Abstract
The hypothalamus is among the most phylogenetically conserved regions in the vertebrate brain, reflecting its critical role in maintaining physiological and behavioural homeostasis. By integrating signals arising from both the brain and periphery, it governs a litany of behaviourally important functions essential for survival. In particular, the lateral hypothalamic area (LHA) is central to the orchestration of sleep–wake states, feeding, energy balance and motivated behaviour. Underlying these diverse functions is a heterogeneous assembly of cell populations typically defined by neurochemical markers, such as the well‐described neuropeptides hypocretin/orexin and melanin‐concentrating hormone. However, anatomical and functional evidence suggests a rich diversity of other cell populations with complex neurochemical profiles that include neuropeptides, receptors and components of fast neurotransmission. Collectively, the LHA acts as a hub for the integration of diverse central and peripheral signals and, through complex local and long‐range output circuits, coordinates adaptive behavioural responses to the environment. Despite tremendous progress in our understanding of the LHA, defining the identity of functionally discrete LHA cell types, and their roles in driving complex behaviour, remain significant challenges in the field. In this review, we discuss advances in our understanding of the neurochemical and cellular heterogeneity of LHA neurons and the recent application of powerful new techniques, such as opto‐ and chemogenetics, in defining the role of LHA circuits in feeding, reward, arousal and stress. From pioneering work to recent developments, we review how the interrogation of LHA cells and circuits is contributing to a mechanistic understanding of how the LHA coordinates complex behaviour.
Keywords: cell types, behavior, lateral hypothalamus, neural circuits, neuropeptides
Abbreviations
- Arc
arcuate nucleus
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- BNSTGABA
(GABAergic neurons in the BNST)
- CART
cocaine‐ and amphetamine regulated transcript
- CeA
central nucleus of the amygdala
- ChR2
channelrhodopsin‐2
- CRH
corticotropin‐releasing hormone
- DA
dopamine
- DR
dorsal raphe
- Dyn
dynorphin
- Enk
enkephalin
- Fx
fornix
- Gal
galanin
- Hcrt/Ox
hypocretin/orexin
- HPA
hypothalamic‐pituitary‐adrenal
- Iapp
islet amyloid polypeptide
- IR
immunoeactive
- LC
locus coeruleus
- LCNA
noradrenergic neurons in the LC
- LepRb
long‐form of the leptin receptor
- LHA
lateral hypothalamic area
- LHAGABA
GABAergic neurons in the LHA
- LHAGlu
glutamatergic neurons in the LHA
- LHALepRb
LepRb‐expressing neurons in the LHA
- LHb
lateral habenula
- LS
lateral septal nuclei
- MC4R
melanocortin receptor 4
- MCH
melanin‐concentrating hormone
- MRF
midbrain reticular formation
- MSN
medium spiny neuron
- MT
mammillothalamic tract
- NA
noradrenaline
- NAc
nucleus accumbens
- NAcD1R/GABA
(GABAergic D1R‐expressing medium spiny neurons in the NAc)
- NTS
nucleus of the solitary tract
- Nts
neurotensin
- N/OFQ
nociceptin/orphanin FQ
- PAG
periaqueductal grey
- PB
parabrachial nucleus
- PeF
perifornical
- PFC
prefrontal cortex
- POAGABA
(GABAergic neurons in the hypothalamic preoptic area)
- PV
parvalbumin
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- RMg
nucleus raphe magnus
- TH
tyrosine hydroxylase
- TMN
tuberomammillary nucleus
- TMNHA
(histaminergic neurons in the TMN)
- TRH
thryotropin‐releasing hormone
- TRNGABA
(GABAergic neurons in the thalamic reticular nucleus)
- UNC3
urocortin‐3
- VGAT
vesicular GABA transporter
- VGLUT2
vesicular glutamate transporter‐2
- VTA
ventral tegmental area
- VTADA
dopaminergic neurons in the VTA
The hypothalamus maintains homeostatic balance in physiology and behaviour by integrating vast amounts of neural and humoral information and conveying appropriate instructions to downstream effector systems. Hypothalamic function relies on the coordinated output of a highly differentiated network of hypothalamic nuclei best understood at the level of cells, circuits and transmitter systems (Saper et al. 2005, 2010; Sternson 2013; Gautron et al. 2015). Through classical neuroanatomical and behavioural work, the lateral hypothalamic area (LHA) emerged as a linchpin in the regulation of key aspects of vertebrate behaviour including arousal, feeding, energy balance, stress, reward and motivated behaviour. Steady progress over decades has been punctuated by seminal discoveries concerning the molecular, cellular and systems‐level substrates of LHA function (Berthoud & Münzberg, 2011; Burdakov et al. 2013; Hurley & Johnson, 2014; Brown et al. 2015; Stuber & Wise, 2016).
Despite significant inroads, the basic mechanisms underlying the function of LHA circuits at the cellular and systems levels are poorly understood, a gap attributable to the daunting heterogeneity, cytoarchitectural complexity and behavioural state‐dependent function of these circuits. Now, all the firepower of modern neuroscience is rapidly advancing our understanding of LHA circuits. New molecular and genetic tools, together with evolving multidisciplinary approaches such as optogenetics, chemogenetics, in vivo electrophysiology and deep brain calcium imaging, are transforming our understanding of LHA circuits and their role in health and disease. Our goal in this review is to overview the cellular and circuit‐level architecture of the LHA, with a focus on recent advances in the field that are beginning to disentangle the multifaceted identity and function of its constituent neuronal cell types and circuits.
Deconstructing the cellular diversity of LHA neurons
Despite its reticular cytoarchitecture, the LHA is a functionally discrete structure within the hypothalamus comprised of an intricate assembly of cell types and circuits, with unique patterns of inputs and outputs that interconnect with numerous physiological systems (Saper et al. 1979; Nieuwenhuys et al. 1982; Luiten et al. 1987; Geeraedts et al. 1990; Bernardis & Bellinger 1993; Bernardis & Bellinger 1996; Swanson et al. 2005; Hahn & Swanson 2010). A central question in attempting to disentangle cell types and circuits in the LHA, and in fact any brain region, is defining what constitutes a discrete cell type. Neuronal cell types may be defined by some confluence of cell type‐specific molecular markers, developmental history, capacity for intercellular signalling, intrinsic electrophysiological properties, unique morphology and patterns of local and long‐range synaptic inputs and outputs (Nelson et al. 2006; Bota & Swanson 2007). LHA neurons are broadly identified by their expression of specific markers, such as the neuropeptides hypocretin/orexin (Hcrt/Ox) (de Lecea et al. 1998; Sakurai et al. 1998) or melanin‐concentrating hormone (MCH) (Bittencourt et al. 1992). However, accumulating evidence suggests that single markers fail to adequately account for LHA cell type diversity in terms of gene expression, connectivity, circuit‐level function and role in behaviour. Another key question is precisely how circuit interactions within the LHA integrate central and peripheral signals and mobilize downstream targets in a concerted, specific and adaptive manner during different behavioural states. What follows is a summary of major LHA cell populations, with an emphasis on classifying neurons according to neurochemical phenotype, and recent advances in our understanding of local circuit interactions among defined cell types.
Two major neuropeptidergic neuron populations in the LHA
Hypocretin/orexin neurons
Neuropeptidergic projection neurons expressing Hcrt/Ox appear to be a distinct cell population, uniquely found within the LHA and particularly concentrated in the perifornical (PeF) region of the LHA (de Lecea et al. 1998; Sakurai et al. 1998). Hcrt/Ox neurons are the best characterized LHA population at the molecular, cellular and systems levels (Sakurai, 2007, 2014; Bonnavion & de Lecea, 2010; Sakurai & Mieda, 2011; Alexandre et al. 2013; Jones & Hassani, 2013; Shan et al. 2015). While defined by their expression of Hcrt/Ox, these neurons overlap significantly with other neuropeptidergic LHA populations and are likely to be capable of co‐release of multiple neuropeptides and transmitters. A majority of Hcrt/Ox neurons co‐express the neuropeptide dynorphin (Dyn) (Chou et al. 2001; Muschamp et al. 2014). Hcrt/Ox neurons are also thought to co‐express nociceptin/orphanin FQ (N/OFQ) (Maolood & Meister, 2010), although other evidence suggests that N/ORQ fibers innervate Hcrt/Ox neurons (Xie et al. 2008) and that these populations are intermingled but non‐overlapping within the LHA (Geraschenko et al. 2011). A small subpopulation of Hcrt/Ox neurons appears to overlap with galanin (Gal) (Hakansson et al. 1999), although this finding was not recapitulated in a Gal‐EGFP transgenic mouse (Laque et al. 2013). Recent evidence also suggests that Hcrt/Ox neurons co‐express neurotensin (Nts) (Furutani et al. 2013); however, these results are inconsistent with data describing no overlap between these populations (Watts & Sanchez‐Watts, 2007; Leinninger et al. 2011). Interestingly, Hcrt/Ox neurons were recently reported to co‐express the pancreatic peptide islet amyloid polypeptide (Iapp), a precursor to amylin (Li et al. 2015) (Fig. 1). Further work will be required to resolve the full extent of neuropeptide co‐expression within Hcrt/Ox neurons.
Figure 1. Neuronal cell type diversity in the LHA .
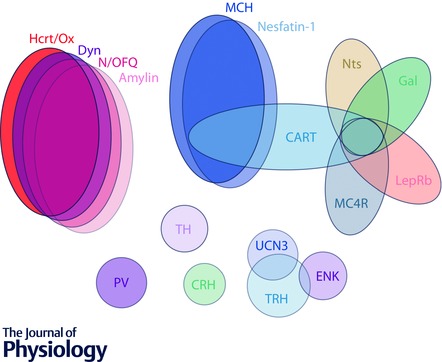
Schematic representation of numerous overlapping neuronal cell types in the LHA that can be classified on the basis of specific neurochemical markers. Three major subpopulations can be identified based on the expression of neuropeptides: Hcrt/Ox, MCH and an apparently separate, though mixed population that represents a confluence of multiple neuropeptide and receptor markers. The Hcrt/Ox population significantly overlaps with Dyn, N/OFQ and amylin, the MCH population largely overlaps with nesfatin and CART and the third population includes overlapping subpopulatons of LepRb, Nts, MC4R, CART and Gal‐expressing neurons. Also included are poorly characterized cell types found in the LHA; UNC3, TRH, Enk, TH, CRH and PV. Furthermore, these largely neuropeptidergic subpopulations are thought to also express the machinery for the release of fast amino acid transmitters GABA or glutamate. The varying degree to which these three main subpopulations, and others, can be characterized as functionally GABAergic or glutamatergic is still the subject of debate and not represented in this diagram. Note that the representation is meant to be illustrative and not quantitative with regard to cell numbers or degree of co‐expression. The following abbreviations are used: CART, cocaine‐ and amphetamine regulated transcript; CRH, corticotropin‐releasing hormone; Dyn, dynorphin; Enk, enkephalin; Gal, galanin; Hcrt/Ox, hypocretin/orexin; LepRb, long‐form of the leptin receptor; MC4R, melanocortin receptor 4; MCH, melanin‐concentrating hormone; Nts, neurotensin, N/OFQ, nociceptin–orphanin FQ; PV, parvalbumin; TH, tyrosine hydroxylase; TRH, thyrotropin‐releasing hormone; UCN3, urocortin‐3.
Importantly, Hcrt/Ox neurons also appear capable of fast, amino acid neurotransmitter release. The synaptic terminals of Hcrt/Ox neurons co‐localize with markers of glutamatergic neurotransmission, including the vesicular glutamate transporter VGLUT2, suggesting they are capable of rapid, synaptic glutamate release (Abrahamson et al. 2001; Rosin et al. 2003; Torrealba et al. 2003; Henny et al. 2010). Additionally, Hcrt/Ox varicosities in the locus coeruleus (LC) were found to end in excitatory asymmetric synapses (Horvath et al. 1999; Henny et al. 2010). Functional evidence for co‐release of glutamate and Hcrt/Ox was recently demonstrated using optogenetics, whereby low‐frequency photostimulation of channelrhodopsin‐2 (ChR2)‐expressing Hcrt/Ox fibres induced glutamate release onto histaminergic (HA) neurons in the tuberomammillary nucleus (TMN) (Schöne et al. 2012) and high‐frequency stimulation could additionally induce Hcrt/Ox release (Schöne et al. 2014). Co‐transmission among these neurons has been proposed to play a critical role in the stability and time course of postsynaptic excitability (Schöne & Burdakov, 2012; Schöne et al. 2014; Kosse & Burdakov, 2014).
While Hcrt/Ox neurons are generally considered to have a glutamatergic phenotype, some evidence suggests that a subpopulation expresses the machinery for GABA synthesis, based on gene expression (Harthoorn et al. 2005) and the morphology of Hcrt/Ox varicosities (Guan et al. 2002; Balcita‐Pedicino & Sesack, 2007), raising the possibility of some variation in neurotransmitter phenotype. Surprising recent work demonstrated that optogenetic activation of Hcrt/Ox neurons profoundly suppressed firing in nearby MCH neurons through light‐evoked inhibitory postsynaptic currents that were unaffected by glutamate receptor blockade, attenuated by Hcrt/Ox receptor blockade and abolished by gabazine (Apergis‐Schoute et al. 2015). Such inhibition could be explained by local Hcrt/Ox release, leading to the activation of local GABAergic neurons, or the release of GABA by Hcrt/Ox neurons themselves, although this result would need to be evaluated under conditions of exclusively monosynaptic release. Interestingly, immunohistochemical detection of GABA was found in close to 20% of Hcrt/Ox neurons, although these cells were devoid of the vesicular GABA transporter, VGAT (Apergis‐Schoute et al. 2015). Evidence for the regulation of excitability through local feed‐forward inhibitory (Matsuki et al. 2009) and excitatory (Li et al. 2002) inputs has been put forward; however, further work is required to parse the role of local circuits from differential transmitter release with regard to synaptic interactions between Hcrt/Ox and MCH neurons.
Beyond neuropeptides and transmitters, the cellular identity of Hcrt/Ox neurons can further be defined by other key markers such as transcription factors. Lhx9, a LIM‐homeodomain‐containing transcription factor, has emerged as a potential specifier for Hcrt/Ox neurons, as revealed though molecular profiling and developmental analysis in multiple species (Shimogori et al. 2010; Dalal et al. 2013; Liu et al. 2015; Sokolowski et al. 2015). Recent RNA‐sequencing of zebrafish Hcrt neurons confirmed the presence of Lhx9 and identified other key markers enriched in these neurons (Yelin‐Bekerman et al. 2015). Additionally, many orexigenic LHA subpopulations including Hcrt/Ox neurons express Dbx1, another developmentally regulated homeodomain‐containing transcription factor (Sokolowski et al. 2015).
Accumulating evidence suggests that even the small population of Hcrt/Ox neurons may be diverse, evidenced by divergent anatomical and electrophysiological characteristics. For example, Hcrt/Ox neurons exhibit two distinct electrophysiological phenotypes, so‐called D‐type and H‐type (Schöne et al. 2011). Also, Hcrt/Ox cells exhibit differential responses to neurotransmitters such as cholinergic agonists (Sakurai et al. 2005; Ohno et al. 2008). Some investigators have proposed a dichotomy in Hcrt/Ox function based on their topology and projection patterns between medial and lateral zones (Harris & Aston‐Jones, 2006), although others report a rather diffuse pattern of Hcrt/Ox projections (González et al. 2012). In addition, gene expression analysis in both mice and zebrafish suggests cell type heterogeneity (Appelbaum et al. 2007; Dalal et al. 2013; Yelin‐Bekerman et al. 2015). Given the diversity of cellular properties, neurochemical content and the myriad roles that they play in behaviour, Hcrt/Ox neurons are unlikely to be monolithic. Further work is required to put cell type heterogeneity in register with functional diversity.
Melanin‐concentrating hormone
A separate population of LHA projection neurons expresses MCH (Bittencourt et al. 1992), which is well‐described anatomically and physiologically (Broberger et al. 1998; Elias et al. 1998; Peyron et al. 1998; Bayer et al. 2002; Guan et al. 2002; van den Pol et al. 2004). MCH and Hcrt/Ox neurons are intermingled throughout the LHA and surrounding regions, but each population exhibits a distinct pattern of localization (Hahn, 2010; Hahn & Swanson, 2010). Similar to Hcrt/Ox neurons, MCH neurons co‐express a variety of neuropeptides, such as nesfatin‐1 (Oh‐I et al. 2006; Foo et al. 2008; Fort et al. 2008). Additionally, approximately 50% of MCH neurons co‐express cocaine‐ and amphetamine‐regulated transcript (CART) (Broberger, 1999; Vrang et al. 1999; Elias et al. 2001; Hathroon et al. 2005; Hanriot et al. 2007) (Fig. 1). The transcription factor Dbx1 can also be found in MCH‐expressing neurons (Sokolowski et al. 2015), while Lhx9 is absent (Dalal et al. 2013).
MCH neurons are generally considered to be GABAergic owing to their co‐localization with two isoforms of glutamic acid decarboxylase, GAD65 and GAD67, the synthetic enzymes for GABA (Elias et al. 2001, 2008; Harthoorn et al. 2005; Sapin et al. 2010). In rat, the vast majority of MCH immunoreactive (IR) neurons co‐express GAD67 (Elias et al. 2008; Sapin et al. 2010). Approximately 67% of hypothalamic CART‐expressing neurons express GAD67 mRNA and LHA CART neurons exhibit extensive overlap with MCH neurons (Elias et al. 2001). In addition, VGAT was found to be co‐localized in approximately 6% of MCH‐IR varicosities found in the LC (del Cid‐Pellitero & Jones, 2012). Finally, optogenetic stimulation of MCH cells has been shown to result in GABA release onto HA neurons in the TMN (Jego et al. 2013), lending support to the anatomical evidence that MCH neurons are functionally GABAergic.
However, some MCH neurons have been shown to express the vesicular glutamate transporters VGLUT1 and VGLUT2 (Abrahamson et al. 2001; Harthoorn et al. 2005; del Cid‐Pellitero, 2012). In support of this anatomical evidence, recent work shows that MCH neurons projecting to the lateral septum co‐express VGLUT2 and surprisingly exhibit photostimulation‐evoked monosynaptic release of glutamate, not GABA (Chee et al. 2015). Additionally, LHA GABAergic neurons, as revealed using Vgat‐Cre (Vong et al. 2011) or Gad65‐EGFP transgenic mice, do not overlap with MCH‐IR neurons (Chee et al. 2015; Jennings et al. 2015; Karnani et al. 2013). These data raise intriguing questions about neurochemical phenotype diversity and perhaps target‐specific signalling of MCH neurons. MCH and other neuropeptide‐expressing LHA cell populations thus appear more diverse and dynamic than previously appreciated.
Identification of a third, distinct LHA neuron population with multiple phenotypes
Leptin‐sensitive neuropeptidergic neurons in the LHA
A recent body of work has elucidated a novel population of LHA neurons identified through both hormone receptor and neuropeptide expression. The anorexigenic, adipose tissue‐derived hormone leptin, produced by the ob gene, is a major peripheral signal modulating hypothalamic functions including feeding, energy expenditure and endocrine function. Leptin crosses the blood–brain barrier, binds to the long isoform of the leptin receptor (LepRb) and engages the Janus kinase–signal transducer and activator of transcription (JAK–STAT) signal transduction pathway (Leinninger & Myers, 2008). LepRb‐expressing neurons are widely expressed but are enriched in the hypothalamus and brainstem (Elmquist et al. 1998). The generation of strains of Lepr‐Cre driver lines (DeFalco et al. 2001; Leshan et al. 2006) and identification of labelled LepRb‐expressing neurons in the brain (Scott et al. 2009; Louis et al. 2010; Patterson et al. 2011; Leinninger et al. 2011) opened the door to more detailed anatomical analysis and cell type‐specific genetic manipulations of these neurons.
Interestingly, LHA LepRb neurons may comprise a unique cell population that modulates LHA network function and behaviour (Myers et al. 2009; Leinninger et al. 2009, 2011; Louis et al. 2010; Leinninger, 2011; Goforth et al. 2014; Bonnavion et al. 2015). LepRb‐expressing neurons are diverse and, within the LHA, overlap with multiple populations of known neuropeptidergic neurons. Approximately 60% of the LHA LepRb population co‐expresses Nts; conversely, about 30% of LHA Nts neurons co‐express LepRb (Leinninger et al. 2011). Interestingly, 95% of this Nts–LepRb population co‐localizes with Gal, whereas the proportion of LHA LepRb neurons expressing Gal is only around 20–44% (Laque et al. 2013) (Fig. 1).
LepRb neurons in the LHA are also thought to be GABAergic. Studies using Gad67‐EGFP reporter mice revealed leptin‐induced activation of STAT3 signalling in EGFP‐positive LHA neurons (Leinninger et al. 2009). Furthermore, in leptin‐injected Vgat‐Cre mice, phosphorylated STAT3 (pSTAT3)‐IR cells could be detected in putative LHA GABAergic neurons, similar to the arcuate nucleus (Arc), and dorsomedial hypothalamus (DMH) (Vong et al. 2011). This work suggests that LepRb neurons in the LHA act via GABAergic mechanisms to exert inhibitory influence on their targets. Interestingly, given the strong co‐localization between LHA LepRb and Nts neurons (Leinninger et al. 2011), one might predict that specific Nts‐ergic inputs to the VTA are GABAergic. However, in the presence of GABAAR blockers, optogenetic stimulation induced the release of glutamate from ChR2‐expressing LHA Nts projections onto VTA targets (Kempadoo et al. 2013), underscoring the apparent neurochemical diversity of LHA Nts neurons.
Adding further complexity to our understanding of LHA LepRb neurons is the existence of LHA neurons expressing the melanocortin‐4 receptor (MC4R), a G‐protein‐coupled receptor for α‐melanocyte stimulating hormone (α‐MSH) (Elmquist et al. 1999; Schwartz et al. 2000; Cone, 2005). This MC4R‐positive population is distinct from Hcrt/Ox, MCH and nesfatin‐1‐positive neurons (Liu et al. 2003; Cui et al. 2012). Approximately 75% of MC4R‐positive neurons co‐localize with Nts; this Nts–MC4R‐expressing neuron population appears unique to the LHA. Cui and co‐authors showed that ∼80% of MC4R cells co‐localize with pSTAT3 following leptin injection, indicating that these cells are also leptin sensitive (Cui et al. 2012) and suggesting a strong overlap between neurons defined by LepRb and those defined by MC4R. Interestingly, while Nts–LepRb‐positive cells send strong projections to the dorsal raphe (DR) and ventral tegmental area (VTA) (Leinninger et al. 2011), MC4R–pSTAT3‐positive cells do not send projections to either region (Cui et al. 2012). This latter result suggests that LHA LepRb neurons co‐expressing MC4R are a distinct subpopulation, as only about one‐third of LHA LepRb neurons co‐expresses MC4R (Cui et al. 2012) (Fig. 1). MCR4 neurons are also thought to exhibit a GABAergic phenotype evidenced by their co‐localization with GAD67 (Liu et al. 2003).
Using trans‐synaptic tracing techniques in a Lepr‐Cre knock‐in mouse, Louis and co‐workers showed that local LHA LepRb neurons appear to make direct synaptic contact with a subpopulation of Hcrt/Ox neurons (30%) (Louis et al. 2010). Subsequently, we found that optogenetic photostimulation of LepRb neurons in slices yielded GABAAR‐mediated synaptic inputs in approximately 27.5% of identified Hcrt/Ox neurons in slices (Bonnavion et al. 2015). Although virally transduced LepRb neurons could also be found in nearby hypothalamic regions outside of the LHA, our data demonstrate that LepRb‐expressing GABAergic neurons make functional synaptic contact with a subpopulation of Hcrt/Ox neurons. This functional connection may contribute to the leptin‐mediated suppression in neuronal activity observed in Hcrt/Ox neurons following both leptin infusion and optogenetic activation of LepRb neurons in vivo (Bonnavion et al. 2015).
While signalling between LepRb‐expressing neurons and Hcrt/Ox neurons may, in part, be mediated by GABAergic mechanisms, recent evidence points to a contribution of GABA‐independent mechanisms. A population of LepRb–Nts neurons within the LHA, described earlier, appears to be in direct synaptic contact with a subpopulation of Hcrt/Ox neurons, not MCH neurons (Leinninger et al. 2011). Goforth and colleagues examined the functional connectivity between LHA Nts neurons and Hcrt/Ox neurons and found that leptin, which activates a large subpopulation of LHA Nts neurons (Leinninger et al. 2011), hyperpolarizes Hcrt/Ox neurons even in the presence of blockers of GABAergic transmission (Goforth et al. 2014). As mentioned, the LHA Nts population overlaps significantly with Gal‐expressing neurons in the LHA (Laque et al. 2013). Accordingly, bath application of Gal inhibits Hcrt/Ox neurons and Gal receptor blockade prevents leptin‐induced inhibition of Hcrt/Ox neurons (Goforth et al. 2014). Interestingly, chemogenetic activation of LHA Nts neurons similarly resulted in hyperpolarization of Hcrt/Ox neurons (Goforth et al. 2014) despite evidence that Nts itself has a strong, excitatory effect on Hcrt/Ox neurons (Tsujino et al. 2005; Furutani et al. 2013). The nature of selective neuropeptide co‐transmission by LHA LepRb/Nts/Gal populations and the relative contribution of neuropeptide and GABA co‐transmission on postsynaptic excitability remain open questions.
A larger population of LHA GABAergic neurons
Neuroanatomical evidence suggests that the LHA contains a large population of GABAergic neurons defined by the presence of components necessary for GABA synthesis and release, including GAD65, GAD67 and VGAT (Rosin et al. 2003; van den Pol et al. 2004; Shin et al. 2007; Hassani et al. 2010; Karnani et al. 2013; Jennings et al. 2015; Chee et al. 2015). How this heterogeneous population of GABAergic neurons tracks with known neuropeptide‐expressing LHA populations, corresponds to specific inputs and outputs of the LHA, and interacts locally with other cell types remains poorly understood. Recently, a population of GAD65‐expressing neurons was identified in the LHA that is distinct from both MCH and Hcrt/Ox populations. These cells exhibit a range of intrinsic firing patterns, sub‐divided into four subtypes based on their distinct electrical signatures (Karnani et al. 2013). Furthermore, a role for local inhibition of LHA projection neurons is suggested by glutamate drop experiments (Huang et al. 2007) and transgenic mice lacking GABABRs in Hcrt/Ox neurons (Matsuki et al. 2009). Recent electrophysiological, imaging and behavioural data, discussed in the second half of this review, have begun to illuminate the physiological function and significance of these LHA GABAergic neurons.
Other neurochemically diverse neuron populations in the LHA
The LHA is home to a variety of other neuron populations that do not appear to track with better known cell types. These include neuron populations defined by their expression of neuropeptides such as thyrotropin‐releasing hormone (TRH), enkephalin (Enk) and urocortin‐3 (UCN3) (Fig. 1). For example, LHA TRH‐expressing neurons do not appear to co‐localize with the neuropeptides that define other major cell populations; however, a significant population overlap with Enk‐IR (16%) and UCN3‐IR (42%) cells in the juxtaparaventricular region (Horjales‐Araujo et al. 2014). Other markers appear to specify other unique cell populations in the LHA such as tyrosine hydroxylase (TH) (van den Pol et al. 1984; Abrahamson & Moore, 2001; Horjales‐Araujo et al. 2014), an RF‐amide peptide (QRFP) (Takayasu et al. 2006; Chen et al. 2016) or the calcium‐binding protein parvalbumin (PV), which defines a small population of glutamatergic neurons in the LHA (Mészár et al. 2012).
Furthermore, recent evidence using a Cre driver line to identify neurons expressing VGLUT2 (Vong et al. 2011) has revealed a population of glutamatergic LHA neurons that are functionally important (Jennings et al. 2013; Nieh et al. 2015; Stamatakis et al. 2016), as discussed in the following section. Like their GABAergic counterparts, LHA glutamatergic neurons are likely to be highly diverse and future work will be necessary to parse the neurochemical phenotype of functionally distinct LHA glutamatergic cell populations and their role in behaviour.
Finally, an important caveat in defining LHA cell type heterogeneity is the distinct possibility that the neurochemical identity of LHA neurons may be dynamic and depend on developmental factors, behavioural state and metabolic conditions. This form of neurotransmitter switching or respecification, in response to changing environmental conditions, has been described in a variety of species, systems and preparations (Spitzer, 2015). Thus, the boundaries between distinct cell types in the LHA may be blurred by, for example, behavioural state‐dependent changes in activity and shifts in gene expression. The extent to which this occurs in the LHA is still an open question but future work may require an examination of coordinated patterns of gene expression in order to identify discrete cell populations.
Disentangling long‐range LHA connectivity and its role in complex behaviour
Pioneering early lesion and electrical stimulation experiments established the key role of the LHA in feeding, drinking, motivated behaviour and arousal. Lesions of the LHA induced the ‘lateral hypothalamic syndrome’ characterized by hypophagia, adipsia, hypoactivity and weight loss. LHA lesions were also associated with somnolence, sensory neglect, decrease in sucrose consumption and neuroendocrine and autonomic defects (Anand & Brobeck, 1951; Teitelbaum & Epstein, 1962; Hoebel, 1965; Boyle & Keesey, 1975; Luiten et al. 1987; Bernardis & Bellinger, 1996; Hurley & Johnson, 2014; Brown et al. 2015; Castro et al. 2015; Stuber & Wise, 2016). Conversely, electrical stimulation of the LHA resulted in voracious eating and weight gain (Delgado & Anand, 1953; Hoebel & Teitelbaum, 1962; Margules & Olds, 1962), promoted self‐stimulation and had potent rewarding effects (Olds & Milner, 1954; Gallistel et al. 1981; Bernardis & Bellinger, 1996; Stuber & Wise, 2016). Stimulation‐evoked hyperphagia in rats was also associated with increased motivation and improved learning in food reward tasks, suggesting that the LHA is a ‘pacemaker for food motivation’ (Bernardis & Bellinger, 1993).
Given its central role in a wide range of behaviours, it is unsurprising that the LHA extensively interconnects with multiple brain regions. The first descriptions of LHA efferent projections revealed a broad and diffuse arborization from the telencephalon to the medulla and spinal cord. Ascending fibres project to forebrain targets that have functional implications in cognitive and emotional processes. In contrast, descending fibres project to nuclei involved in wakefulness, feeding, autonomic and neuroendocrine functions, in addition to prominent intrahypothalamic projections (Saper et al. 1979; Luiten & Room, 1980; Berk & Finkelstein, 1982; Ter Horst & Luiten, 1987; Luiten et al. 1987; Bernardis & Bellinger, 1993, 1996; Hahn & Swanson, 2010; Berthoud & Münzberg, 2011; Brown et al. 2015) (Fig. 2). Many of the structures targeted by the LHA, in turn, exert feedback control onto the LHA. Major forebrain afferents to the LHA originate in the medial prefrontal cortex (mPFC), lateral septal nuclei (LS), nucleus accumbens (NAc), bed nucleus of the stria terminalis (BNST), basolateral amygdala (BLA) and lateral habenular nucleus (LHb). Midbrain and brainstem afferents are also substantial, notably from the mesencephalic and central grey, VTA, DR, LC and lateral parabrachial area (LPB) (Saper et al. 1979; Barone et al. 1981; Kita & Oomura, 1982; Luiten et al. 1987; Bernardis & Bellinger, 1993, 1996; Sakurai et al. 2005; Yoshida et al. 2006; Hahn & Swanson, 2010; Berthoud & Münzberg, 2011; Brown et al. 2015; Castro et al. 2015; González et al. 2016) (Fig. 2). The advent of powerful new genetic tools has transformed our ability to specifically probe LHA circuitry and its role in complex behaviours. What follows is a summary of the role of defined LHA cell populations in feeding, reward, arousal and stress. The roles of Hcrt/Ox and MCH neurons in behaviour have been detailed in many excellent reviews, cited below, and we will discuss some highlights of this work. Emphasis, however, is placed on recent advances in our understanding of other LHA cell types, including LHA GABAergic and glutamatergic neurons among others, as revealed through opto‐ and chemogenetic approaches.
Figure 2. Long‐range connectivity of the LHA as a substrate for complex behaviour .
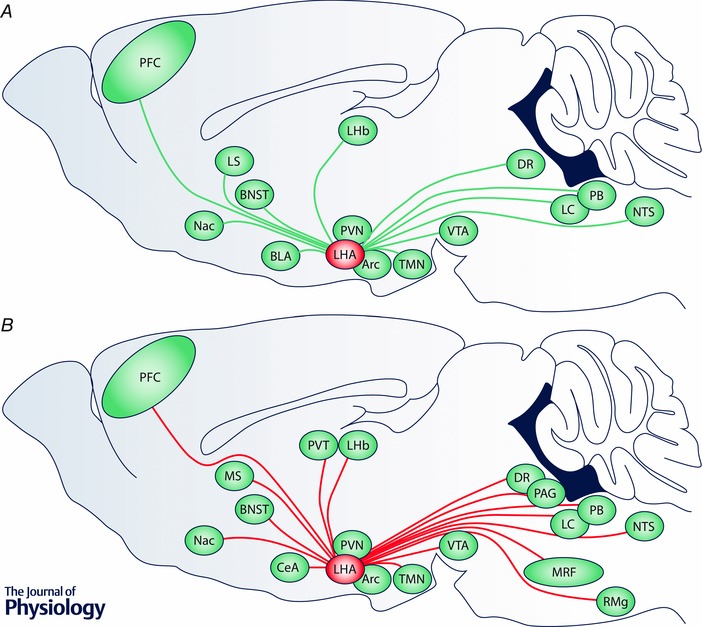
Long‐range anatomical inputs and outputs of the LHA reflect the engagement of multiple physiological systems to regulate fundamental behavioural programs. A, schematic of a parasagittal rodent brain illustrating major long‐range inputs (green lines) to the LHA. B, similar schematic illustrating major long‐range outputs (red lines) from the LHA. The following abbreviations are used: Arc, arcuate nucleus; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DR, dorsal raphe; Fx, fornix; LC, locus coeruleus; LHA, lateral hypothalamic area; LHb, lateral habenula; LS, lateral septal nuclei; MRF, midbrain reticular formation; MT, mammillothalamic tract; NAc, nucleus accumbens; NTS, nucleus of the solitary tract; PAG, periaqueductal grey; PB, parabrachial nucleus; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; RMg, nucleus raphe magnus; TMN, tuberomammillary nucleus; VTA, ventral tegmental area.
Coordination of feeding and reward
The MCH and Hcrt/Ox systems were first examined for their role in feeding and regulating energy balance using pharmacological and genetic deletion approaches (Pissios et al. 2006; Sakurai, 2007, 2014; Bonnavion & de Lecea, 2010; Sakurai & Mieda, 2011; Alexandre et al. 2013; Shan et al. 2015; Burdakov et al. 2013; Brown et al. 2015). In particular, accumulating evidence suggests that the MCH system critically regulates energy homeostasis and reward. Intracerebral delivery of MCH promotes feeding (Qu et al. 1996) while genetic overexpression of MCH results in a hyperphagic and obese phenotype (Ludwig et al. 2001). Genetic deletion of MCH (Shimada et al. 1998) or loss of MCH neurons (Alon & Friedman, 2006) both result in a hypophagic and lean phenotype. In addition, loss of MCH also leads to perturbed mesolimbic dopamine (DA) function (Pissios et al. 2008). One important pathway through which the MCH system may be acting is on the NAc shell, where local administration of MCH enhanced feeding and an MCHR‐1 antagonist reduced feeding (Georgescu et al. 2005) and reduced cocaine self‐administration (Chung et al. 2009). In animal models of obesity, specific MCH antagonists have consistently shown efficacy in acutely reducing food intake and inhibiting weight gain (Macneil, 2013). However, a recent study dissecting the neural substrates of LHA‐induced feeding and reward excluded the involvement of MCH neurons in the voracious feeding and self‐stimulation resulting from optogenetic activation of LHA GABAergic neurons (LHAGABA) (Jennings et al. 2015). Furthermore, the activity of MCH neurons has been implicated in the rewarding effects of sugar consumption, through its modulation of striatal DA release (Domingos et al. 2013).
The role of the Hcrt/Ox system in feeding is the subject of debate. Although the Hcrt/Ox system is sensitive to metabolic signals (Burt et al. 2011; Berthoud & Münzberg, 2011; Burdakov et al. 2013), effects of Hcrt/Ox dysfunction on energy homeostasis may rather be attributed to motivational deficits or decreased locomotor activity resulting from diminished arousal (Siegel, 2004; Bonnavion & de Lecea, 2010). Interestingly, one striking phenotype in Hcrt/Ox‐ablated mice is the absence of food‐anticipatory arousal resulting from food restriction (Yamanaka et al. 2003). These data suggest that the Hcrt/Ox system orchestrates adaptive motor and arousal‐related behaviour through the integration of metabolic changes and food cues. Further work is necessary to elucidate the circuitry and specific roles of Hcrt/Ox and MCH neurons in feeding by also focusing on associative learning, which may encode incentive responses to food reward. Evidence for the role of Hcrt/Ox neurons in reward includes the presence of excitatory connections to reward circuitry, notably dopamine (DA) and GABAergic neurons of the VTA (Korotkova et al. 2003; Beier et al. 2015). Further behavioural studies demonstrate the influence of Hcrt/Ox neurons on the rewarding effects of food (Borgland et al. 2009; Sharf et al. 2010), drugs of abuse (Borgland et al. 2006; Boutrel & de Lecea, 2008) and sexual behaviour (Muschamp et al. 2007). Recently, investigation of the LHAHcrt/Ox→VTADA pathway indicates that both Hcrt/Ox and Dyn are involved in modulation of VTADA activity and reward. These peptides are co‐expressed, and may be co‐released, by the same LHA neurons, but appear to have opposing actions on the excitability of VTADA neurons in vitro, and on reward in vivo (Muschamp et al. 2014). Finally, recent in vivo single‐unit recordings from Hcrt/Ox neurons revealed enhanced firing and cortical activation upon presentation of cues associated with delivery of appetitive (sucrose) vs. aversive (quinine) solutions, characteristic of neurons that encode both reward and arousal (Hassani et al. 2016).
Recent studies highlighted the important role of LHA glutamatergic and GABAergic neurons in the control of feeding and reward. First, Jennings and co‐workers dissected an inhibitory projection from the BNST to the LHA in the context of food intake using optogenetic techniques. Photostimulation of terminals in the LHA originating from BNST GABAergic neurons (BNSTGABA→LHA) induced both rapid and voracious feeding and self‐stimulation (Jennings et al. 2013). Interestingly, these responses varied with the nutritional status of the animals, similar to early LHA electrical stimulation experiments (Bernardis & Bellinger, 1996). Importantly, BNSTGABA neurons appeared to preferentially contact glutamatergic, VGLUT2‐expressing LHA neurons (LHAGlu). They went on to show that direct photostimulation of LHAGlu neurons suppressed feeding behaviour in food‐deprived mice and that their photoinhibition promoted feeding in well‐fed mice (Jennings et al. 2013). These data suggest that inhibitory BNST projections directly suppress the activity of anorexigenic LHAGlu neurons, while disinhibiting the activity of orexigenic neurons that initiate feeding behaviour (Stuber & Wise, 2016). Subsequent work revealed that LHAGABA neurons may in fact be orexigenic. Optogenetic stimulation of non‐Hcrt/Ox, non‐MCH, VGAT‐expressing LHAGABA neurons enhances both food consumption and self‐stimulation. Conversely, their optogenetic inhibition or ablation results in decreased food intake. Through in vivo deep brain calcium imaging, the authors could divide these LHAGABA neurons into two subgroups based on differential activity during feeding behaviour: one activated during the action to obtain the food reward (appetitive responses), and the other during food consumption itself (consummatory responses) (Jennings et al. 2015) (Fig. 3 A).
Figure 3. Summary of optogenetic interrogation of LHA circuits in feeding, arousal and stress behaviour .
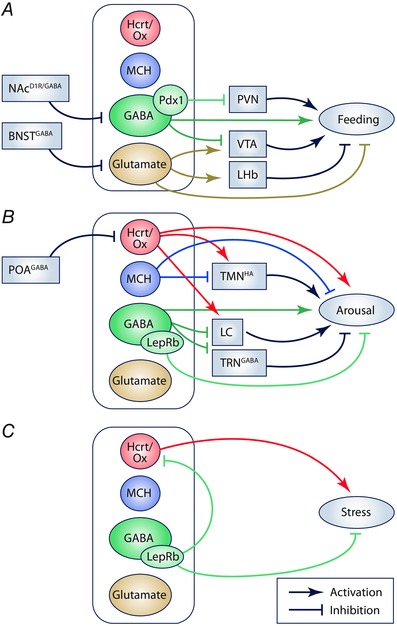
Schematic illustration summarizing recent in vivo optogenetic manipulation of specific LHA cell types and circuits in behaviour using Cre driver lines or promoter‐driven viral expression. In each case, the outputs of Hcrt/Ox, MCH, GABAergic and glutamatergic populations, and putative subpopulations, are illustrated along with some relevant inputs. A, optogenetic investigation of feeding behaviour, overlapping with reward‐related behaviour. Note a putative subpopulation of LHAGABA neurons defined by Pdx1. B, optogenetic interrogation of sleep and wake behaviour. Note a putative subpopulation of LHAGABA neurons defined by LepRb. C, optogenetic manipulation of stress‐related behaviour, including LepRb neurons. The following abbreviations are used: BNSTGABA (GABAergic neurons in the bed nucleus of the stria terminalis); LC, locus coeruleus; LHb, lateral habenula; NAcD1R/GABA (GABAergic D1R‐expressing medium spiny neurons in the nucleus accumbens); POAGABA (GABAergic neurons in the hypothalamic preoptic area); PVN, paraventricular nucleus of the hypothalamus; TMNHA (histaminergic neurons in the tuberomamillary nucleus); TRNGABA (GABAergic neurons in the thalamic reticular nucleus); VTA, ventral tegmental area.
Consistent with the orexigenic effects of LHAGABA neurons, recent work has highlighted the role of specific NAc→LHA projections in feeding. GABAergic dopamine D1 receptor (D1R)‐expressing medium spiny neurons (MSNs) in the NAc shell were found to preferentially innervate LHAGABA neurons and, when these projections were optogenetically activated, feeding was rapidly suppressed, even in hungry animals (O'Connor et al. 2015) (Fig. 3 A). Inhibitory inputs from the NAc to the LHA have also been investigated with regard to addictive behaviour. Larson and co‐authors found that stimulation of this pathway in vivo resulted in increased motivation for cocaine self‐administration and a long‐lasting enhancement in cocaine‐seeking behaviour after withdrawal (Larson et al. 2015). NAc MSNs were reported to also make direct contact with an unknown population of glutamatergic neurons in the anterior LHA, distinct from MCH and Hcrt/Ox neurons (Sano & Yokoi, 2007). The differential roles of inhibitory NAc→LHA circuits involved in enhanced drug‐seeking (Larson et al. 2015) and those involved in the cessation of feeding (O'Connor et al. 2015) may depend on the nature of the NAc projections, neurochemistry of their LHA targets and behavioural context.
The nature of glutamatergic and GABAergic projections from LHA to the VTA were further examined in sucrose reward‐seeking behaviour (Nieh et al. 2015) and in the control of feeding (Barbano et al. 2016). The first study confirmed that LHA→VTA projecting neurons target both DA and non‐DA GABAergic neurons, with both excitatory and inhibitory inputs. Within the LHA, in vivo single‐unit recording in mice trained to respond to a cue predicting sucrose reward revealed that at least two patterns of activity could be distinguished during a sucrose‐seeking task: Type 1 cells showed steady, phasic responses to the sucrose dispenser once the conditional training was acquired, and Type 2 cells exhibited phasic responses to both dispenser and stimulus, which were systematically affected by unexpected reward or unexpected absence of reward (Nieh et al. 2015). These data suggest that two different subsets of LHA→VTA projecting neurons differentially encode conditioned responses and sucrose reward expectation. However, the identity of these cells has not yet been identified. Interestingly, activation of Type 1 LHA→VTA neurons is associated with increased feeding and counterbalances stressful foot‐shock effects to obtain further sucrose rewards (Nieh et al. 2015). We can thus question whether LHA stimulation influences and modifies the value of reward (Stuber & Wise, 2016). It has been shown that LHA neurons display different types of firing responses to taste stimuli that differ in palatability, suggesting that encoding of hedonic and incentive responses to food and taste aversion also occur in the LHA (Li et al. 2013). More recently, Barbano and co‐workers showed that eating and rewarding effects of LHAGABA→VTA neurons were differentially evoked depending on the frequency of stimulation used to synchronize their activity. Low frequency (5‐10 Hz) stimulation of LHAGABA→VTA terminals preferentially induced feeding, while higher frequencies (40 Hz) preferentially induced rewarding effects (Barbano et al. 2016). The authors hypothesized that these effects may depend on differential neuropeptide co‐release (Barbano et al. 2016; Stuber & Wise, 2016). Furthermore, Stamatakis and co‐workers recently found that optogenetic inhibition of an excitatory projection of LHAGlu neurons to the LHb enhanced consumption of a palatable reward whereas its activation mediated avoidance behaviour (Stamatakis et al. 2016) (Fig. 3 A). This LHAGlu→LHb circuit may be a pathway through which the LHA negatively regulates both feeding and reward.
The properties of LHAGABA and LHAGlu neurons may be compared with the distinctive features of LHALepRb neurons and their functional role in feeding, energy balance and reward (Leinninger et al. 2011; Leinninger, 2011; Laque et al. 2015). LHALepRb/Nts/Gal neurons directly modulate Hcrt/Ox and mesolimbic DA functions (Leinninger et al. 2009; Louis et al. 2010; Leinninger et al. 2011; Kempadoo et al. 2013; Goforth et al. 2014; Bonnavion et al. 2015; Laque et al. 2015) and convey leptin's inhibitory effects on food intake (Leinninger et al. 2009; Leinninger et al. 2011). Increased concentration of Nts in the VTA, through chemogenetic activation of LHANts cells, facilitates prolonged DA release in the NAc and increased locomotor activity (Patterson et al. 2015). LHANts neurons contact both VTA DA and GABA neurons, but display twice as many synapses onto the former than the latter (Beier et al. 2015). However, recent work suggests that LHAGal neurons, do not innervate the VTA (Laque et al, 2015). Optical self‐stimulation, induced by activation of the LHA→VTA pathway, is partly mediated by Nts and glutamatergic neurotransmission (Kempadoo et al. 2013). Although LHANts/Glu→VTA projecting neurons are likely to express LepRb, most LHALepRb/Nts→VTA neurons express GABAergic markers (Leinninger et al. 2011). In light of the recent work describing the orexigenic effects of LHAGABA neurons (Jennings et al. 2015, Wu et al. 2015; Nieh et al. 2015; Barbano et al. 2016), we can question whether those various effects on feeding and reward behaviour can be explained by multiple LHAGABA/LHALepRb/GABA subpopulations, or by the same neurons that exhibit differential neurotransmitter/neuropeptide co‐release driving one behaviour over another. The VTA may be a relay for LHA‐induced feeding and self‐stimulation, but many other downstream targets remain unexplored including key intrahypothalamic feeding centres interconnected with the LHA (Sternson, 2013; Krashes et al. 2014; Sternson et al. 2016). Indeed, stimulation of a GABAergic projection from the LHA to the paraventricular nucleus of the hypothalamus (PVN), LHAPdx1/GABA→PVN, was shown to promote feeding behaviour (Wu et al. 2015) (Fig. 3 A). How these inhibitory neurons track with identified LHA subpopulations, respond to circulating signals of metabolic status, and effect postsynaptic signalling in identified targets will no doubt be the focus of intense future investigation.
Control of sleep and wakefulness
Our understanding of how LHA neuron populations and their efferents regulate sleep and wakefulness primarily resulted from work on the MCH and Hcrt/Ox systems. MCH and Hcrt/Ox neurons exhibit in vivo firing patterns that correlate tightly with vigilance state in rodents, i.e. waking (W), rapid‐eye movement (REM) sleep and non‐REM sleep (NREM) stages. In vivo recordings reveal that MCH neurons are silent during waking, fire occasionally during NREM sleep and fire maximally (from 3 to 12 Hz) during REM sleep, suggesting that they have a critical role in promoting and maintaining REM sleep (Hassani et al. 2009). Indeed, optogenetic activation of MCH neurons selectively modulates REM sleep state (Jego et al. 2013; Konadhode et al. 2013; Tsunematsu et al. 2014) (Fig. 3 B). Activation of MCH neurons during NREM sleep promotes the transition to REM sleep, during which their high‐frequency stimulation extends REM sleep duration (Jego et al. 2013). Chronic activation of MCH neurons over 24 h leads to an increase in both NREM and REM sleep durations (Konadhode et al. 2013) while selective ablation of MCH neurons results in decreased NREM sleep without affecting REM (Tsunematsu et al. 2014). These results indicate that MCH neurons can influence all stages of sleep; however, their impact on sleep as it relates to behavioural and cognitive function requires further investigation. MCH control of REM sleep state is thought to result from multiple downstream pathways involving brainstem GABAergic neurons (Clément et al. 2012) and two distinct forebrain pathways, including a projection from GABAergic MCH neurons to HA‐containing neurons in the TMN (LHAMCH/GABA→TMNHA) and to neurons in the medial septum (MS) (LHAMCH/GABA →MS) (Jego et al. 2013). Each of these connections is involved in inducing and stabilizing REM sleep. Activation of the LHAMCH → brainstemGABA pathway facilitates REM sleep by shutting off REM suppressor neurons (Clément et al. 2012). Photostimulation of the LHAMCH/GABA →TMNHA pathway extends REM sleep duration by maintaining inhibitory tone on HA neurons, which in turn delays the transition to wakefulness (Fig. 3 B). Activation of the LHAMCH/GABA →MS pathway also extends REM sleep duration and is hypothesized to stabilize the septo‐hippocampal regulation of REM sleep‐associated theta rhythm (Jego et al. 2013).
Elucidating the functions of the Hcrt/Ox system has significantly informed our understanding of the basic biology of sleep and the aetiology of sleep disorders (Scammell, 2003; Burgess & Scammell, 2012). Hcrt/Ox deficiency or depletion results in narcolepsy in humans and animals (Chemelli et al. 1999; Peyron et al. 2000; Thannickal et al. 2000; Hara et al. 2001). In animal models, narcoleptic attacks – characterized by brief periods of wakefulness, frequent transitions to REM sleep, and behaviour resembling cataplexy – can result from Hcrt/Ox cell ablation or genetic mutations that impair normal synthesis of Hcrt/Ox or its receptors (Scammell, 2003). In contrast to MCH neurons, in vivo recordings from rodent Hcrt/Ox neurons revealed that they are primarily active during wakefulness (from 3 to 13 Hz), less active during quiet waking and silent during NREM and REM sleep, but start firing prior to the onset of wakefulness (Lee et al. 2005; Mileykovskiy et al. 2005; Takahashi et al. 2008). Furthermore, the firing rate of Hcrt/Ox neurons can vary depending on behavioural state, showing phasic activity reaching frequencies up to 15 Hz during sleep‐to‐wake transitions or high‐vigilance states associated with increased muscle tone and/or EEG desynchronization (Lee et al. 2005; Mileykovskiy et al. 2005; Takahashi et al. 2008). In addition, recent fibre photometry experiments revealed a rapid increase in the activity of Hcrt/Ox neurons, but not MCH neurons, in response to an air‐puff (González et al. 2016). These findings indicate that the in vivo firing patterns of Hcrt/Ox neurons are dynamic across sleep–wake states, similar to other systems that promote wakefulness and arousal, but with the particularity of being activated prior to the return of cortical activity and muscle tone during REM sleep‐to‐wake transitions.
Optogenetic methods were also used to interrogate Hcrt/Ox neurons and their causal role in sleep‐to‐wake transitions (Adamantidis et al. 2007; Carter et al. 2009, 2012; Rolls et al. 2011; Bonnavion et al. 2015) (Fig. 3 B). These studies demonstrate that directly driving the activity of Hcrt/Ox neurons during sleep disrupts sleep and promotes transitions to wakefulness (Adamantidis et al. 2007), which may substantially contribute to the mechanisms underlying forms of insomnia. Furthermore, prolonged stimulation of the Hcrt/Ox system impairs memory consolidation during sleep (Rolls et al. 2011), and alters hypothalamic–pituitary axis (HPA) endocrine function (Bonnavion et al. 2015). From the known targets of Hcrt/Ox, the LC is a critical downstream effector of Hcrt/Ox‐mediated sleep‐to‐wake transitions (Carter et al. 2012). In addition, optogenetic activation of sleep‐active GABAergic preoptic (POA) neurons were found to suppress firing in Hcrt/Ox neurons (Saito et al. 2013). These findings suggest that Hcrt/Ox neurons act as a gatekeeper of behavioural state stability.
In vivo electrophysiological recordings of single LHA neurons across natural sleep–wake cycles also identified a population of non‐Hcrt/Ox, non‐MCH LHAGABA neurons that preferentially fire during sleep states (Hassani et al. 2010). Recent work showed that optogenetic stimulation of LHAGABA neurons, identified in Vgat‐Cre mice, that project to thalamic reticular nucleus (TRN) GABA neurons, evokes rapid arousal when the stimulation occurs specifically during NREM sleep (Herrera et al. 2016) (Fig. 3 B). While 1 Hz stimulation evokes rapid transitioning from NREM sleep to wakefulness, only 20 Hz stimulation also suppressed the in vivo firing rate of TRNGABA neurons, the transient inhibition of which allows fast cortical arousal during NREM sleep. LHAGABA → TRN neurons were shown to exhibit spontaneous activity during sleep and increase their firing when the animals switch from sleep to wakefulness (Herrera et al. 2016). The authors note that these LHAGABA neurons do not overlap with either Hcrt/Ox neurons, which induce a much slower transition to wake (Adamantidis et al. 2007), or MCH neurons. Wake‐promoting LHAGABA neurons were also shown to evoke arousal through other targets including the LC and the paraventricular nucleus of the thalamus (PVT). Stimulation of LHAGABA terminals in both targets favours wakefulness (Herrera et al. 2016), suggesting that LHAGABA projections may act through disinhibition of arousal systems. Interestingly, LHALepRb/Gal neurons were found to densely project directly to noradrenergic LC neurons (LCNA) neurons (Laque et al. 2015), but the functional implications of this pathway for sleep–wake regulation and cortical arousal remain to be investigated. Finally, Herrera and co‐workers found that the selective optogenetic activation of LHALepRb neurons, a putative subpopulation of LHAGABA neurons, delays the transition from sleep to wake, suggesting that these neurons may recruit a circuit that favours sleep promotion and/or maintenance (Herrera et al. 2016). This work suggests functional diversity among LHAGABA populations, which remains to be fully elucidated.
Regulation of stress and anxiety
An increasing body of literature suggests that the Hcrt/Ox system has a key role in stress, anxiety and other high‐arousal states (Winsky‐Sommerer et al. 2004; España et al. 2003; Furlong et al. 2009; Berridge et al. 2010; Johnson et al. 2011; Giardino & de Lecea, 2014). Collectively, these studies suggest that Hcrt/Ox neurons are capable of integrating multiple stress‐related central and peripheral inputs, and are critical modulators of the neural circuitry of stress. Furthermore, Hcrt/Ox neurons exhibit increased firing rates in vivo in novel environments or when exposed to an unpredictable sound stimulus, which can be associated with stress (Mileykovskiy et al. 2005). In addition, fibre photometry experiments showed a rapid increase in Hcrt/Ox neuron activity during immobilization stress (González et al. 2016). However, the in vivo firing dynamics of single Hcrt/Ox neurons during states of stress and anxiety remain unknown, and a causal relationship between Hcrt/Ox activation and stress initiation had not been established until recently.
Interestingly, elevated Hcrt/Ox neurotransmission has been associated with anxiety/panic attacks in humans and the development of panic‐like responses (Johnson et al. 2010). Additional studies highlighted the involvement of Hcrt/Ox neurons in modulating fear and fear memory (Flores et al. 2015). Mice lacking the Hcrt‐1 receptor showed impaired freezing responses and reduced neuronal activation in the lateral amygdala in fear‐conditioning paradigms (Soya et al. 2013). Interestingly, the restoration of Hcrt‐1 receptor expression in LCNA neurons normalized cue‐induced fear behaviour and increased lateral amygdala activity. Further investigation using optogenetics showed that Hcrt/Ox activity in the LC enhanced threat memory formation in an amygdala‐dependent process (Sears et al. 2013). These studies suggest that activity of the Hcrt/Ox system can influence the dysregulation of stress responses and emotional memory formation, which may lead to the development of anxiety disorders.
Recently, we demonstrated a causal relationship between prolonged activation of the Hcrt/Ox system and neuroendocrine and behavioural stress responses. We showed that in vivo optogenetic stimulation of Hcrt/Ox neurons resulted in a state of hypercorticosteronaemia corresponding to increased HPA activity, which was associated with enhanced cardiovascular responses, sleep fragmentation and disrupted exploratory behaviour with signs of freezing (Bonnavion et al. 2015) (Fig. 3 C). These effects likely involve the PVN, the PVT and brainstem nuclei including the LC and DR. This study also demonstrated that the Hcrt/Ox‐evoked increase in corticosterone concentrations is influenced by the nutritional status of the mice, notably in a leptin‐dependent manner. Food deprivation enhanced Hcrt/Ox‐dependent HPA axis activation, while local infusion of leptin into the LHA blunted Hcrt/Ox neuronal activity, as did the optogenetic activation of LHALepRb neurons. Indeed, optogenetic stimulation of LHALepRb neurons reduced both corticosterone release and suppressed Hcrt/Ox neuron activation in response to stress. Interestingly, we found that the activation of LHALepRb neurons in obese leptin‐deficient mice exhibiting endogenous hypercorticosteronaemia acutely normalized corticosterone levels, without affecting food intake or body weight (Bonnavion et al. 2015) (Fig. 3 C). How these LHALepRb neurons correspond to LHA peptidergic populations is not yet known. In addition, we did not find any direct projections from LHALepRb neurons to the PVN, which controls the neuroendocrine stress response through the secretion of corticotropin‐releasing factor (CRF). This observation indicates that the inhibitory influence of LHALepRb neurons on stress‐induced activation of the PVN is indirect. We showed that synaptic input from LHALepRb/GABA neurons may account for inhibition of Hcrt/Ox neurons, which would in turn weaken PVN activation through diminished excitatory Hcrt/Ox inputs (Bonnavion et al. 2015). Modulation of other targets of LHALepRb neurons, such as LCNA neurons (Laque et al. 2015) may also have a role in modulating the neuroendocrine stress response. Evidence for direct LHAPdx1/GABA → PVN projections (Wu et al. 2015) suggests that LHAPdx1/GABA neurons may be distinct from LHALepRb/GABA neurons; however, their roles in the regulation of HPA activity and stress responses have not yet been investigated.
Further study is required to fully dissect the role of LHALepRb neurons in stress and anxiety. For instance, does the in vivo firing rate of LHALepRb neurons mirror that of Hcrt/Ox neurons across the sleep–wake cycle? Do they participate in the regulation of sleep, appetite and/or reward (Jennings et al. 2015; Nieh et al. 2015; O'Connor et al. 2015; Herrera et al. 2016; Stuber & Wise, 2016)? How much do they account for the full spectrum of leptin effects in the central nervous system? These LHALepRb neurons appear to modulate stress, arousal, reward and feeding in response to changes in metabolic status, thereby positioning these neurons to shape the comorbidity of anxiety disorders and eating disorders, such as anorexia nervosa or binge eating (Kaye et al. 2004). Such questions are fertile ground for future investigation.
Concluding remarks
The application of new technology is rapidly advancing our understanding of the cell types and circuits that comprise the mammalian hypothalamus – a structure that accounts for only ∼0.3% of the volume of the human brain (Hofman & Swaab, 1992), yet is critical to survival. The LHA in particular has long been a target of investigation owing to its pivotal role in regulating arousal, feeding and motivated behaviour. The LHA is uniquely positioned at the intersection of multiple neural and humoral systems that drive essential behavioural programmes and maintain homeostatic balance in physiology and behaviour. However, the cell‐type and circuit‐level heterogeneity of the LHA has made it a challenging structure to study.
An ever‐increasing number of innovative tools and reagents are being employed to deconstruct the constituent circuits of the LHA. These studies are beginning to provide detailed insight into anatomical inputs and outputs of the LHA and the ability to visualize specific classes of neurons for electrophysiological recording and imaging. Opto‐ and chemogenetic interrogation is providing critical complementary insight into the necessity and sufficiency of these circuits in complex behaviour. Emerging from these discoveries is a mechanistic understanding of the LHA and its constituent neural circuits. Nonetheless, fundamental questions remain concerning the nature of LHA circuits. What is the overarching taxonomy of functional cell types within the LHA? What is the synaptic organization of specific local and long‐range LHA circuits? How do the network dynamics of LHA circuits encode and drive specific facets of behaviour? How might cell type identity, synaptic relationships and larger scale circuit organization shift during development and in the pathogenesis of disease states? Furthering our understanding of the basic biology of LHA cell types and circuits holds the promise of identifying potential targets for selective therapeutic interventions in a variety of disease processes, including neuropsychiatric illness, addiction, sleep disorders and obesity.
Additional information
Competing interests
None declared.
Funding
P.B. was supported by an IBRO Research Fellowship, a NARSAD Young Investigator grant and by the Hilda and Preston Davis Foundation. L.dL. was supported by grants from the NIMH (R01MH087592061), NIA (R01AG04767102) and Klarman Family Foundation. A.C.J. was supported by a grant from the NIMH (R00MH097792).
Acknowledgements
We gratefully acknowledge Dr. Anna Lisa Lucido for valuable comments on the manuscript.
Biographies
Patricia Bonnavion received her PhD training in neuroscience at the Université Pierre et Marie Curie Paris VI, followed by a postdoctoral fellowship in the laboratory of Luis de Lecea, Professor in the Department of Psychiatry and Behavioural Sciences at Stanford University School of Medicine. Currently, she is a Marie Curie fellow researcher in the Laboratory of Neurophysiology, Université Libre de Bruxelles (ULB) and her research focuses on monoamine control of arousal and cognitive processes.

Laura Mickelsen received her undergraduate degree in Integrative Neuroscience at State University of New York at Binghamton. She and Akie Fujita are PhD students in the laboratory of Alexander Jackson, Assistant Professor in the Department of Physiology and Neurobiology at the University of Connecticut. Her research concerns the cellular and synaptic neurophysiology of cell types and circuits in the lateral hypothalamus.
References
- Abrahamson EE, Leak RK & Moore RY (2001). The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12, 435–440. [DOI] [PubMed] [Google Scholar]
- Abrahamson EE & Moore RY (2001). The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889, 1–22. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K & de Lecea L (2007). Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre C, Andermann ML & Scammell TE (2013). Control of arousal by the orexin neurons. Curr Opin Neurobiol 23, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T & Friedman JM (2006). Late‐onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci 26, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand B & Brobeck J (1951). Hypothalamic control of food intake in rats and cats. Yale J Biol Med 42, 123–140. [PMC free article] [PubMed] [Google Scholar]
- Apergis‐Schoute XJ, Iordanidou P, Faure C, Jego S, Scho C, Aitta‐Aho T & Burdakov D (2015). Optogenetic evidence for inhibitory signalling from orexin to MCH neurons via local microcircuits. J Neurosci 35, 5435–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum L, Skariah G, Mourrain P, Mignot E (2007). Comparative expression of p2x receptors and ecto‐nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174, 66–75. [DOI] [PubMed] [Google Scholar]
- Balcita‐Pedicino JJ & Sesack SR (2007). Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma‐aminobutyric acid neurons. J Comp Neurol 503, 668–684. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Wang HL, Morales M & Wise RA (2016). Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J Neurosci 36, 2975–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Wayner MJ, Scharoun SL, Guevara‐Aguilar R & Aguilar‐Baturoni HU (1981). Afferent connections to the lateral hypothalamus: a horseradish peroxidase study in the rat. Brain Res Bull 7, 75–88. [DOI] [PubMed] [Google Scholar]
- Bayer L, Mairet‐Coello G, Risold PY & Griffond B (2002). Orexin/hypocretin neurons: Chemical phenotype and possible interactions with melanin‐concentrating hormone neurons. Regul Pept 104, 33–39. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC & Luo L (2015). Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk ML & Finkelstein JA (1982). Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull 8, 511–526. [DOI] [PubMed] [Google Scholar]
- Bernardis LL & Bellinger LL (1993). The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci Biobehav Rev 17, 141–193. [DOI] [PubMed] [Google Scholar]
- Bernardis LL & Bellinger LL (1996). The lateral hypothalamic area revisited: ingestive behaviour. Neurosci Biobehav Rev 20, 189–287. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA & Vittoz NM (2010). Hypocretin/orexin in arousal and stress. Brain Res 1314, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR & Münzberg H (2011). The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self‐stimulation to opto‐genetics. Physiol Behav 104, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W & Sawchenko PE (1992). The melanin‐concentrating hormone system of the rat brain: An immuno‐ and hybridization histochemical characterization. J Comp Neurol 319, 218–245. [DOI] [PubMed] [Google Scholar]
- Bonnavion P & de Lecea L (2010). Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep 10, 174–179. [DOI] [PubMed] [Google Scholar]
- Bonnavion P, Jackson AC, Carter, ME & de Lecea L (2015). Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun 6, 6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT & Bonci A (2009). Orexin A/hypocretin‐1 selectively promotes motivation for positive reinforcers. J Neurosci 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL & Bonci A (2006). Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioural sensitization to cocaine. Neuron 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Bota M & Swanson LW (2007). The neuron classification problem. Brain Res Rev 56, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B & de Lecea L (2008). Addiction and arousal: The hypocretin connection. Physiol Behav 93, 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PC & Keesey RE (1975). Chronically reduced body weight in rats sustaining lesions of the lateral hypothalamus and maintained on palatable diets and drinking solutions. J Comp Physiol Psychol 88, 218–223. [DOI] [PubMed] [Google Scholar]
- Broberger C (1999). Hypothalamic cocaine‐ and amphetamine‐regulated transcript (CART) neurons: Histochemical relationship to thyrotropin‐releasing hormone, melanin‐concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res 848, 101–113. [DOI] [PubMed] [Google Scholar]
- Broberger C, de Lecea L, Sutcliffe JG & Hokfelt T (1998). Hypocretin/orexin‐ and melanin‐concentrating hormone‐expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to the neuropeptide Y and agouti gene‐related protein systems. J Comp Neurol 402, 460–474. [PubMed] [Google Scholar]
- Brown JA, Woodworth HL & Leinninger GM (2015). To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front Syst Neurosci 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Karnani MM & González A (2013). Lateral hypothalamus as a sensor‐regulator in respiratory and metabolic control. Physiol Behav 121, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR & Scammell TE (2012). Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci 32, 12305–12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt J, Alberto CO, Parsons MP & Hirasawa M (2011). Local network regulation of orexin neurons in the lateral hypothalamus. J Physiol 301, 578–580. [DOI] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K & de Lecea L (2009). Sleep homeostasis modulates hypocretin‐mediated sleep‐to‐wake transitions. J Neurosci 29, 10939–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R & de Lecea L (2012). Mechanism for Hypocretin‐mediated sleep‐to‐wake transitions. Proc Natl Acad Sci USA 109, 2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Cole SL & Berridge KC (2015). Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci 9, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJS, Arrigoni E & Maratos‐Flier E (2015). Melanin‐concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci 35, 3644–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB & Yanagisawa M (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Chen A, Chiu CN, Mosser EA, Kahn S, Spence R, Prober DA (2016). QRFP and its receptors regulate locomotor activity and sleep in zebrafish. J Neurosci 36, 1823–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT & Scammell TE (2001). Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O (2009). The melanin‐concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci USA 106, 6772–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément O, Sapin E, Libourel P‐A, Arthaud S, Brischoux F, Fort P & Luppi PH (2012). The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci 32, 16763–16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD (2005). Anatomy and regulation of the central melanocortin system. Nat Neurosci 8, 571–578. [DOI] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK & Lutter M (2012). Neuroanatomy of melanocortin‐4 receptor pathway in the lateral hypothalamic area. J Comp Neurol 520, 4168–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal J, Roh JH, Maloney SE, Akuffo A, Shah S, Yuan H & Dougherty JD (2013). Translational profiling of hypocretin neurons identifies candidate molecules for sleep regulation. Genes Dev 27, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L and Friedman JM (2001). Virus‐assisted mapping of neural inputs to a feeding centre in the hypothalamus. Science 291, 2608–2613. [DOI] [PubMed] [Google Scholar]
- del Cid‐Pellitero E & Jones BE (2012). Immunohistochemical evidence for synaptic release of GABA from melanin‐concentrating hormone containing varicosities in the locus coeruleus. Neuroscience 223, 269–276. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM & Sutcliffe JG (1998). The hypocretins: hypothalamus‐specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, JMR & Anand BK (1953). Increase of food intake induced by electrical stimulation of the lateral hypothalamus. J Physiol 172, 162–168. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM (2013). Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife 2, e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M & Saper CB (2001). Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol 432, 1–19. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos‐Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Huffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M & Elmquist JK (1998). Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402, 442–459. [PubMed] [Google Scholar]
- Elias CF, Sita LV, Zambon BK, Oliveira ER, Vasconcelos LA & Bittencourt JC (2008). Melanin‐concentrating hormone projections to areas involved in somatomotor responses. J Chem Neuroanat 35, 188–201. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF & Saper CB (1999). From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Maratos‐Flier E, Saper CB & Flier JS (1998). Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1, 445–450. [DOI] [PubMed] [Google Scholar]
- España RA, Valentino RJ & Berridge CW (2003). Fos immunoreactivity in hypocretin‐synthesizing and hypocretin‐1 receptor‐expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin‐1 administration. Neuroscience 121, 201–217. [DOI] [PubMed] [Google Scholar]
- Flores Á, Saravia R, Maldonado R & Berrendero F (2015). Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci 38, 550–559. [DOI] [PubMed] [Google Scholar]
- Foo KS, Brismar H & Broberger C (2008). Distribution and neuropeptide coexistence of nucleobindin‐2 mRNA/nesfatin‐like immunoreactivity in the rat CNS. Neuroscience 156, 563–579. [DOI] [PubMed] [Google Scholar]
- Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K & Luppi PH (2008). The satiety molecule nesfatin‐1 is co‐expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 155, 174–181. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L & Carrive P (2009). Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30, 1603–1614. [DOI] [PubMed] [Google Scholar]
- Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M & Sakurai T (2013). Neurotensin co‐expressed in orexin‐producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness states. PLoS One 8 e62391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Shizgal P & Yeomans JS (1981). A portrait of the substrate for self‐stimulation. Psychol Rev 88, 228–273. [PubMed] [Google Scholar]
- Gautron L, Elmquist JK & Williams KW (2015). Neural control of energy balance: translating circuits to therapies. Cell 161, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraedts LM, Nieuwenhuys R & Veening JG (1990). Medial forebrain bundle of the rat: IV. Cytoarchitecture of the caudal (lateral hypothalamic) part of the medial forebrain bundle bed nucleus. J Comp Neurol 294, 537–568. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos‐Flier E, Nestler EJ & DiLeone RJ (2005). The hypothalamic neuropeptide melanin‐concentrating hormone acts in the nucleus accumbens to modulate feeding behaviour and forced‐swim performance. J Neurosci 25, 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Horvath T L, & Xie X (2011). Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociceptin/orphanin FQ blocks stress‐induced analgesia in rats. Neuropharmacology 60, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ & de Lecea L (2014). Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol 29, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Leinninger GM, Patterson CM, Satin LS & Myers MG Jr (2014). Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA‐independent mechanisms. J Neurosci 34, 11405–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Jensen LT, Fugger L & Burdakov D (2012). Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci 35, 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Iordanidou P, Strom M, Adamantidis A & Burdakov D. (2016) Awake dynamics and brain‐wide direct inputs of hypothalamic MCH and orexin networks. Nat Commun 7:11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T & Shioda S (2002). Reciprocal synaptic relationships between orexin‐ and melanin‐concentrating hormone‐containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord 26, 1523–1532. [DOI] [PubMed] [Google Scholar]
- Hahn JD (2010). Comparison of melanin‐concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett 468, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD & Swanson LW (2010). Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev 64, 14–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson ML, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B & Ha M (1999). Leptin receptor‐ and STAT3‐immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 11, 653–663. [DOI] [PubMed] [Google Scholar]
- Hanriot L, Camargo N, Courau AC, Leger L, Luppi PH & Peyron C (2007). Characterization of the melanin‐concentrating hormone neurons activated during paradoxical sleep hypersomnia in rats. J Comp Neurol 505, 147–157. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami KI, Goto K, Yanagisawa M & Sakurai T (2001). Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354. [DOI] [PubMed] [Google Scholar]
- Harris GC & Aston‐Jones G (2006). Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29, 571–577. [DOI] [PubMed] [Google Scholar]
- Harthoorn LF, Sañé A, Nethe M & Van Heerikhuize JJ (2005). Multi‐transcriptional profiling of melanin‐concentrating hormone and orexin‐containing neurons. Cell Mol Neurobiol 25, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Henny P, Lee MG & Jones BE (2010). GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during slee Eur J Neurosci 32, 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Krause MR, Mainville L, Cordova CA, Jones BE (2016). Orexin neurons respond differentially to auditory cues associated with appetitive versus aversive outcomes. J Neurosci 36, 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG & Jones BE (2009). Melanin‐concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep‐wake cycle. Proc Natl Acad Sci USA 106, 2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T & Jones BE (2010). Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169, 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T & Adamantidis A (2016). Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci 19, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG (1965). Hypothalamic lesions by electrocauterization: disinhibition of feeding and self‐stimulation. Science 149, 452–453. [DOI] [PubMed] [Google Scholar]
- Hoebel BG & Teitelbaum P (1962). Hypothalamic control of feeding and self‐stimulation. Science 135, 375–377. [DOI] [PubMed] [Google Scholar]
- Hofman MA & Swaab DF (1992). The human hypothalamus: comparative morphometry and photoperiodic influences. Prog Brain Res 93, 133–147. [DOI] [PubMed] [Google Scholar]
- Horjales‐Araujo E, Hellysaz A & Broberger C (2014). Lateral hypothalamic thyrotropin‐releasing hormone neurons: distribution and relationship to histochemically defined cell populations in the rat. Neuroscience 277, 87–102. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston‐Jones G, Kilduff TS & van den Pol AN (1999). Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415, 145–159. [PubMed] [Google Scholar]
- Huang H, Acuna‐Goycolea C, Li Y, Cheng HM, Obrietan K & van den Pol AN (2007). Cannabinoids excite hypothalamic melanin‐concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci 27, 4870–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SW & Johnson AK (2014). The role of the lateral hypothalamus and orexin in ingestive behaviour: a model for the translation of past experience and sensed deficits into motivated behaviours. Front Syst Neurosci 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D & Adamantidis AR (2013). Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci 16, 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL & Stuber GD (2013). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak P, Aita M, Shilling‐scrivo K, Ramakrishnan C, Deisseroth K, Otte S & Stuber GD (2015). Article visualizing hypothalamic network dynamics for appetitive and consummatory behaviours. Cell 160, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA & Shekhar A (2011). Orexin, stress, and anxiety/panic states. Prog Brain Res 198, 133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman‐Bendz L, Goddard AW, Brundin L & Shekhar A (2010). A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE & Hassani OK (2013). The role of Hcrt/Orx and MCH neurons in sleep–wake state regulation. Sleep 36, 1769–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Szabó G, Erdélyi F & Burdakov D (2013). Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin‐ and melanin‐concentrating hormone neurons. J Physiol 591, 933–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K (2004). Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 161, 2215–21. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG Jr, Deisseroth K, de Lecea L & Bonci A (2013). Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci 33, 7618–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H & Oomura Y (1982). An HRP study of the afferent connections to rat lateral hypothalamic region. Brain Res Bull 8, 63–71. [DOI] [PubMed] [Google Scholar]
- Konadhode RR, Pelluru D, Blanco‐Centurion C, Zayachkivsky A, Liu M, Uhde T & Shiromani PJ (2013). Optogenetic stimulation of MCH neurons increases sleep. J Neurosci 33, 10257–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL & Brown RE (2003). Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosse C & Burdakov D (2014). A unifying computational framework for stability and flexibility of arousal. Front Syst Neurosci 8, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe‐Uchida M, Uchida N, Liberles SD & Lowell BB (2014). An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laque A, Yu S, Qualls‐Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud H‐R, Morrison CD, Richards BK & Münzberg H (2015). Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laque A, Zhang Y, Gettys S, Nguyen T‐A, Bui K, Morrison CD & Münzberg H (2013). Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab 304, E999–E1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wissman AM, Loriaux AL & Self DW (2015). Optogenetic stimulation of accumbens shell or shell projections to lateral hypothalamus produce differential effects on the motivation for cocaine. J Neurosci 35, 3537–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK & Jones BE (2005). Discharge of identified orexin/hypocretin neurons across the sleep‐waking cycle. J Neurosci 25, 6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM (2011). Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiol Behav 104, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo Y‐H, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H & Myers MG Jr (2009). Leptin acts via leptin receptor‐expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM & Myers MG Jr (2008). LRb signals act within a distributed network of leptin‐responsive neurones to mediate leptin action. Acta Physiol 192, 49–59. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci La, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC & Myers MG Jr (2011). Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab 14, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Björnholm M, Münzberg H & Myers MG Jr (2006). Leptin receptor signalling and action in the central nervous system. Obesity (Silver Spring) 14 (Suppl. 5), 208S–212S. [DOI] [PubMed] [Google Scholar]
- Li JX, Yoshida T, Monk KJ & Katz DB (2013). Lateral hypothalamus contains two types of palatability‐related taste responses with distinct dynamics. J Neurosci 33, 9462–9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T & van den Pol AN (2002). Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron – A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36, 1169–1181. [DOI] [PubMed] [Google Scholar]
- Li Z, Kelly L, Heiman M, Greengard P & Friedman JM (2015). Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metab 22, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM & Elmquist JK (2003). Transgenic mice expressing green fluorescent protein under the control of the melanocortin‐4 receptor promoter. J Neurosci 23, 7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J , Merkle FT, Gandhi AV, Gagnon J, Woods IG, Chiu CN & Prober D (2015). Evolutionarily conserved regulation of hypocretin neuron specification by Lhx9. Development 142, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GW, Leinninger GM, Rhodes CJ & Myers MG Jr (2010). Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci 30, 11278–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS & Maratos‐Flier E (2001). Melanin‐concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest 107, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten PG & Room P (1980). Interrelations between lateral, dorsomedial and ventromedial hypothalamic nuclei in the rat: An HRP study. Brain Res 190, 321–332. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Ter Horst GJ & Steffens AB (1987). The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol 28, 1–54. [DOI] [PubMed] [Google Scholar]
- Macneil DJ (2013). The role of melanin‐concentrating hormone and its receptors in energy homeostasis. Front Endocrinol 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maolood N & Meister B (2010). Nociceptin/orphanin FQ peptide in hypothalamic neurones associated with the control of feeding behaviour. J Neuroendocrinol 22, 75–82. [DOI] [PubMed] [Google Scholar]
- Margules DL & Olds J (1962). Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 135, 374–375. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M & Sakurai T (2009). Selective loss of GABA(B) receptors in orexin‐producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci USA 106, 4459–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészár Z, Girard F, Saper CB & Celio MR (2012). The lateral hypothalamic parvalbumin‐immunoreactive (PV1) nucleus in rodents. J Comp Neurol 520, 798–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI & Siegel JM (2005). Behavioural correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen R‐Y & Hull EM (2007). A role for hypocretin (orexin) in male sexual behaviour. J Neurosci 27, 2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander J, Thompson, JL , Voren G, Hassinger, LC , Onvani S & Carlezon W (2014). Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA 11, 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG Jr, Münzberg H, Leinninger GM & Leshan RL (2009). The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Sugino K & Hempel CM (2006). The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci 29, 339–345. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Matthews G, Allsop S, Presbrey KN, Leppla C, Wichmann R, Neve R, Wildes CP & Tye KM (2015). Article decoding neural circuits that control compulsive sucrose seeking. Cell 160, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LM & Veening JG (1982). The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol 206, 49–81. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C & Lüscher C (2015). Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564. [DOI] [PubMed] [Google Scholar]
- Oh‐I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M & Mori M (2006). Identification of nesfatin‐1 as a satiety molecule in the hypothalamus. Nature 443, 709–712. [DOI] [PubMed] [Google Scholar]
- Ohno K, Hondo M & Sakurai T (2008). Cholinergic regulation of orexin/hypocretin neurons through M3 muscarinic receptor in mice. J Pharmacol Sci 106, 485–491. [DOI] [PubMed] [Google Scholar]
- Olds J & Milner P (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of the rat brain. J Comp Physiol Psychol 47, 419–427. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Leshan RL, Jones JC & Myers MG Jr (2011). Molecular mapping of mouse brain regions innervated by leptin receptor‐expressing cells. Brain Res 1378, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Wong J‐MT, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT & Myers MG Jr (2015). Ventral tegmental area neurotensin signalling links the lateral hypothalamus to locomotor activity and striatal dopamine. Endocrinol 156, 1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay, Y & Mignot, E (2000). A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6, 991–997. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissios P, Bradley RL & Maratos‐Flier E (2006). Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev 27, 606–620. [DOI] [PubMed] [Google Scholar]
- Pissios P, Frank L, Kennedy AR, Porter DR, Marino FE, Liu FF, Pothos EN & Maratos‐Flier E (2008). Dysregulation of the mesolimbic dopamine system and reward in MCH‐/‐ mice. Biol Psychiatry 64, 184–191. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R & Maratos‐Flier E (1996). A role for melanin‐concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247. [DOI] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre‐Amos T, Heller HC & de Lecea L (2011). Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA 108, 13305–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL & Guyenet PG (2003). Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol 465, 593–603. [DOI] [PubMed] [Google Scholar]
- Saito YC, Tsujino N, Hasegawa E, Akashi K, Abe M, Mieda M, Sakimura K, Sakurai T (2013). GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front Neural Circuits 7, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ & Yanagisawa M (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behaviour. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M (2005). Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46, 297–308. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8, 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2014). The role of orexin in motivated behaviours. Nat Rev Neurosci 15, 719–731. [DOI] [PubMed] [Google Scholar]
- Sakurai T & Mieda M (2011). Connectomics of orexin‐producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci 32, 451–462. [DOI] [PubMed] [Google Scholar]
- Sano H & Yokoi M (2007). Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin‐ or melanin‐concentrating hormone‐containing neurons. J Neurosci 27, 6948–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J & Scammell TE (2010). Sleep state switching. Neuron 68, 1023–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE & Lu J (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW & Cowan WM (1979). An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol 183, 689–706. [DOI] [PubMed] [Google Scholar]
- Sapin E, Bérod A, Léger L, Herman P, Luppi PH & Peyron C (2010). A very large number of GABAergic neurons are activated in the tuberal hypothalamus during paradoxical (REM) sleep hypersomnia. PLoS One 5, e11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE (2003). The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 53, 154–166. [DOI] [PubMed] [Google Scholar]
- Schöne C, Apergis‐Schoute J, Sakurai T, Adamantidis A & Burdakov D (2014). Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Reports 7, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C & Burdakov D (2012). Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front Behav Neurosci 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Cao ZFH, Apergis‐Schoute J, Adamantidis A, Sakurai T & Burdakov D (2012). Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ . J Neurosci 32, 12437–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Venner A, Knowles D, Karnani MM & Burdakov D (2011). Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol 589, 2767–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ & Baskin DG (2000). Central nervous system control of food intake. Nature 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM & Elmquist JK (2009). Leptin targets in the mouse brain. J Comp Neurol 514, 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears, RM , Fink AE, Wigestrand MB, Farb CR, de Lecea L & LeDoux JE (2013). Orexin/hypocretin system modulates amygdala‐dependent threat learning through the locus coeruleus. Proc Natl Acad Sci USA 110, 20260–202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Dauvilliers Y & Siegel JM (2015). Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol 11, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR & DiLeone RJ (2010). Orexin signalling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry 67, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS & Maratos‐Flier E (1998). Mice lacking melanin‐concentrating hormone are hypophagic and lean. Nature 396, 670–674. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda‐Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J & Blackshaw S (2010). A genomic atlas of mouse hypothalamic development. Nat Neurosci 13, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Yang JH, Lee H, Erdélyi F, Szabó G, Lee SY & Ryu PD (2007). Identification of the adrenoceptor subtypes expressed on GABAergic neurons in the anterior hypothalamic area and rostral zona incerta of GAD65‐eGFP transgenic mice. Neurosci Lett 422, 153–157. [DOI] [PubMed] [Google Scholar]
- Siegel JM (2004). Hypocretin (orexin): role in normal behaviour and neuropathology. Annu Rev Psychol 55, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski K, Esumi S, Hirata T, Kamal Y, Tran T, Lam A, Oboti L, Brighthaupt S‐C, Zaghlula M, Martinez J, Ghimbovschi S, Knoblach S, Pierani A, Tamamaki N, Shah NM, Jones KS, Corbin JG (2015). Specification of select hypothalamic circuits and innate behaviours by the embryonic patterning gene dbx1 . Neuron 86, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M & Sakurai T (2013). Orexin receptor‐1 in the locus coeruleus plays an important role in cue‐dependent fear memory consolidation. J Neurosci 33, 14549–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC (2015). Neurotransmitter switching? No surprise. Neuron 86, 1131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD (2016). Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci 36, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM (2013). Hypothalamic survival circuits: blueprints for purposive behaviours. Neuron 77, 810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S (2016). An emerging technology framework for the neurobiology of appetite. Cell Metab 23, 234–253. [DOI] [PubMed] [Google Scholar]
- Stuber GD & Wise RA (2016). Lateral hypothalamic circuit for feeding and reward. Nat Neurosci 19, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sanchez‐Watts G & Watts AG (2005). Comparison of melanin‐concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett 387, 80–84. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS & Sakai K (2008). Neuronal activity of orexin and non‐orexin waking‐active neurons during wake‐sleep states in the mouse. Neurosci 153, 860–870. [DOI] [PubMed] [Google Scholar]
- Takayasu S, Sakurai T, Iwasaki S, Teranishi H, Yamanaka A, Williams SC, Iguchi H, Kawasawa YI, Ikeda Y, Sakakibara I, Ohno K, Ioka RX, Murakami S, Dohmae N, Xie J, Suda T, Motoike T, Ohuchi T, Yanagisawa M, Sakai J (2006). A neuropeptide ligand of the G protein‐coupled receptor GPR103 regulates feeding, behavioural arousal, and blood pressure in mice. Proc Natl Acad Sci USA 103, 7438–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P & Epstein AN (1962). The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69, 74–90. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ & Luiten PG (1987). Phaseolus vulgaris leuco‐agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res Bull 18, 191–203. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M & Siegel JM (2000). Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M & Saper CB (2003). Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K & Sakurai T (2005). Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci 25, 7459–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, Kilduff TS, Terao A & Yamanaka A (2014). Optogenetic manipulation of activity and temporally controlled cell‐specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci 34, 6896–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Acuna‐Goycolea C, Clark KR & Ghosh PK (2004). Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron 42, 635–652. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Herbst RS & Powell JF (1984). Tyrosine hydroxylase‐immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience 13, 1117–1156. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S Jr & Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Clausen JT & Kristensen P (1999). Neurochemical characterization of hypothalamic cocaine‐ amphetamine‐regulated transcript neurons. J Neurosci 19, RC5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG & Sanchez‐Watts G (2007). Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration‐anorexia. J Comp Neurol 502, 768–782. [DOI] [PubMed] [Google Scholar]
- Winsky‐Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL & de Lecea L (2004). Interaction between the corticotropin‐releasing factor system and hypocretins (orexins). J Neurosci 24, 11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kim ER, Sun H, Xu Y, Mangieri LR., Li D‐P & Tong Q (2015). GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J Neurosci 35, 3312–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, Yamanaka A, Diano S, Horvath TL, Sakurai T, Toll L, Kilduff TS (2008). Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress‐induced analgesia. J Clin Invest 118, 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami KI, Sugiyama F, Goto K, Yanagisawa M & Sakurai T (2003). Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713. [DOI] [PubMed] [Google Scholar]
- Yelin‐Bekerman L, Elbaz I, Diber A, Dahary D & Gibbs L (2015). Hypocretin neuron‐specific transcriptome profiling identifies the sleep modulator Kcnh4a. eLife 4, e08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España R, Crocker A & Scammell TE (2006). Afferents to the orexin neurons of the rat brain. J Comp Neurol 494, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]


