 were used30. Additionally, human USP15 and OTUD1 were subcloned from pDEST-cDNA constructs (Addgene) into 2×FLAG-C1 vector (Clontech) at HindIII/XbaI and XhoI/EcoRI respectively, and mutagenesis of catalytic residues C269S in USP15 and C320S in OTUD1 was performed using standard protocols (Stratagene)). DNA was delivered into HEK cells using polyethylenimine (PEI, Polysciences, Inc.) according to manufacturer’s instructions. 24h following transfection, cells were harvested by scraping in lysis buffer (150 mM NaCl, 50 mM Tris HCl pH 7.5, 0.5% Triton-X100, Protease Inhibitor tablet (Roche)) and lysate was clarified by centrifugation. Reactions were incubated in the presence of indicated components with either Cy5-Ub-PA (1.0 µg/reaction), stationary for 30–45 min at RT or Cy5-Ub-Dha (1.5 µg/reaction) with gentle agitation for 70 min at 37°C in the presence of 10 mM ATP and 10 mM MgCl2. 10 mM NMM was added where indicated. Pre-incubation with unlabeled Ub-PA (1.5 µg/reaction) was performed as for Cy5-Ub-PA, followed by Cy5-Ub-Dha chase as above. Reactions were stopped by the addition of sample loading buffer supplemented with 100 mM DTT, followed by boiling for 10 min. Samples were resolved using standard SDS-PAGE, and probe reactivity was assessed by Cy5 fluorescence scanning (λex= 625 nm; λem= 680 nm), followed by gel transfer onto Nitrocellulose membranes and immunoblotting using either rabbit anti-GFP serum52, mouse anti-Flag (1:1000 dilution; Sigma Aldrich, Sigma F3165) or mouse anti-β-actin (1:10000 dilution; Sigma A544), as indicated. Fluorescent secondary antibodies anti-mouse-800 (1: 10000 dilution; LiCOR, 926–3210) and anti-rabbit-800 (1: 10000 dilution; LiCOR, 926–3211) were used for visualization of labeled proteins on LICOR Odyssey system v3.0.
Cell lysate labeling
Cell lysates were prepared by resuspending cell pellets in 3 pellet volumes of HR buffer (50mM TRIS, pH 7.4, 5 mM MgCl2, 250 mM sucrose, 1 mM DTT) and lysed by sonication. After clarification by centrifugation (20,000 rpm, 4°C, 20 min), total protein concentration was determined by Nanodrop. For the labeling experiments, 100 µg of lysate was incubated with 0.5µg Cy5-Ub-Dha, 10 mM ATP, 10 mM MgCl2, in Labeling Buffer (50 mM HEPES, 100 mM NaCl, pH 7.5) at 37°C for 1h or as indicated. Additional 1 mM ATP and MgCl2 were added to the reaction every 20 minutes to replenish consumed ATP. In case of the negative control, lysates were treated with 2 Units of Apyrase (Sigma Aldrich) prior to addition of the Cy5-UbDha probe (ATP and MgCl2 were omitted). The reaction was terminated by the addition of 3× SDS-PAGE Loading Buffer (Invitrogen) containing beta-mercaptoethanol. Samples were resolved by SDS-PAGE and visualized by fluorescence scanning (λex = 625 nm; λem = 680 nm). For visualizing the reactivity of endogenous UBE1 and UBA6 western blotting was performed as previously described and membranes were probed with rabbit UBE1 (1:1000 dilution; Abcam, ab 181225) or rabbit UBA6 antibody (1:1000 dilution; Abcam, ab 177514).
Activity-based Ub cascade profiling in intact cells
Electroporation of UbDha into living cells
HeLa cells cultured under standard growth conditions were transfected with GFP, GFP-UBE153, GFP-UBE2J1, GFP-UBE2J1 or mutants C91A or C91S, Flag-UBE2J1 or mutant Flag-C91A using Effectene (Qiagen), according to manufacturer’s instructions. To facilitate the incorporation of the probe, the growth medium was refreshed 4–6 hours following transfection and again 1–2 hours prior to electroporation. For inhibition of UBE1, cells were treated with 50 µM PYR-41 for 30 min prior to introducing the probe. Following removal of the growth medium, cells were kept on ice for the duration of the protocol. Cells were washed twice with cold electroporation buffer (2mM HEPES, pH 7.4, 15 mM K2HPO4/KHPO4, 250 mM Mannitol, 1mM MgCl2). Next, 1.5 mL of a solution of the Cy5-UbDha, Cy5-Ub or Rho-UbDha probe in electroporation buffer (0.4 mg/mL) was added to each of the wells and electroporation was performed on ice using a Biorad GenePulser Xcell with CE and PE module Pulse Generator equipped with a Petri Pulser™ electroporation applicator (BTX) using the following settings: square wave, voltage=75V, pulse length=3ms, pulse interval=1.5s, number of pulses=5, cuvette width=2mm. The electroporation applicator was turned 90 degrees and electroporation was repeated once. The probe solution was replaced by cold electroporation buffer and cells were allowed to recover on ice for 2 min. Following treatment, cells were washed twice with ice-cold PBS and allowed to recover under standard growth conditions as indicated (15 –120 min).
For gel-based analysis, samples were lysed using reducing SDS-PAGE loading buffer followed by brief sonication, heated at 98°C for 10 min before being analyzed on SDS-PAGE gel, and subsequently visualized by fluorescence scanning scanning (λex = 625 nm; λem = 680 nm (Cy5-UbDha) or λex = 473 nm; λem = 530 nm (Rho-UbDha)). Western blotting was performed as previously described and membranes were probed with either rabbit anti-GFP serum52 or mouse anti-Flag (1:1000 dilution; Sigma Aldrich, Sigma F3165).
Confocal microscopy
For microscopy experiments, the samples were fixed in 4% Formaldehyde (Merck) in PBS and mounted onto glass slides (Thermo Scientific) using Prolong Gold mounting medium with DAPI (Invitrogen). Z stacks of 5 images per cell were collected on a Leica SP5 confocal microscope equipped with HyD detectors, using a 63× magnification lens in combination with 2.5–4× digital zoom and represented as maximum z projections. Image processing and fluorescence intensity analysis was performed using ImageJ64 software, and colocalization was expressed in the form of Mander’s overlap coefficients calculated using JACoP. Pixel plot analyses were generated using Leica LASAF software.
Statistics and reproducibility
Statistical data are presented as standard deviation (s.d.). Comparisons between the samples were made using a two-sided t-test (assessed against the control), with the p values reported above the corresponding data in the graphs. Data analyzed was derived from at least two biologically independent experiments (n) as indicated in figure legends.
Proteomic activity profiling of Ub-conjugating enzymes
Sample preparation
For identification of enzymes reactive towards the Biotin-UbDha probe, 15 cm dishes of subconfluent HeLa or MelJuSo cells were cultured under standard conditions. Cells were washed with PBS, trypsinized and centrifuged at 1500 rpm. The pellet was washed once with PBS, resuspended in 3 pellet volumes HR lysis buffer (50 mM Tris pH 7.4, 5 mM MgCl2, 250 mM sucrose, 1 mM DTT, and Protease Inhibitor Tablet (Roche)) and lysed by sonication. After sonication, the cell extracts were clarified by centrifugation (20.000 rpm, 4°C, 20 min) and the total protein concentration determined using the Nanodrop. Labeling was performed by adding 10 µg Biotin-UbDha, 10mM ATP, 10mM MgCl2 to 10 mg cell lysates (total protein concentration) in Labeling Buffer (50mM HEPES, 100mM NaCl, 1mM DTT, pH 8.0) in a volume of 400µL for 1h at 37°C while gently shaking. To ensure that the ATP supply is constant, 1mM ATP and 1mM MgCl2 were added to the reaction every 15 min. For the negative controls, ATP was depleted prior to the reaction using 2 Units of Apyrase (Sigma Aldrich) for 15 min at 37°C. In case of the negative controls, the labeling reaction was performed as previously described, but with the omission of ATP and MgCl2. After reaction with the probe, the lysates were incubated with pre-equilbrated High Capacity Neutravidin Agarose (Thermo Fisher) for 3h at 4°C in a total volume of 700 µL, while rotating. Subsequently, the supernatant was removed and the resin was washed with 1 mL each of the following wash buffers54: buffer 1 (2% SDS in dH2O), buffer 2 (50 mM HEPES, pH 7.5, 1 mM EDTA, 500 mM NaCl, 1% Triton-X 100, 0,1% deoxycholate), buffer 3 (10 mM Tris, pH 8.0, 1 mM EDTA, 0,5% NP-40, 250 mM LiCl) and buffer 4 (50 mM Tris, pH 7.4, 50 mM NaCl). After washing, the samples were eluted in 40 µL 3× SDS PAGE Loading Buffer (Invitrogen) with additional 2-mercaptoethanol and boiled at 95°C for 5 min before loading onto a 10% gel (NuPAGE,Invitrogen). The proteins were run 1cm into the gel, and subsequently stained overnight with Coomassie Brilliant Blue before being destained in H2O. After destaining, the band was cut into small pieces using a sterile scapel and processed further for LC-MS/MS analysis.
LC-MS/MS analysis
In-gel digested peptides were cleaned and desalted by 1 ml 0.1% formic acid twice on OASIS HLB cartridge (Waters), before being eluted twice with 100 µL 80% acetonitrile in 0.1% formic acid. Desalted peptides were vacuum centrifuged at room temperature until complete dryness then dissolved in 0.1% formic acid, prior to online nanoflow liquid chromatography-tandem mass spectrometry. The analysis of in-solution digested peptides was performed using an EASY-nLC system (Proxeon) connected to a Q-Exactive (Thermo) using Higher-Collisional Dissociation (HCD) fragmentation. Separation of peptides was performed using 13 cm long analytical columns (ID 75 µm, Polymicro Avantes) packed in-house with 1.8 µm C18 beads (Reprospher-DE, Pur, Dr. Maisch), using a 30 minute gradient from 2% to 95% acetonitrile in 0.1% formic acid and a flow rate of 200 nL per minute. The mass spectrometer was operated in data-dependent acquisition mode using a top 10 method. Full-scan MS spectra were acquired with a target value of 3E6 and a resolution of 70,000, with a scan range from 400 to 1,400 m/z. HCD tandem MS/MS spectra were acquired using a target value of 1E5, a resolution of 17,500, and a normalized collision energy of 25%. All charges lower than two and higher than six were rejected, and all unknown charges were rejected. The underfill ratio was set to 1.0%, and a dynamic exclusion of 60 seconds was used.
Data processing
MaxQuant version 1.5.1.2 was used to analyze all RAW data.55,56 The experiment was performed in biological triplicate, and measured as three technical replicates. MS/MS spectra were filtered and deisotoped and the 18 most abundant fragments for each 100 m/z were retained. MS/MS spectra were filtered for a mass tolerance of six ppm for precursor masses, and a mass tolerance of 20 ppm was used for fragment ions. Peptide and protein identification was performed through matching the identified MS/MS spectra versus a target-decoy version of the complete human Uniprot database (03 December 2015, 92040 proteins), in addition to a database of 245 commonly observed mass spectrometry contaminants. Up to two missed tryptic cleavages were allowed. Cysteine carbamidomethylation was set as a fixed peptide modification. Protein N-terminal acetylation and methionine oxidation were set as variable peptide modifications. Peptides were accepted with a minimum length of six amino acids, a maximum size of 6kDa, and a maximum charge of six. The processed data was filtered by posterior error probability (PEP) to achieve a protein false discovery rate (FDR) of below 1%, a peptide-spectrum match FDR of below 1%. Modified peptides were additionally filtered to have an Andromeda score of at least 40.
Label-Free Quantification (LFQ)
Label-Free Quantification was performed using MaxQuant LFQ. Quantification was performed over three biological replicates. The fast LFQ algorithm was used with default settings (min. ratio count of two, min. neighbors of three, average neighbors of six). Protein LFQ values were Log2 transformed for further processing. Proteins identified by site only, reverse sequence only, and potential contaminants were removed. Proteins identified by at least 4 MS/MS spectra, at least 2 razor+unique peptides and at least one unique peptide were retained. To ensure biological reproducibility, only proteins detected in at least two out of the three biological replicates in at least one group were retained. Subsequently, missing values were imputed using Perseus software version 1.5.1.6, with standard settings. LFQ values were averaged within all treatment conditions. Proteins were considered to have a specific interaction with the ubiquitin ligase probe when they were enriched at least two-fold compared to the negative control with a T-test p value of less than 0.05.
Supplementary Material
Acknowledgments
We thank members of the Ovaa lab for helpful discussion and reagents, Dr Jason Brown and Sian Armour (Ubiquigent) for providing the E2 scan kit and Dris El Atmioui for solid phase peptide synthesis. We acknowledge beam-line staff at Diamond I04-1 for expert help. Work was supported by a VICI grant from the Netherlands Organization for Scientific Research (N.W.O.) [724013002] to H.O., a Marie Curie ITN fellowship [290257] to K.W. and EMBO long term fellowships to I.B. and J.N.P. Work in the DK lab is funded by Medical Research Council [U105192732], the European Research Council [309756], and the Lister Institute for Preventive Medicine. Work in the BAS lab is funded by ALSAC, HHMI, and NIH grant R37GM069530. Work in the A.C.O.V. lab is funded by N.W.O [93511037] and the European Research Council [310913]. Work in the M.G. lab is funded by the German Research Foundation (DFG) CRC969 - project C01. J.B. received a stipend from the Graduate School Chemical Biology Ko-RSCB.
H.O., M.P.C.M and F.E. are entitled to royalties that may result from licensing patent application WO2016/032332 according to IP policies of the Netherlands Cancer Institute. D.K. is a consultant for FORMA Therapeutics.
Footnotes
Author contributions
M.P.C.M. and F.E. designed the study. M.P.C.M., K.W. and I.B. carried out all labeling experiments. I.B. and K.W. designed and executed in-cell labeling experiments with assistance from R.M, and I.B. collected and analyzed confocal microscopy data. Mass spectrometry and relevant data analysis were performed by J.C. and A.C.O.V. on samples prepared by K.W. and I.B. J.N.P. and D.K. performed structural and competition studies and analyzed NMR and X-ray data. K.P.W. and B.A.S. generated the panel of purified HECT and NEDD8 pathway enzymes and helped with data analysis. J.B. and M.G. provided UBA6. M.P.C.M., F.E. and H.O. managed the study. M.P.C.M. and I.B. wrote the manuscript with input from other authors.
Competing financial interests
H.O. and F.E. declare competing financial interests as co-founder and shareholder of UbiQ Bio BV. D.K. and H.O. are part of the DUB Alliance that includes Cancer Research Technology and FORMA Therapeutics.
Accession codes
The structure of the thioether-linked UBE2D3-UbDha adduct has been deposited to the Protein Data Bank (PDB) under PDB accession code 5IFR.
References
References
- 1.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 2.Steele-Mortimer O. Exploitation of the ubiquitin system by invading bacteria. Traffic. 2011;12:162–169. doi: 10.1111/j.1600-0854.2010.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadaghiani AM, Verhelst SH, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr Opin Chem Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 5.Borodovsky A, et al. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borodovsky A, et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9:1149–1159. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 7.Ekkebus R, Flierman D, Geurink PP, Ovaa H. Catching a DUB in the act: novel ubiquitin-based active site directed probes. Curr Opin Chem Biol. 2014;23:63–70. doi: 10.1016/j.cbpa.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer HB, Nicholson B, Kessler BM, Altun M. Detection of ubiquitin-proteasome enzymatic activities in cells: application of activity-based probes to inhibitor development. Biochim Biophys Acta. 2012;1823:2029–2037. doi: 10.1016/j.bbamcr.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, et al. Designed semisynthetic protein inhibitors of Ub/Ubl E1 activating enzymes. J Am Chem Soc. 2010;132:1748–1749. doi: 10.1021/ja9088549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen SK, Capili AD, Lu X, Tan DS, Lima CD. Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature. 2010;463:906–912. doi: 10.1038/nature08765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An H, Statsyuk AV. Development of activity-based probes for ubiquitin and ubiquitin-like protein signaling pathways. J Am Chem Soc. 2013;135:16948–16962. doi: 10.1021/ja4099643. [DOI] [PubMed] [Google Scholar]
- 12.An H, Statsyuk AV. Facile synthesis of covalent probes to capture enzymatic intermediates during E1 enzyme catalysis. Chem Commun. 2016;52:2477–2480. doi: 10.1039/c5cc08592f. [DOI] [PubMed] [Google Scholar]
- 13.Wiener R, Zhang X, Wang T, Wolberger C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature. 2012;483:618–622. doi: 10.1038/nature10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamadurai HB, et al. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol Cell. 2009;36:1095–1102. doi: 10.1016/j.molcel.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruneda JN, et al. Structure of an E3:E2~Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol Cell. 2012;47:933–942. doi: 10.1016/j.molcel.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott DC, et al. Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin-like protein NEDD8. Cell. 2014;157:1671–1684. doi: 10.1016/j.cell.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plechanovova A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489:115–120. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgins RR, Ellison KS, Ellison MJ. Expression of a ubiquitin derivative that conjugates to protein irreversibly produces phenotypes consistent with a ubiquitin deficiency. J Biol Chem. 1992;267:8807–8812. [PubMed] [Google Scholar]
- 20.Pickart CM, Kasperek EM, Beal R, Kim A. Substrate properties of site-specific mutant ubiquitin protein (G76A) reveal unexpected mechanistic features of ubiquitin-activating enzyme (E1) J Biol Chem. 1994;269:7115–7123. [PubMed] [Google Scholar]
- 21.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 22.El Oualid F, et al. Chemical synthesis of ubiquitin, ubiquitin-based probes, and diubiquitin. Angew Chem Int Ed Engl. 2010;49:10149–10153. doi: 10.1002/anie.201005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardes GJ, Chalker JM, Errey JC, Davis BG. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J Am Chem Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]
- 24.Chalker JM, et al. Methods for converting cysteine to dehydroalanine. Chem Sci. 2011;2:1666–1676. [Google Scholar]
- 25.Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- 26.Schafer A, Kuhn M, Schindelin H. Structure of the ubiquitin-activating enzyme loaded with two ubiquitin molecules. Acta Crystallogr D Biol Crystallogr. 2014;70:1311–1320. doi: 10.1107/S1399004714002910. [DOI] [PubMed] [Google Scholar]
- 27.Olsen SK, Lima CD. Structure of a ubiquitin E1–E2 complex: insights to E1–E2 thioester transfer. Mol Cell. 2013;49:884–896. doi: 10.1016/j.molcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen PL, et al. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J Cell Biol. 2005;170:745–755. doi: 10.1083/jcb.200502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kee Y, Lyon N, Huibregtse JM. The Rsp5 ubiquitin ligase is coupled to and antagonized by the Ubp2 deubiquitinating enzyme. EMBO J. 2005;24:2414–2424. doi: 10.1038/sj.emboj.7600710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekkebus R, et al. On terminal alkynes that can react with active-site cysteine nucleophiles in proteases. J Am Chem Soc. 2013;135:2867–2870. doi: 10.1021/ja309802n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walden H, et al. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol Cell. 2003;12:1427–1437. doi: 10.1016/s1097-2765(03)00452-0. [DOI] [PubMed] [Google Scholar]
- 32.Pruneda JN, Stoll KE, Bolton LJ, Brzovic PS, Klevit RE. Ubiquitin in motion: structural studies of the ubiquitin-conjugating enzyme approximately ubiquitin conjugate. Biochemistry. 2011;50:1624–1633. doi: 10.1021/bi101913m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page RC, Pruneda JN, Amick J, Klevit RE, Misra S. Structural insights into the conformation and oligomerization of E2~ubiquitin conjugates. Biochemistry. 2012;51:4175–4187. doi: 10.1021/bi300058m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Wijk SJ, Timmers HT. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, et al. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2–E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 36.Nakada S, et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 38.Dantuma NP, Groothuis TA, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–845. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Menon MB, et al. Endoplasmic reticulum-associated ubiquitin-conjugating enzyme Ube2j1 is a novel substrate of MK2 (MAPKAP kinase-2) involved in MK2-mediated TNFalpha production. Biochem J. 2013;456:163–172. doi: 10.1042/BJ20130755. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, et al. Targeting the ubiquitin pathway for cancer treatment. Biochim Biophys Acta. 2015;1855:50–60. doi: 10.1016/j.bbcan.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Silva SR, Paiva SL, Lukkarila JL, Gunning PT. Exploring a new frontier in cancer treatment: targeting the ubiquitin and ubiquitin-like activating enzymes. J Med Chem. 2013;56:2165–2177. doi: 10.1021/jm301420b. [DOI] [PubMed] [Google Scholar]
- 43.Sommer S, Ritterhoff T, Melchior F, Mootz HD. A stable chemical SUMO1-Ubc9 conjugate specifically binds as a thioester mimic to the RanBP2-E3 ligase complex. Chembiochem. 2015;16:1183–1189. doi: 10.1002/cbic.201500011. [DOI] [PubMed] [Google Scholar]
- 44.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Favier A, Brutscher B. Recovering lost magnetization: polarization enhancement in biomolecular NMR. J Biomol NMR. 2011;49:9–15. doi: 10.1007/s10858-010-9461-5. [DOI] [PubMed] [Google Scholar]
- 46.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams PD, et al. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson JP, Demmer-Dieckmann M, Meo T, Hadam MR, Riethmuller G. Surface antigens of human melanoma cells defined by monoclonal antibodies. I. Biochemical characterization of two antigens found on cell lines and fresh tumors of diverse tissue origin. Eur. J. Immunol. 1981;11:825–831. doi: 10.1002/eji.1830111015. [DOI] [PubMed] [Google Scholar]
- 52.van der Kant R, et al. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J Cell Sci. 2013;126:3462–3474. doi: 10.1242/jcs.129270. [DOI] [PubMed] [Google Scholar]
- 53.Sugaya K, Ishihara Y, Inoue S, Tsuji H. Characterization of ubiquitin-activating enzyme Uba1 in the nucleus by its mammalian temperature-sensitive mutant. PLoS One. 2014;9:e96666. doi: 10.1371/journal.pone.0096666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox J, et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 56.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.