Abstract
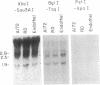
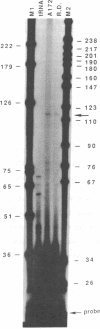
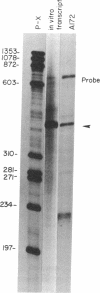
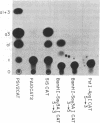
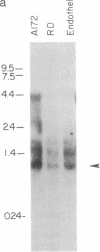
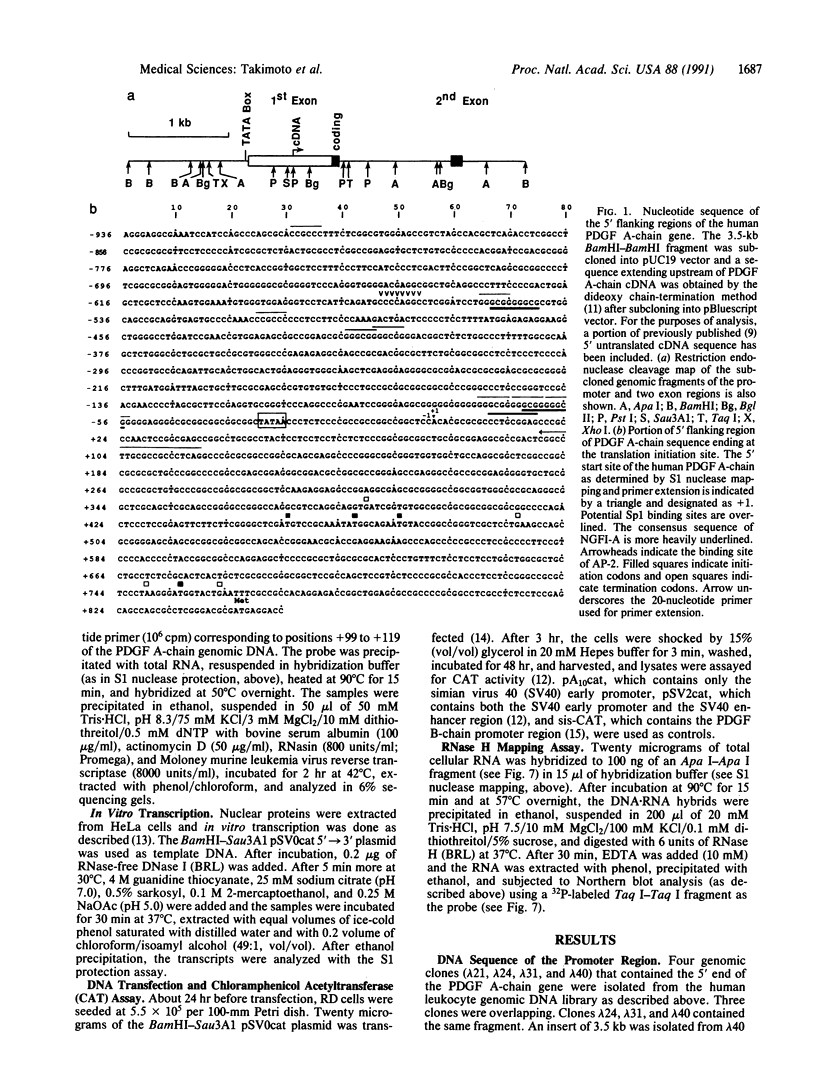
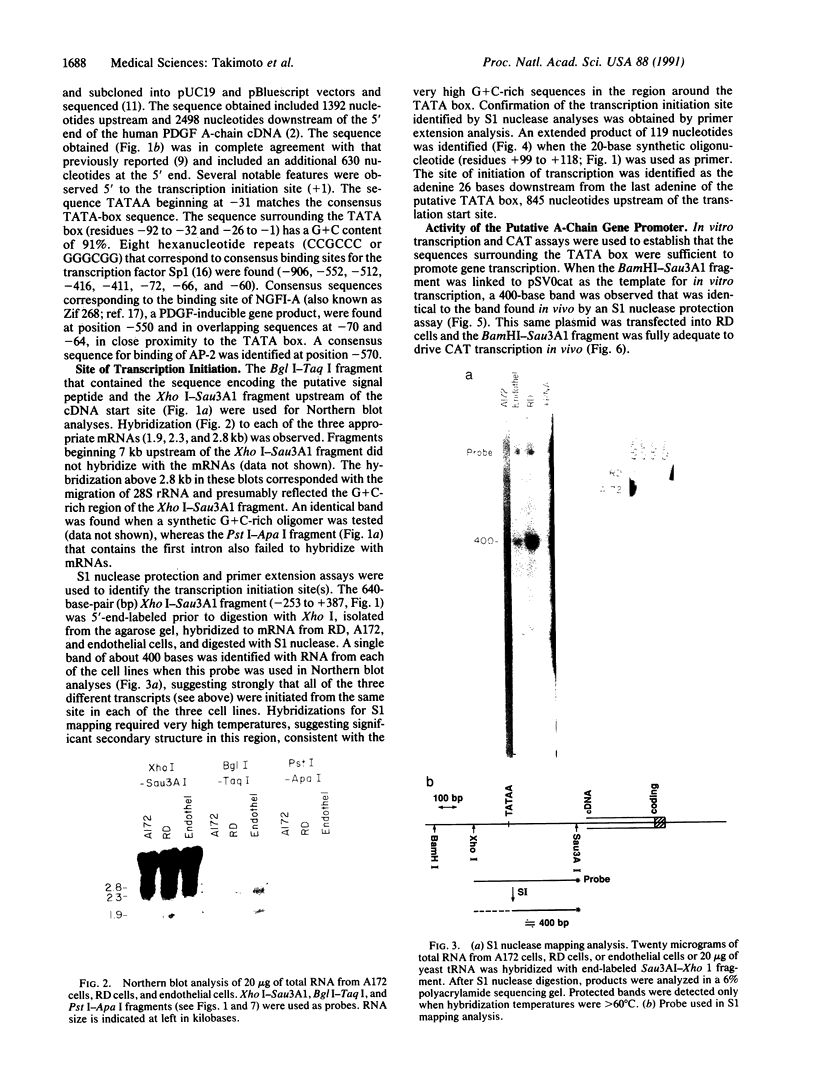
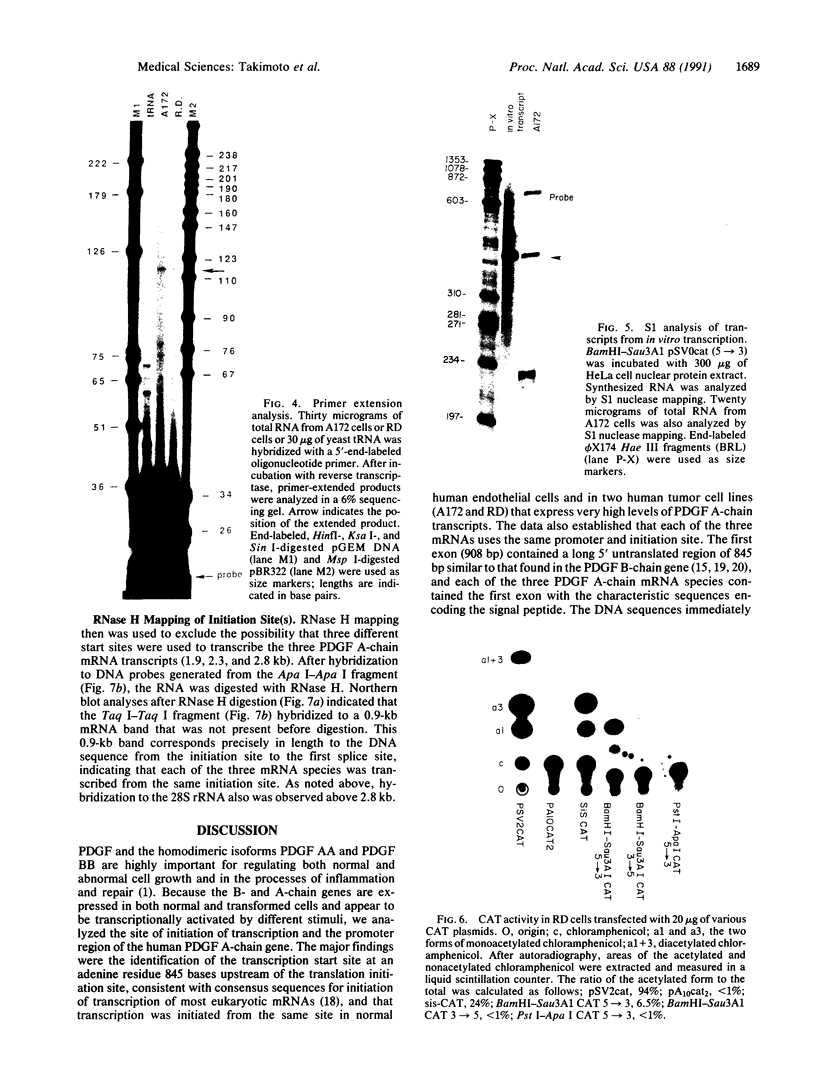
The platelet-derived growth factor (PDGF) A- and B-chain genes are widely expressed in mammalian tissues and their homodimeric gene products appear to regulate the autocrine growth of both normal and transformed cells. In this study, we analyzed the 5' flanking sequences of the human PDGF A-chain gene to seek elements important to regulating its transcription. The promoter region was exceptionally G + C-rich and contained a "TATA box" but no "CAAT box." The transcription start site was identified 845 base pairs 5' to the translation initiation site by S1 nuclease mapping and by primer extension. Both in vitro transcription and transient expression of the chloramphenicol acetyltransferase gene linked to the PDGF A-chain 5' flanking sequences established that the putative promoter region was active, and RNase H mapping established that the three characteristic mRNAs (1.9, 2.3, and 2.8 kilobases) used the same transcription start site, which was used in normal endothelial cells and in two human tumor cell lines that express high levels of A-chain transcripts. The results established an exceptionally G + C-rich promoter region and a single transcription start site active for each of the three mRNAs of the PDGF A-chain gene. DNA sites of potential importance in mediating the activation of the PDGF A-chain gene in normal cells and in transformed cell lines expressing high levels of PDGF A chain were identified.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Bonthron D. T., Orkin S. H. Alternative RNA splicing affects function of encoded platelet-derived growth factor A chain. Nature. 1987 Aug 13;328(6131):621–624. doi: 10.1038/328621a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh W. M., Harsh G. R., 4th, Starksen N. F., Rocco C. M., Williams L. T. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J Biol Chem. 1988 Jun 15;263(17):8470–8472. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Kanehisa M., Krönke M., Peffer N. J., Svetlik P. B., Sullivan M., Greene W. C. Structure of the human interleukin-2 receptor gene. Science. 1985 Nov 8;230(4726):633–639. doi: 10.1126/science.2996141. [DOI] [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Rao C. D., Igarashi H., Chiu I. M., Robbins K. C., Aaronson S. A. Structure and sequence of the human c-sis/platelet-derived growth factor 2 (SIS/PDGF2) transcriptional unit. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2392–2396. doi: 10.1073/pnas.83.8.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Thielan B., Collins T. Sequences of the 5' portion of the human c-sis gene: characterization of the transcriptional promoter and regulation of expression of the protein product by 5' untranslated mRNA sequences. Nucleic Acids Res. 1987 Aug 11;15(15):6017–6036. doi: 10.1093/nar/15.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Rorsman F., Bywater M., Knott T. J., Scott J., Betsholtz C. Structural characterization of the human platelet-derived growth factor A-chain cDNA and gene: alternative exon usage predicts two different precursor proteins. Mol Cell Biol. 1988 Feb;8(2):571–577. doi: 10.1128/mcb.8.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Jaffer F. E., Abboud H. E. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund M., Hedin U., Sejersen T., Heldin C. H., Thyberg J. Arterial smooth muscle cells express platelet-derived growth factor (PDGF) A chain mRNA, secrete a PDGF-like mitogen, and bind exogenous PDGF in a phenotype- and growth state-dependent manner. J Cell Biol. 1988 Feb;106(2):403–413. doi: 10.1083/jcb.106.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong B. D., Auer D. E., Jaye M., Kaplow J. M., Ricca G., McConathy E., Drohan W., Deuel T. F. cDNA clones reveal differences between human glial and endothelial cell platelet-derived growth factor A-chains. Nature. 1987 Aug 13;328(6131):619–621. doi: 10.1038/328619a0. [DOI] [PubMed] [Google Scholar]
- Van den Ouweland A. M., Roebroek A. J., Schalken J. A., Claesen C. A., Bloemers H. P., Van de Ven W. J. Structure and nucleotide sequence of the 5' region of the human and feline c-sis proto-oncogenes. Nucleic Acids Res. 1986 Jan 24;14(2):765–778. doi: 10.1093/nar/14.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leen R. W., Kastrop P. M., van Roozendaal K. E., Schoenmakers J. G. Sequence divergence and selection of cap sites in the rat gamma-crystallin gene family. Eur J Biochem. 1986 May 15;157(1):203–208. doi: 10.1111/j.1432-1033.1986.tb09657.x. [DOI] [PubMed] [Google Scholar]