The fast emerging technological advances that are enabling biologists to convert stem cells from various sources into an ever‐increasing variety of cell types unquestionably will have far‐reaching implications for the future of basic bioscientific research. In time, such technologies will also undoubtedly fundamentally change the nature of clinical intervention in multiple disease areas. Indeed stem cell‐related therapies are already a widespread feature of the management of lymphoproliferative disorders and leukaemias and interventions in other diseases are increasingly being investigated both in preclinical studies and in formal clinical trials.
One area of great interest, and also one of potentially substantial beneficial impact, is the generation and exploitation of various cells of the neural lineage. The scale of this impact is, however, matched by a range of substantial scientific challenges that arise from multiple sources, not least the immense complexity of the mammalian nervous system. However, the goal of producing replacement neurons for those lost in disease could clearly have vast potential, and has led to the greatest efforts being directed towards conditions, such as Parkinson's disease, Huntington's disease and motor neuron disease, in which single neuronal classes dominate the overall population of lost cells and thus the symptom spectrum.
For experimental neurophysiologists, the idea of assembling a chosen neural circuit from stem cell‐based building blocks is certainly attractive, especially if this can be done using human neural cells which, for obvious reasons, most neuroscientists cannot readily access in a living, viable state. This ‘build a brain’ concept is certainly one stem cell proponents are keen to promote as realizable, and although things are moving in the right direction, one only has to consider for a moment the immense complexity of the mammalian CNS to appreciate the challenge these worthy goals present. By way of example, just the CA1 area of the rodent hippocampus is thought to contain round 25 distinct types of neuron as well as a variety of glia (Klausberger & Somogyi, 2008). It is currently hard to conceive how each of these could be specified individually from a stem cell source and then mixed in the appropriate ratios in the appropriate anatomical relationships to construct a ‘synthetic’ area CA1. A recent exciting approach that potentially circumvents the difficulties posed by the neural ‘pick and mix’ approach to circuit construction is that of CNS organoid culture (Lancaster et al. 2013; Lancaster & Knoblich,. 2014 a, 2014 b). These stem cell‐derived cultures produced in spinning bioreactors display a self‐organizing capability that recapitulates many aspects of the early development of the mammalian CNS, thereby producing multiple cell types and layering characteristic of early cortical development. The original work that produced structures reminiscent of cortex has recently been complemented by the production of a midbrain‐like organoid (Jo et al. 2016).
The many developmental, cell and molecular biologists who have driven stem‐cell research to its current state‐of‐art have predominantly relied on characterizations of gene and protein expression, combined with assessments of general cellular morphology to monitor the production of specific cell types. Thus, a ‘neuron’ would be expected to have processes formed into some form of arborisation and should be immunopositive for certain neuron‐specific markers (for example TUJ1 – class III β‐tubulin or neurofilament), whilst also lacking immunoreactivity to markers for other cell types (including glia and neural precursors). Using similar methods, a different range of criteria would need to be met by cells deemed to be, for example, astroglia or oligodendrocytes.
As opposed to a cell or molecular biologist, a neurophysiologist, such as myself, may consider an entirely different suite of factors to be cardinal defining features of a neuron or astrocyte. This standpoint is based on what over a century of real‐time in vivo and in vitro electrical and other physiological measurements have taught us. For example, practically all mammalian neurons have a resting membrane potential (V m) more negative than –40 mV. Indeed, in CNS neurones resting V m is more typically between –65 and –85 mV. Furthermore, with the exception of very specialized neurons such as rods and cones, mature neurons are capable for firing Na+‐dependent action potentials, and most can do this repeatedly at high frequencies. Additionally pretty much every neuron responds to the major amino acid neurotransmitters that mediate fast excitatory and inhibitory synaptic communication via activation of a range of ligand‐gated ion channels; and relating to this, all neurons form presynaptic terminals of some form and almost all additionally possess postsynaptic specializations that allow synaptic input. At a more detailed biophysical level we know much about how neurons behave. For example, action potentials in most adult CNS neurons, including those in neocortical structures, rise at 300–600 V s−1. These rates of rise as well as the ability to fire repeatedly, arise mainly from massive Na+ channel expression that yields peak inward somatic current densities as high as 1 nA pF−1 (around 15 nS pF−1 of conductance). These levels of Na+ channel expression are matched or even surpassed by the levels of accompanying voltage‐gated K+ conductances required for rapid spike repolarization (e.g. Brown et al. 2011).
My own experiences of neurophysiologically characterizing what were proposed to be neurons made from stem cell sources go back to the early years of the millennium, at which time I was working in the pharmaceutical industry. At this time small start‐up companies were keen to sell pharmaceutical companies embryonic stem cell‐based technologies. They held up the enticing possibility that their products would enable drug discoverers to test their compound equity on human neurons. Unfortunately, at least in our hands, this proved to be a false dawn. So although the cells expressed some of the claimed neuronal marker proteins, neurophysiologically they were wholly disappointing. For example membrane potentials were circa –15 mV (rather than –60 to –85 mV), action potentials were impossible as there were no Na+ currents in most cells, and when we could occasionally find a hint of a Na+ current it was no bigger than those you can find in many HEK 293 cells. Similarly we could find little in the way of electrical responses to applications of either GABA or glutamate.
Years passed and stem cell researchers beavered away around the world, including those bringing the induced pluripotent stem cell (iPSC) methodologies that have revolutionized this area. Now back in academia, I was asked to record from some putative stem cell‐derived neurons made by a colleague. Things were better ... at least a bit. Cells looked a bit more ‘neurony’, membrane potentials were perhaps –20 to –35 mV, and more cells (but certainly not all) had Na+ currents – and although these were very far from the levels in ‘real’ neurons, in the occasional cell (the sort that is used for the ‘example cell’ in papers) they were just about big enough to produce a single, what might be generously called, ‘spikelet’. This second dip into the stem cell world certainly did not give me the compelling urge to throw away my brain slicers and turn the laboratory in to the Randall stem cell neurophysiology group.
A few more years passed without our micropipettes approaching anything in a dish that was produced by a stem cell scientist. However, from talks and posters at conferences, papers in top journals and the grant applications I saw going through the boards I sat on, I began to get the impression that the ability to create at least somewhat physiologically credible neurons from stem cells was developing to new levels. So even with my well‐founded scepticism that the figures presented in talks, papers and grants represented the authors’ best ever ‘examples’, it seemed a new foray into this area could be worthwhile.
This was readily borne out when next I dipped my scientific toes into the waters of stem‐cell‐derived neuron physiology. This work was through collaboration with Pfizer, who were not making CNS‐like neurons but were instead primarily working with new protocols designed to produce peripheral sensory neurons, including those involved in transduction of nociceptive signalling (Chambers et al. 2012; Young et al. 2014). From the first recording this proved something of a road to Damascus experience. These cells had the right sorts of resting potentials, as well as the sorts of huge voltage‐gated Na+ and K+ current densities that are required to produce repetitively firing, very fast rising, spikes, which they did with ease (especially when studied at physiological temperature). To me, someone more bothered by whether these cells could make the correct electrical signals than by which cytoskeletal proteins were expressed, these were ‘proper’ neurons. Some data from these Pfizer sensory neurons were presented in the session by my then PhD student, Dr Malgozata Mis. A subset of these data have appeared in two recent publications (Alexandrou et al. 2016; Cao et al. 2016) and examples are shown in Fig. 1 A–D.
Figure 1. Electrophysiological recordings from iPSC‐derived neurons at physiological temperature .
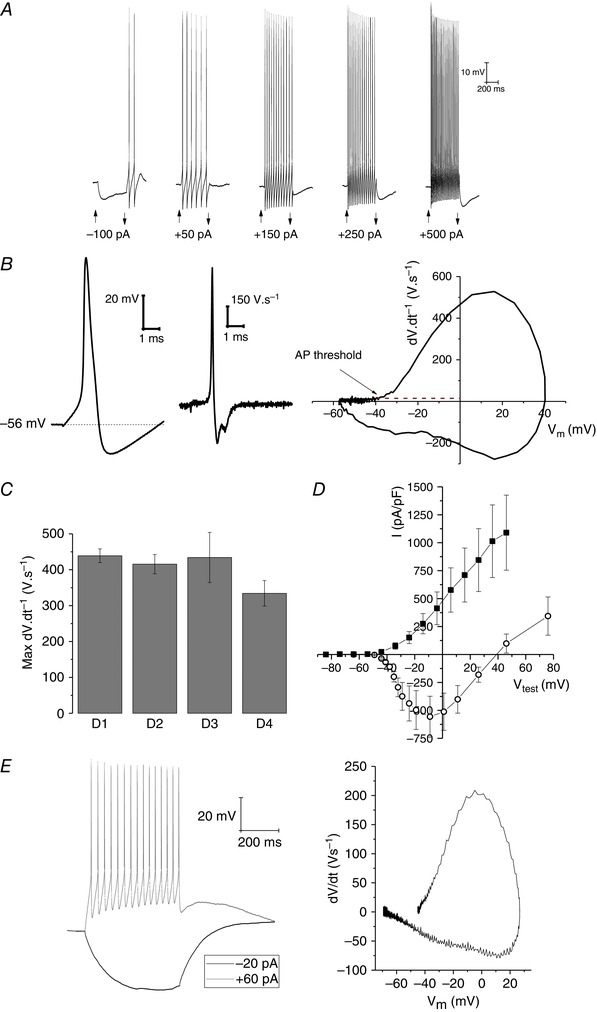
A, example sweeps from a current clamp recording from a typical human iPSC‐derived sensory neuron. Current pulses of 500 ms duration of the indicated amplitudes were applied to cells at rest for the times shown by the arrowheads; note the ability to support robust repetitive firing. B, an example first spike elicited by a depolarizing current injection (left), its first derivative (centre), and a standard phase‐plane plot (right) from a typical iPSC‐derived sensory neuron. Note the fast rising spikes (almost 500 V s−1) and threshold of approximately –40 mV. C, pooled data plotting rate of action potential rise in many iPSC‐derived neurons created from four different control human donors. D, pooled current–voltage relationships for the voltage‐gated Na+ current (open circles) and K+ current (filled squares) recorded from 12 nucleated macropatches isolated from the cell body of iPSC‐derived sensory neurons. E, right, a current clamp recording from an iPSC‐derived forebrain neuron grown for ∼3 weeks in the media described in Telezhkin et al. (2016) within a PuraMatrix‐based 3D culture. Left, a phase plane plot from this neuron for the first action potential fired in response to the depolarizing current injection. Panels A–D, data from A.R. and Malgorzata Mis (University of Bristol); panel E, data from A.R. and Jane Hancock (University of Bristol).
Leaving aside peripheral neurons, what about stem cells converted to represent the neural cells of the CNS? This issue of The Journal of Physiology contains three symposium reviews contributed by participants in a session at the Physiology 2015 meeting in Cardiff entitled Are stem cell‐derived neural cells physiologically credible? The session started with a presentation by Dr Manuel Peter from Rick Livesey's laboratory in Cambridge, a group that have played a key role in developing widely adopted protocols that produce various subtypes for cortical neurons from iPSC sources (Shi et al. 2012 a, b ). Given sufficient time (many weeks) these protocols give rise to cells that can fire multiple action potentials and produce functional synapses. Our second speaker, Matthew Livesey, described his work with Edinburgh colleagues that has analysed various electrophysiological indices of neuronal functionality including the development of responsivity to amino acid receptor ligands (Livesey et al. 2014, 2016; James et al. 2014). In this issue they review their work and that of others looking at how functionally mature human stem cell‐derived neurons really are (Livesey et al. 2016). They conclude that current methods, although producing credible neurons, generally produce cells that are reminiscent of immature cortical neurons based on a range of indices. These include measurements of intrinsic properties such as resting potential and input resistance as well as the receptor complement of some key ionotropic receptors. This conclusion is perhaps not surprising given the developmental time scales of the human nervous system in utero and following birth. Livesey and colleagues round off their review by considering the future challenge of making more mature neurones from stem cells sources.
The second symposium‐related review in this issue (Kemp et al. 2016) concerns the challenges of both improving and accelerating the functional maturation of stem cell‐derived neurons. This work in Cardiff has been based on harnessing the changing physiological features of brain milieu during the developmental time course. This has led to the formulation of culture media that can significantly accelerate the appearance of action potentials and synaptic connectively in iPSC‐derived neurons (Telezhkin et al. 2016), something which could save time and money for many adopters of stem cell‐based neuronal preparations. As someone who has recently contributed experimental work to the functional assessment of their media, I can state it unquestionably speeds things up compared to any previous approaches we have tested. As a consequence, the neurons produced are functionally more credible on a number of fronts (see example in Fig. 1 E). However, based on my almost 30 years of studying adult neurons in brain slices, I can also attest these cells have not yet attained what I would deem a fully mature phenotype, although this may simply be because we have not waited long enough before studying them.
Finally, anyone wishing to study neural function in stem cell‐derived model systems should not forget the importance of glial cells. The final talk in our session was by Eric Hill, who with colleagues at Aston University has been focusing on the generation and analysis of stem cell‐derived astroglia. Such cells can be produced from the same neural precursors as neurons. In their review in this issue (Hill et al. 2016) they first outline the various roles of astroglia in the CNS before highlighting the key publications describing the specification of astrocytes from stem cells courses. Finally they describe how such cells are starting to be considered in the study of CNS diseases including amyotrophic lateral sclerosis and Rett syndrome. They also touch on the important question of astrocyte diversity, which to date has received less attention than neuronal diversity, but will be a key factor for those seeking to develop stem cell‐based approaches to the study of glial cells.
In conclusion, in the last 15 years there has been significant progress in improving the physiological credibility of neural cells derived from stem cell sources. It has become reasonably straightforward to produce neurons with passable levels of electrogenesis and functioning synaptic connections. There is, however, still much to be done if we are to produce mammalian neurones and glia that resemble their adult counterparts embedded within mature circuits. This goal will best be met by starting with clear ideas of the criteria we aim to achieve. For example, if we aim to produce cells that functionally resemble dissociated primary cultures made from embryonic or neonatal rodents, this is where we will arrive; however, as such cultures do not represent especially close facsimiles of adult neurons in situ, this may not be the route that best serves neuroscientific progress.
Additional information
Competing interests
None declared.
Acknowledgements
Supported by Parkinson's UK, NC3Rs and The Biotechnology and Biological Sciences Research Council. AR would like to thank Malgorzata Mis and Jane Hancock for the recordings and data used in Figure 1 and Pfizer Neusentis for the cells used by Dr Mis for the work shown.
References
- Alexandrou AJ, Brown AR, Chapman ML, et al (2016). Subtype‐selective small molecule inhibitors reveal a fundamental role for Nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One 11, e0152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JT, Chin J, Leiser SC, Pangalos MN & Randall AD (2011). Altered intrinsic neuronal excitability and reduced Na+ currents in a mouse model of Alzheimer's disease. Neurobiol Aging 32, 2109e1–2109.e14. [DOI] [PubMed] [Google Scholar]
- Cao L, McDonnell A, Nitzsche A, et al (2016). Pharmacological reversal of a pain phenotype in iPSC‐derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 8, 335ra56. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang X‐J, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi S‐H & Studer L (2012). Combined small‐molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Nagel D, Parri R & Coleman M (2016). Stem cell‐derived astrocytes: are they physiologically credible? J Physiol 594, 6595–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James OT, Livesey MR, Qiu J, Dando O, Bilican B, Haghi G, Rajan R, Burr K, Hardingham GE, Chandran S, Kind PC & Wyllie DJA (2014). Ionotropic GABA and glycine receptor subunit composition in human pluripotent stem cell‐derived excitatory cortical neurones. J Physiol 592, 4353–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun AX et al (2016). Midbrain‐like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin‐producing neurons. Cell Stem Cell 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PJ, Rushton DJ, Yarova PL, Schnell C, Geater C, Hancock JM, Wieland A, Hughes A, Badder L, Cope E, Riccardi D, Randall AD, Brown JT, Allen ND & Telezhkin V (2016). Improving and accelerating the differentiation and functional maturation of human stem cell‐derived neurons: role of extracellular calcium and GABA. J Physiol 594, 6583–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T & Somogyi P (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA & Knoblich JA (2014. a). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Lancaster MA & Knoblich JA (2014. b). Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9, 2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C‐A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP & Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Bilican B, Qiu J, Rzechorzek NM, Haghi G, Burr K, Hardingham GE, Chandran S & Wyllie DJA (2014). Maturation of AMPAR composition and the GABAAR reversal potential in hPSC‐derived cortical neurons. J Neurosci 34, 4070–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Magnani D, Hardingham GE, Chandran S & Wyllie DJA (2016). Functional properties of in vitro excitatory cortical neurons derived from human pluripotent stem cells. J Physio 594, 6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P & Livesey FJ (2012. a). Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7, 1836–1846. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HPC & Livesey FJ (2012. b). Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 15, 477–486, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telezhkin V, Schnell C, Yarova P et al (2016). Forced cell cycle exit and modulation of GABAA, CREB, and GSK3β signalling promote functional maturation of induced pluripotent stem cell‐derived neurons. Am J Physiol Cell Physiol 310, C520–C541. [DOI] [PubMed] [Google Scholar]
- Young GT, Gutteridge A, Fox HDE, Wilbrey AL, Cao L, Cho LT, Brown AR, Benn CL, Kammonen LR, Friedman JH, Bictash M, Whiting P, Bilsland JG & Stevens EB (2014). Characterizing human stem cell‐derived sensory neurons at the single‐cell level reveals their ion channel expression and utility in pain research. Mol Ther 22, 1530–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]


