Abstract
Neurons differentiated from pluripotent stem cells using established neural culture conditions often exhibit functional deficits. Recently, we have developed enhanced media which both synchronize the neurogenesis of pluripotent stem cell‐derived neural progenitors and accelerate their functional maturation; together these media are termed SynaptoJuice. This pair of media are pro‐synaptogenic and generate authentic, mature synaptic networks of connected forebrain neurons from a variety of induced pluripotent and embryonic stem cell lines. Such enhanced rate and extent of synchronized maturation of pluripotent stem cell‐derived neural progenitor cells generates neurons which are characterized by a relatively hyperpolarized resting membrane potential, higher spontaneous and induced action potential activity, enhanced synaptic activity, more complete development of a mature inhibitory GABAA receptor phenotype and faster production of electrical network activity when compared to standard differentiation media. This entire process – from pre‐patterned neural progenitor to active neuron – takes 3 weeks or less, making it an ideal platform for drug discovery and disease modelling in the fields of human neurodegenerative and neuropsychiatric disorders, such as Huntington's disease, Parkinson's disease, Alzheimer's disease and Schizophrenia.
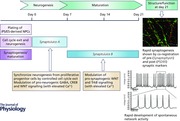
Introduction
Since the original isolation and characterization of human embryonic stem cells (ESCs) in 1998 (Thomson et al. 1998), followed by the discovery some 10 years later of human somatic cell reprogramming into induced pluripotent stem cells (iPSCs) (Takahashi et al. 2007), pluripotent stem cells have been regularly hailed as a panacea for understanding and, potentially, treating an ever increasing list of human disorders, providing the raw material for cell replacement therapies, disease modelling and drug screening. Review of clinical trials shows that pluripotent stem cells have been employed with variable success as cell replacements in a variety of disorders, either directly or via a population of artificial support matrices, including tracheal replacement (Jungebluth et al. 2011), retinal dysfunction (Zarbin, 2016), spinal cord injury (Lukovic et al. 2014) and several neurodegenerative diseases (Levy et al. 2016). Unlike replacement therapies, which require the use of either the pluripotent stem cells themselves or pre‐patterned/pre‐specified cell lineage‐specific precursors, disease modelling and drug screening frequently have the explicit requirement for a population of terminally differentiated cells of the type affected in the disease in question. This requirement has led to the rapid and spirited development of protocols aimed at directing differentiation of pluripotent stem cells to correctly specified cells. Such developments have been particularly competitive and often controversial in the field of neuroscience where the scope for mixed populations of neurons is high but often undesirable when the exact cellular composition in vitro may not yet be fully defined.
Independently of specification of a particular neuronal cell type, it is absolutely imperative that differentiated neurons demonstrate appropriate cell biological characteristics and functional activities. In the field of neuronal differentiation, the efficiency and efficacy of the majority of protocols is assessed using an array of established cell‐specific markers employing a combination of immunocytochemistry (ICC), reverse transcription polymerase chain reaction (RT‐PCR) and, more recently, RNA sequencing (RNAseq) and proteomic analyses. However, except in a handful of exceptional studies (Johnson et al. 2007; Song et al. 2013; Livesey et al. 2014; Telias et al. 2014; Bardy et al. 2015), integration of functional assays of neuronal function is rare and, where reported, is normally restricted to single examples of induced action potentials and/or miniature synaptic potentials, with no further analyses. This relative dearth of functional information regarding appropriate neuronal activity is troublesome when such protocols are used to develop meaningful disease models and high‐throughput drug screens. In other words, it is unclear whether particular protocols consistently generate functionally active neurons whose activity has not been robustly characterized or, more worryingly, that the protocols do not produce neurons with robust electrophysiological activity characteristic of the neurons being studied. Indeed, we and others have found that published neuronal differentiation protocols generate neurons which express the appropriate marker molecules but which often fail to generate authentic neuronal activity with any consistency across the population. With this in mind, recent work in several laboratories, including our own, has focused on improving basic neuronal function during the in vitro differentiation process using small molecule regulators of specific cell biological cascades and manoeuvres aimed at mimicking physiological and environmental cues which are known to be important during neuronal differentiation in vivo in order to improve function and to stimulate synaptogenesis and consequent neuronal network activity in vitro.
Regulation of physiological and of extracellular free ionized calcium and their potential roles in neuronal differentiation
Neuronal specification and differentiation in vivo occur prenatally in an environment which is markedly different from that postnatally (Mohyeldin et al. 2010). Of particular significance is the partial pressure of oxygen (), which rapidly changes from around 20–30 to 150–160 mmHg following the first breath of the emerging neonate (Ward, 2008). Such low , or relative hypoxia, has been successfully employed to improve the efficiency of the generation of iPSCs (Bilican et al. 2012; Hawkins et al. 2013) and a handful of studies have investigated the effect of such relative hypoxia on subsequent neuralization, specification and differentiation of neurons (e.g. Studer et al. 2000; Bilican et al. 2014).
Less well appreciated is the parallel switch in extracellular free ionized Ca2+ concentration ([Ca2+]o) which occurs in the perinatal period. Thus, human fetal plasma [Ca2+]o is ∼1.6–1.7 mm (Kovacs & Kronenberg, 1997) and this is rapidly reduced within 24 h postnatally to the mean adult level of 1.1–1.3 mm (Brown, 1991); the relative hypercalcaemia of the developing fetus compared to maternal levels is retained independently of any changes in the maternal [Ca2+]o (Kovacs et al. 1998). In health, the relatively low postnatal [Ca2+]o persists and is strictly regulated within a very narrow range by the action of parathyroid hormone on the kidney, intestine and bone, a homeostatic process which is critically dependent upon the activation by of the extracellular Ca2+‐sensing receptor (CaSR) expressed in the parathyroid gland (Brown et al. 1993; Riccardi & Kemp, 2012). The striking transition from high prenatal to low postnatal [Ca2+]o is mediated by the CaSR (Kovacs et al. 1998).
Many commercially available tissue culture media contain [Ca2+]o broadly embracing the mean value of postnatal [Ca2+]o in humans of 1.1–1.3 mm (Brown, 1991). With this knowledge in mind, it is puzzling that the majority of neuronal specification and differentiation protocols employ these standard culture media which contain relatively low [Ca2+]o, or hypocalcaemic, conditions for the developing fetus. Indeed, where studied, the effect of increasing [Ca2+]o from adult to fetal values is permissive for neurite outgrowth of prenatal primary neurons isolated from the sympathetic cervical ganglion in vitro (Vizard et al. 2008).
Role of astrocytes and astrocyte‐conditioned medium in neuronal differentiation
It has long been recognized that co‐culture of primary neurons with astrocytes promotes neurogenesis and enhances synaptogenesis both directly (Pfrieger & Barres, 1997; Ullian et al. 2001, 2004; Hama et al. 2004) and indirectly via secreted factors (Chang et al. 2003; Ullian et al. 2004; Chou et al. 2008). Furthermore, maturation of human stem cell‐derived neurons can be enhanced by co‐culture with astrocytes (Johnson et al. 2007) or astrocyte conditioned medium (ACM) (Rushton et al. 2013; Tang et al. 2013). Indeed, we have shown that ACM has the effect of accelerating the functional maturation rate of iPSC‐derived neurons during differentiation by hyperpolarizing the resting membrane potential, thereby increasing the spontaneous activity of the developing neurons, often generating neurons with complex, biphasic patterns of excitability (Rushton et al. 2013). Such relative hyperpolarization, which is seen during differentiation of human iPSCs to neurons in vitro, appears to be physiologically relevant as evidenced by the fact that it can also be observed in vivo, where we have preliminary evidence to show that developmentally regulated expression of Kv7.2/7.3 (M‐type) currents appears to underlie a gradual hyperpolarization of striatal neurons during the second and third trimesters of mouse gestation (Telezhkin et al. 2014).
Previous observations have reported that there are increases in Ca2+ channel expression and/or function early in stem cell‐derived and native NPC differentiation (Arnhold et al. 2000; Mazzanti & Haydon, 2003; D'Ascenzo et al. 2006). This led us to investigate voltage‐gated Ca2+ entry, and the potential role of Ca2+ channels, in the ACM‐evoked enhancement of human iPSC neuronal differentiation (Rushton et al. 2013). Differentiation in ACM evoked a robust enhancement of depolarization‐evoked Ca2+ entry, principally via L‐type, N‐type and R‐type Ca2+ channels, with little contribution of P/Q‐type channels (Aga toxin‐sensitive) (Fig. 1 A). Consistent with the selective upregulation of Ca2+ influx through voltage‐gated Ca2+ channels, chronic blockade of L‐type (nifedipine), N‐type (conotoxin), or R‐type (SNX‐482) Ca2+ channels during the differentiation process resulted in complete abolishment of the ACM‐evoked enhancements of spontaneous activity and significantly depolarized resting membrane potential (Fig. 1 B). Furthermore, similar diminutions were seen when ionotropic GABAA receptors were inhibited using bicuculline, the first suggestion that a major contributor to Ca2+ influx during differentiation might be provided by excitatory GABAergic synaptic input in these developing neuronal networks (Fig. 1 B). However, it should be noted that no direct comparison of [Ca2+]i in the two conditions was attempted in these studies.
Figure 1. Potential mechanisms of ACM‐evoked enhancement of neuronal function.
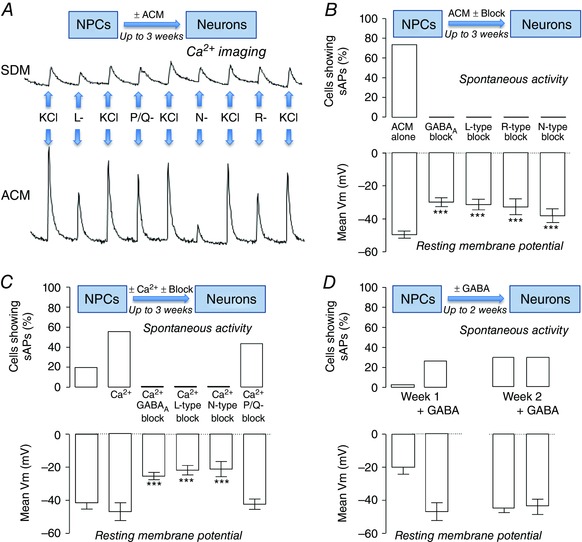
A, Fura‐2‐derived relative intracellular Ca2+ concentration measurements for iPSC‐derived NPCs after 1 week of differentiation in either standard differentiation medium (SDM, upper trace) or (ACM, lower trace). Ca2+ influx was evoked by depolarization using 50 mm KCl in the absence and presence of 10 μm nifedipine (L‐type Ca2+ channel blockade), 100 nm conotoxin (N‐type Ca2+ channel blockade), 100 nm agatoxin (P/Q Ca2+ channel blockade) or 100 nm SNX482 (R‐type Ca2+channel inhibition). B, bar graphs comparing the proportion of neurons firing spontaneous action potentials (sAPs, upper bars) and mean resting membrane potentials (V m,, lower bars) following 3 weeks of differentiation in ACM alone, or ACM with 10 μm bicuculline, 2 μm nifedipine, 100 nm conotoxin or 100 nm SNX482. C, comparison of the proportion of neurons firing sAPs (upper bars) and mean V m (lower bars) following 3 weeks of differentiation in 1.2 or 1.8 mm [Ca2+]o in the presence of 10 μm bicuculline, 2 μm nifedipine, 100 nm conotoxin or 100 nm agatoxin. D, comparison of the proportion of cells firing sAPs (upper bars) and mean V m (lower bars) following 1 and 2 weeks of differentiation in SDM with and without 300 μm GABA. Different from control, *** P < 0.001. Adapted from Rushton et al. (2013).
Roles for GABAA receptors and extracellular Ca2+ in neuronal differentiation
The fact that the robust enhancement of function supported by ACM was characterized by increased Ca2+ influx through specific Ca2+ channels, and that it could be attenuated by their inhibition, suggested that the mechanism of the ACM effect might involve chronic augmentation of intracellular free ionized calcium concentration ([Ca2+]i) via GABAA‐dependent excitation. In order to test this possibility, we attempted to mimic, at least early in the differentiation process, the permissive effects of ACM by chronically raising the extracellular GABA concentration, and the [Ca2+]o to a level similar to that measured in fetal plasma, namely 1.8 mm (Kovacs & Kronenberg, 1997). These manoeuvres allowed the study of the efficiency and efficacy of differentiation of iPSC‐derived neural progenitor cells (NPCs) to neurons under conditions where the [Ca2+]i was predicted to have been increased tonically (Rushton et al. 2013), although it must be noted that no direct comparison of [Ca2+]i in the two conditions was attempted in these studies.
Thus, differentiating the NPCs for 3 weeks (which is 35 days from the pluripotent stem cell stage), in medium containing 1.8 mm resulted in an increase in spontaneous activity and a significant hyperpolarization. These effects were attenuated by co‐incubation for the entire differentiation with inhibitors of GABAA receptors or blockers of L‐type, N‐type or R‐type Ca2+ channels; consistent with the Ca2+ imaging data presented in Fig. 1 A, blockade of P/Q channels had no effect on the ability of raised [Ca2+]o to augment spontaneous activity and to hyperpolarize the resting membrane potential (Fig. 1 C). Note that the potent Ca2+ channel opener BayK 8644 was also able to enhance spontaneous activity and to hyperpolarize the neurons in standard [Ca2+]o (Rushton et al. 2013).
Chronic incubation of differentiating iPSCs with GABA evoked a significant increase in spontaneous activity and hyperpolarization at week 1. However, this augmentation in function was not further enhanced at week 2 (Fig. 1 D), suggesting that GABA might only be useful in promoting neuronal maturation early in the differentiation programme. The idea that GABA might provide the stimulus for influx early in the neuronal differentiation/maturation process, perhaps both in vivo and in vitro, is consistent with knowledge that ionotropic GABAA receptors are actually excitatory in prenatal neurons. Thus, GABA binding elicits Cl− exit and consequent depolarization (Ganguly et al. 2001; Ben‐Ari, 2002; Tozuka et al. 2005). The postnatal transition from excitatory to inhibitory is a result of the developmentally modulated Cl− equilibrium potential, which is itself controlled by the differential expression of Na+–K+–2Cl− cotransporters (NKCC1) and K+–Cl− cotransporters (KCC2) (Ganguly et al. 2001). Consequently, the GABAA phenotype (excitatory or inhibitory) of differentiating stem cells is a reliable functional readout for immature versus mature neurons. Human stem cell‐derived neurons demonstrate robust GABAA currents and GABA‐evoked influx even at early stages of neuronal differentiation (Joannides et al. 2007; HD iPSC Consortium, 2012) and GABA‐dependent excitation has been shown to promote neuronal differentiation in several systems, including adult hippocampal stem/progenitors (Tozuka et al. 2005) and neuroectodermal stem cells (Jelitai et al. 2004). Therefore, it seemed likely that GABA might promote neuronal differentiation of iPSCs. Interestingly, although there is transition from excitatory to inhibitory GABA phenotype during early differentiation, ACM does not significantly alter the time course of this event (Rushton et al. 2013), suggesting that regulated GABAA, NKCC1 and KCC2 expression do not contribute significantly to ACM‐induced neuronal maturation per se (Rushton et al. 2013). The GABA transition has also been well characterized during differentiation of human stem cells to cortical neurons (Livesey et al. 2014); this study also showed time‐dependent alteration in the expression of AMPA receptor composition. Whether this process is sensitive to [Ca2+]o is not known (Livesey et al. 2014).
Integrating GABA and raised [Ca2+]o into a pro‐synaptogenic differentiation protocol
Having determined that maintaining [Ca2+]o at 1.8 mm throughout the 3 week differentiation period and that GABA stimulation early in the process were both able to support enhanced neuronal function, as determined by observation of the frequency of spontaneous electrical activity and relative hyperpolarization, we began the rational design of a pair of media which would augment and/or accelerate functional maturation of iPSC‐derived neurons. Medium 1 contained the high [Ca2+]o together with GABA (used during the first week following plate‐down of NPCs) whilst medium 2 contained just the high [Ca2+]o (used from 1 week onwards). With the addition of brain‐derived neurotrophic factor (BDNF) to both, these media were termed base media, and to them various small molecules were added. For these particular experiments, pre‐patterning to NPCs took 16 days, meaning the entire process, from pluripotent stem cell stage to mature neurons took 35–37 days.
One of the major problems associated with neuronal differentiation from stem cells is the lack of synchronicity in the process. This means that neurons can be ‘born’ from NPCs at almost any time during the differentiation and this results in distinct lack of neuronal functional homogeneity. To address this issue, we employed inhibitors of both Notch signalling and the cell cycle checkpoint cyclin‐dependent kinases CDK4 and CDK6 in order to promote cell cycle exit (Telezhkin et al. 2016). Blockade of Notch signalling with the γ‐secretase inhibitor N‐[N‐(3,5‐difluorophenacetyl‐l‐alanyl)]‐(S)‐phenylglycine t‐butyl ester (DAPT) or CDK4/6 kinase activity with the selective inhibitor PD0332991(Toogood et al. 2005) significantly reduced the proportion of NPCs actively proliferating at 3 and 7 days post‐plate‐down. In combination, these small molecules evoked synchronous, sustained cell cycle exit (Fig. 2 A and B) and enhanced neural differentiation, as shown by an increase in the proportion of cells expressing the neuronal marker, β‐III‐tubulin, and a marked reduction in the neural progenitor marker nestin at 7 days (Telezhkin et al. 2016). These data suggested that these molecules should be added to the base media.
Figure 2. Synchronous cell cycle exit and differentiation are by promoted γ‐secretase and cyclin dependent kinase 4/6 inhibition.
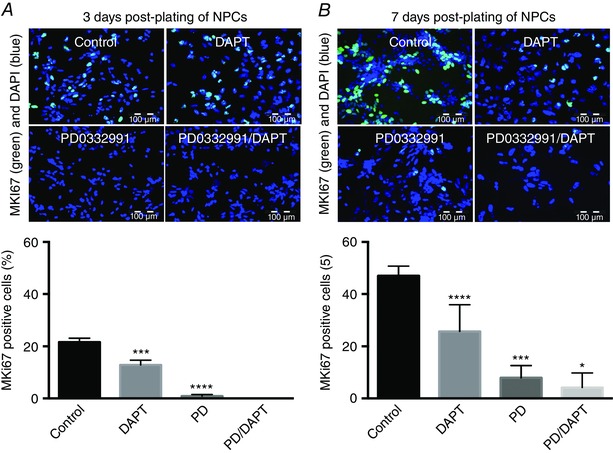
Exemplar fluorescence micrographs (upper) and mean cell counts (lower) of MKI67 immunoreactivity (green) and cell nuclear staining with DAPI (blue) of neural progenitor cells (NPCs) 3 (A) and 7 days (B) post‐plating in base medium with no additions (Control), or base medium supplemented with 2 μm PD0332991 or 10 μm DAPT, or both. Different from control, *** P < 0.001, ** P < 0.01, * P < 0.05. Scale bars: 100 μm. Adapted from Telezhkin et al. (2016).
It is well known that GABAA receptor activation leads to Ca2+‐dependent cAMP response element‐binding protein (CREB) and extracellular signal‐regulated kinase (ERK) phosphorylation, which positively regulates neurogenic gene expression (Merz et al. 2011; Wiegert & Bading, 2011). Furthermore, functional expression of neuronal voltage‐gated Na+ and K+ channels during differentiation can also be affected by agents that elevate cAMP (Aglah et al. 2008; Ravni et al. 2008). Thus, medium 1, which already contained high [Ca2+]o and GABA to drive the GABAA‐dependent increase in [Ca2+]i, was supplemented with forskolin to raise intracellular [cAMP]i, leading to CREB phosphorylation (Telezhkin et al. 2016).
As GABA‐dependent CREB phosphorylation has a transient developmental role in neurogenesis (Jagasia et al. 2009), GABA and forskolin were removed from medium 2, to encourage maturation during the later phase differentiation. Similarly, since the pro‐neurogenic effects of Notch inhibition are only needed early in neurogenesis, and sustained γ‐secretase inhibition can be deleterious (Shen, 2014), DAPT was also removed from medium 2.
A directed screen of growth factors and small molecules was then performed in order to identify compounds which enhance neuronal excitability. Little or no enhancement in excitability was observed with BDNF, fibroblast growth factor 2 (FGF2), insulin‐like growth factor 1 (IGF1) or activin A. However, the glycogen synthase kinase 3β (GSK3β) antagonist CHIR99021 did increase action potential firing, which prompted us to incorporate it into both media for further analysis. CHIR99021 has been previously demonstrated to promote post‐mitotic nociceptor neural differentiation, although this was in combination with DAPT (Chambers et al. 2012).
Functional consequences of neuronal differentiation in the enhanced media
With the components of the two media complete, plated NPCs derived from several iPSC sources, derived using both integrating and non‐integrating reprogramming vectors, were differentiated for 1 week in medium 1, and 2 more weeks in medium 2. Together, these two media are now termed SynaptoJuice (www.synaptojuice.co.uk). The potential benefits of using these media were determined by comparing their effects on electrical activity and neurotransmitter responses with those seen in base medium and standard differentiation medium (Telezhkin et al. 2016). Differentiation in the enhanced media yielded the highest proportion of neurons demonstrating spontaneous electrical activity (Fig. 3 A and B), which was consistent with the data showing that such neurons displayed the most hyperpolarized membrane potentials and suggested that there was a more rapid transition from inactive to active state via evocation of relative hyperpolarization (Fig. 3 C). Such a notion is again entirely consistent with our preliminary observations that M‐currents are developmentally regulated both in vivo and in vitro (Telezhkin et al. 2014).
Figure 3. Enhanced differentiation media (SynaptoJuice) augment the generation of neurons which exhibit spontaneous action potentials.
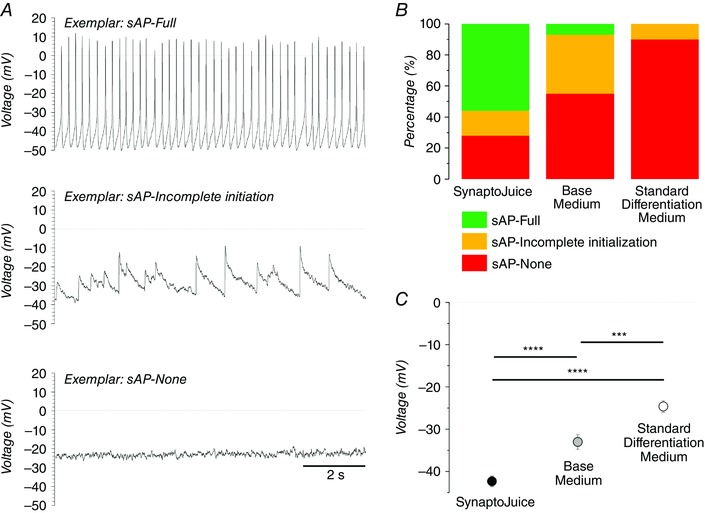
A, typical current‐clamp recordings (I = 0 pA) illustrating the three separate types of activity which were observed spontaneously in the neurons: spontaneous action potentials (sAP‐full, upper); incomplete initiation of spontaneous action potentials (sAP–incomplete initiation, middle); and no spontaneous action potentials (sAP–None, lower). Note that each sAP type is also the modal activity for neurons differentiated in SynaptoJuice, base medium and standard differentiation medium, respectively. B, proportions of neurons which demonstrated each of the individual sAP characteristics when NPCs were differentiated into neurons for 3 weeks in the enhanced media (SynpatoJuice), base medium and standard differentiation medium (i.e. 35 days from pluripotent stem cells). C, mean resting membrane potentials (V m) of neurons differentiated for 3 weeks in SynpatoJuice, base medium and standard differentiation medium. *** P < 0.001, **** P < 0.0001. Time bar applies to all traces. Adapted from Telezhkin et al. (2016).
In terms of induced electrical activity, differentiation in enhanced media supported the highest proportion of neurons exhibiting action potential trains compared to those differentiated in base or standard media (Fig. 4; Telezhkin et al. 2016); and the current injection vs. spike frequency plots showed that the enhanced media supported the greatest excitability (Telezhkin et al. 2016). Differentiation in the enhanced media generated neurons with significantly shorter half‐widths and larger overshoots, afterhyperpolarizations, spike amplitudes and depolarization rates (Telezhkin et al. 2016). Surprisingly, neither voltage‐activated Na+ nor K+ current densities were affected by the differentiation protocol. However, differentiation in the enhanced media generated neurons which displayed significantly altered Na+ current activation/inactivation profiles, resulting in larger availability windows and a higher proportion of neurons with V m values falling within those windows than did either base or standard media resulting in the probability of a neuron firing a spontaneous action potential being increased (Telezhkin et al. 2016). These data suggested that the enhanced media improved functional maturation by facilitating neuronal hyperpolarization (to support higher spontaneous activity), and by increasing Na+ current availability (to enhance regenerative action potential train activity).
Figure 4. Enhanced differentiation media (SynaptoJuice) augment the generation of neurons exhibiting induced action potential train.
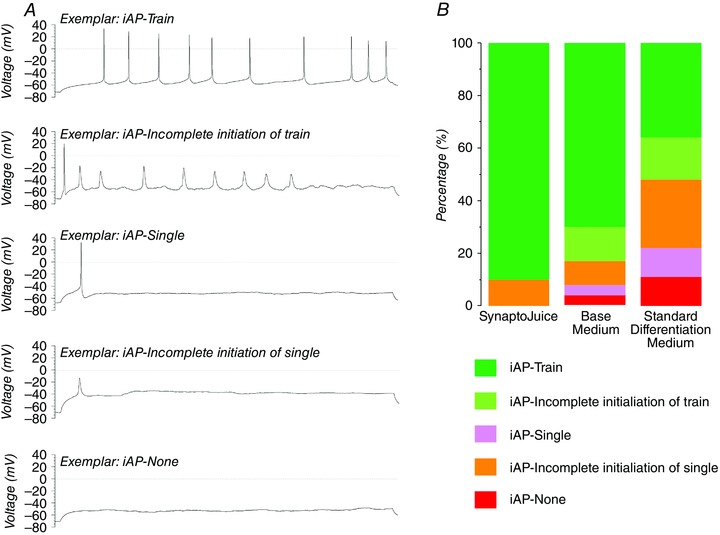
A, exemplar current‐clamp recordings (I = 0 pA) illustrating the five separate types of induced activity which were observed in the neurons: train of induced action potentials (iAP–Train); single action potential with incomplete initiation of action potential trains (iAP–Incomplete initiation); single action potential (iAP–Single); incomplete initiation of single action potential (iPS–Incomplete single); and no induced action potential (iAP–None, lower). Time bar applies to all traces. Adapted from Telezhkin et al. (2016). B, proportions of neurons which demonstrated each of the individual iAP characteristics when NPCs were differentiated into neurons for 3 weeks in the enhanced media (SynpatoJuice), base medium and standard differentiation medium (i.e. for 35 days from pluripotent stem cells).
To examine whether any set of media was able to support functional synaptogenesis, spontaneous miniature synaptic currents (minis), evoked postsynaptic currents, neurotransmitter‐evoked changes in intracellular calcium concentration ([Ca2+]i) and network activity were determined. Cells differentiated in standard medium for 3 weeks never exhibited miniature synaptic currents. Differentiation in base media only rarely supported the generation of GABAergic minis. In contrast, up to 75% of neurons differentiated in the enhanced media exhibited large GABAergic and modest glutamatergic minis. All cells differentiated in any of the media exhibited GABA‐evoked currents, whereas there was a distinct enhancement in the proportion of cells exhibiting, and the magnitude of, NMDA‐evoked currents in the enhanced media. These observations were supported by the demonstration of punctate, presumably synaptic, staining of NR1, NR2A and NR2B NMDA receptor subunits, co‐registration of the pre‐ and postsynaptic markers, synaptophysin and PSD95 and the appearance of dendritic spines exclusively in the neurons differentiated in the enhanced media (Telezhkin et al. 2016).
As the enhanced media appeared to improve the extent of functional neuronal maturation, weekly imaging of the neurotransmitter responses was performed in order to determine the time course of this enhancement. From a very low level at the time of plating the NPCs, most of the cells from all three protocols responded to a depolarizing challenge at 1 week of differentiation and thereafter (Telezhkin et al. 2016). However, from 2 weeks onwards, the magnitude of the responses was significantly lower for neurons differentiated using either standard or base media and this persisted until 3 weeks in standard medium (Telezhkin et al. 2016). This was consistent with augmented expression of voltage‐activated Ca2+ channels being supported by the enhanced media, which has been previously documented to occur with ACM (Rushton et al. 2013). The proportion of neurons responding, and the magnitude of the Ca2+ response, to glutamate/glycine increased during differentiation, but the enhanced media encouraged a more rapid augmentation which was delayed by a week in base medium and was never enhanced in standard differentiation medium (Fig. 5 A–C). Likewise, the proportion of cells responding, and the magnitude of the response, to GABA in low [Cl−]o remained significantly smaller for standard and base media (Fig. 5 A–C). Furthermore, since the response to GABA in normal [Cl−]o is potentially composed of both excitatory GABAA and facilitatory GABAB (Karls & Mynlieff, 2015) receptor responses, it seems reasonable to assume that our enhanced media, which largely abrogate this response to GABA in normal [Cl−]o (Fig. 5 C), are promoting the transition from immature to mature GABAA phenotype and, possibly, causing a reduction in GABAB expression. As little is known about the ontogeny of GABAB‐dependent Ca2+ signalling in developing neurons, but it is known that GABAA responses transition from excitatory to inhibitory, loss of GABAB might also be an indication of maturity in pluripotent stem cell‐derived neurons. This, and the precise timing of the functional switch in GABAA phenotype, which is promoted by the enhanced differentiation media, are currently under investigation.
Figure 5. Enhanced differentiation media (SynaptoJuice) improve time‐dependent maturation of postsynaptic neurotransmitter responses and supports regulatable network activity.
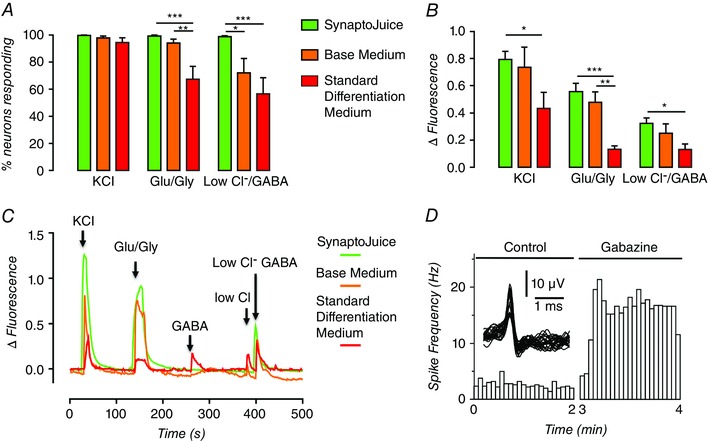
A, proportion of neurons displaying Ca2+ influx in response to 60 mm KCl (KCl), 300 μm glutamate/30 μm glycine (Glu/Gly) and 300 μm GABA in 7.5 mm chloride (Low Cl−/GABA) following differentiation for 3 weeks of NPCs (i.e. 35 days from pluripotent stem cells) from NPCs in SynpatoJuice, base medium, and standard differentiation medium. B, mean magnitude of Ca2+ influx, expressed as change in fluorescence from baseline (ΔFluorescence) of the same neurons used for the analysis shown in panel A. *** P < 0.001, ** P < 0.01, * P < 0.05. C, exemplar fura‐2‐dervied intracellular Ca2+ recordings from individual neurons of the cohort shown in panels A and B. Note the response to 300 μm GABA alone (GABA) was large in neurons in standard differentiation medium, small in base medium and often not detectable in enhanced differentiation media. D, exemplar recording of spontaneous network spike rate before (left) and following (right) the addition of 5 μm gabazine to one of 24 wells in a multi‐well microelectrode array. Note the split time axis for addition of drug. Single unit activity is shown as an insert. Neurons were differentiated from NPCs for 3 weeks in enhanced differentiation medium (i.e. 35 days from pluripotent stem cells. Adapted from Telezhkin et al. (2016).
Finally, evidence that the enhanced media supported the formation of excitatory synaptic networks whose activity was being tonically dampened by inhibitory GABAergic tone was provided by experiments using 24‐well microelectrode array (MEA) plates which showed that large and maintained increases in activity could be evoked by the addition of gabazine, a GABAA receptor inhibitor (Fig. 5 D). Furthermore, glutamate was able to stimulate activity whilst tetrodotoxin completed abolished activity in all wells (Telezhkin et al. 2016).
Comparison with alternative differentiation media and protocols
Thus, our recent published studies support the notion that forced cell cycle exit is able to synchronize neuronal differentiation. That simple manoeuvre, coupled to simulating fetal conditions by increasing [Ca2+]o throughout whilst transiently generating a GABA excitatory drive and tonically applying known pro‐synaptogenic small molecules (CHIR 99021 and forskolin), results in rapid functional maturation of neurons with highly homogeneous response profiles. Although there has been sustained activity for over a decade aimed at producing neurons from a variety of stem cell sources, it is only recently that there has been brisk and highly competitive progress towards generating demonstrably functional neurons exhibiting characteristically appropriate synaptic properties in vitro. There have been several other carefully performed recent studies which have demonstrated maturation by a variety of functional endpoints (Song et al. 2013; Livesey et al. 2014; Telias et al. 2014; Bardy et al. 2015). The study by Song and colleagues analysed the electrophysiological time course of pluripotent stem cell‐derived neuronal differentiation (Song et al. 2013). Although their data are in broad agreement with ours, the homogeneity of the neurons with respect to repetitive action potential train generation is more similar at 3 weeks to those generated by our standard differentiation medium. Even after a further week, the Song protocol still only supported repetitive firing in under 50% of the neurons, likely to be due to the lack of synchronization of the differentiation programme that our enhanced media afford early in neurogenesis. Furthermore, their induced action potentials have lower spike amplitudes, slow depolarization rates and long half‐widths, again more reminiscent of those we see in standard differentiation medium. The Livesey protocol (Livesey et al. 2014) used several important readouts of neuronal maturation, including GABAA phenotype (Ganguly et al. 2001). They observed GABAA response transition from excitatory to inhibitory during the 7 week differentiation, which was due to progressive reduction in chloride equilibrium potential (E Cl) as the expression of NKCC1 and KCC2 was reduced and enhanced, respectively (Rivera et al. 1999; Yamada et al. 2004); no data were available between 1 and 5 weeks. With our enhanced media, inhibition of GABAergic signalling with gabazine resulted in a dramatic increase in neuronal network activity, which suggests that even by 3 weeks synaptic GABA was inhibitory across the network. In the Telias protocol (Telias et al. 2014) neurons differentiated from human ESCs demonstrated significant maturation properties by 3 weeks, including a high proportion of neurons with train activity. However, input resistance was high and individual action potential amplitudes were very low compared to neurons differentiated in our enhanced media. Their use of DAPT was encouraging and might have been expected partially to synchronize the neuronal maturation. However, there were no data presented regarding potential synchronization and it might be more prudent to use PD0332991 with DAPT, as we have now shown (Telezhkin et al. 2016). Finally, and of particular note, there is the exceptionally elegant study of Bardy and colleagues who, using an evidence‐based approach, systematically manipulated the composition of their medium in order to optimize neuronal functional readouts (Bardy et al. 2015). This medium supported high levels of synaptic function in both primary neurons and differentiated stem cells. Now termed BrainPhys (Stem Cell Technologies), this medium contains, in contrast to SynaptoJuice, adult [Ca2+]o. Compared to our enhanced media, activity appears to take longer to develop, but because the authors have shown that it supports neuronal activity for many weeks longer than SynaptoJuice has been assayed so far, it could well be a perfect candidate for the ‘third’ medium for use after our enhanced media, in order to support long‐term ageing of neurons, something that will be important for successful modelling of late‐onset neurodegenerative diseases.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The authors would like to thank the CHDI Foundation, an FP7 collaborative grant (Repair‐HD), and the NC3Rs (Crack‐it challenge) for funding parts of this work.
Biographies
Paul J. Kemp completed his DPhil in Physiology in the Department of Human Anatomy at the University of Oxford in 1990. Since then he has held academic positions in the University of Dundee, the University of Southern California and the University of Leeds. Since 2004, he has been Professor of Physiology at Cardiff University School of Biosciences where his group works on gas sensing by ion channels, novel asthma therapeutics (with Daniela Riccardi) and fate determination of human pluripotent stem cells (with Nicholas D Allen).

Vsevolod Telezhkin was trained in the Bogomoletz Institute in Kiev where he obtained his PhD in 2003. Following several successful post‐doctoral positions at the Universities of Vermont, Bath and Cardiff, he worked at University College London with Professor David Brown, FRS, with whom he published a series of important papers on regulation of M‐channels by lipids. Currently, he is an associate at Cardiff University where his main interest is in the role of M‐channels in pluripotent stem cell differentiation and neuronal maturation.
This review was presented at the symposium “Are stem cell‐derived neural cells physiologically credible?”, which took place at Physiology 2015 in Cardiff, UK, 6–8 July 2015.
References
- Aglah C, Gordon T & Posse de Chaves EI (2008). cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology 55, 8–17. [DOI] [PubMed] [Google Scholar]
- Arnhold S, Andressen C, Angelov DN, Vajna R, Volsen SG, Hescheler J & Addicks K (2000). Embryonic stem‐cell derived neurones express a maturation dependent pattern of voltage‐gated calcium channels and calcium‐binding proteins. Int J Dev Neurosci 18, 201–212. [DOI] [PubMed] [Google Scholar]
- Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Bohnke L, Boyer L, Simon S & Gage FH (2015). Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci USA 112, E2725–E2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari Y (2002). Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3, 728–739. [DOI] [PubMed] [Google Scholar]
- Bilican B, Livesey MR, Haghi G, Qiu J, Burr K, Siller R, Hardingham GE, Wyllie DJ & Chandran S (2014). Physiological normoxia and absence of EGF is required for the long‐term propagation of anterior neural precursors from human pluripotent cells. PloS One 9, e85932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, Park IH, Friedman BA, Daley GQ, Wyllie DJ, Hardingham GE, Wilmut I, Finkbeiner S, Maniatis T, Shaw CE & Chandran S (2012). Mutant induced pluripotent stem cell lines recapitulate aspects of TDP‐43 proteinopathies and reveal cell‐specific vulnerability. Proc Natl Acad Sci USA 109, 5803–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM (1991). Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 71, 371–411. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J & Hebert SC (1993). Cloning and characterization of an extracellular Ca2+‐sensing receptor from bovine parathyroid. Nature 366, 575–580. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH & Studer L (2012). Combined small‐molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MY, Son H, Lee YS & Lee SH (2003). Neurons and astrocytes secrete factors that cause stem cells to differentiate into neurons and astrocytes, respectively. Mol Cell Neurosci 23, 414–426. [DOI] [PubMed] [Google Scholar]
- Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW & Chern Y (2008). Expanded‐polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci 28, 3277–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB & Grassi C (2006). Role of L‐type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci 23, 935–944. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST & Poo M (2001). GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105, 521–532. [DOI] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K & Miyawaki A (2004). PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron 41, 405–415. [DOI] [PubMed] [Google Scholar]
- Hawkins KE, Sharp TV & McKay TR (2013). The role of hypoxia in stem cell potency and differentiation. Regen Med 8, 771–782. [DOI] [PubMed] [Google Scholar]
- HD iPSC Consortium (2012). Induced pluripotent stem cells from patients with Huntington's disease show CAG‐repeat‐expansion‐associated phenotypes. Cell Stem Cell 11, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus‐Kessler T, Saxe M, Gage FH, Song H & Lie DC (2009). GABA‐cAMP response element‐binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci 29, 7966–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitai M, Anderova M, Marko K, Kekesi K, Koncz P, Sykova E & Madarasz E (2004). Role of γ‐aminobutyric acid in early neuronal development: studies with an embryonic neuroectodermal stem cell clone. J Neurosci Res 76, 801–811. [DOI] [PubMed] [Google Scholar]
- Joannides AJ, Webber DJ, Raineteau O, Kelly C, Irvine KA, Watts C, Rosser AE, Kemp PJ, Blakemore WF, Compston A, Caldwell MA, Allen ND & Chandran S (2007). Environmental signals regulate lineage choice and temporal maturation of neural stem cells from human embryonic stem cells. Brain 130, 1263–1275. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA & Zhang SC (2007). Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 27, 3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozoky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, Le Guyader S, Henriksson G, Hermanson O, Juto JE, Leidner B, Lilja T, Liska J, Luedde T, Lundin V, Moll G, Nilsson B, Roderburg C, Stromblad S, Sutlu T, Teixeira AI, Watz E, Seifalian A & Macchiarini P (2011). Tracheobronchial transplantation with a stem‐cell‐seeded bioartificial nanocomposite: a proof‐of‐concept study. Lancet 378, 1997–2004. [DOI] [PubMed] [Google Scholar]
- Karls A & Mynlieff M (2015). GABAB receptors couple to Gαq to mediate increases in voltage‐dependent calcium current during development. J Neurochem 135, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS, Ho‐Pao CL, Hunzelman JL, Lanske B, Fox J, Seidman JG, Seidman CE & Kronenberg HM (1998). Regulation of murine fetal‐placental calcium metabolism by the calcium‐sensing receptor. J Clin Invest 101, 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS & Kronenberg HM (1997). Maternal‐fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18, 832–872. [DOI] [PubMed] [Google Scholar]
- Levy M, Boulis N, Rao M & Svendsen CN (2016). Regenerative cellular therapies for neurologic diseases. Brain Res 1638, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Bilican B, Qiu J, Rzechorzek NM, Haghi G, Burr K, Hardingham GE, Chandran S & Wyllie DJ (2014). Maturation of AMPAR composition and the GABAAR reversal potential in hPSC‐derived cortical neurons. J Neurosci 34, 4070–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukovic D, Stojkovic M, Moreno‐Manzano V, Bhattacharya SS & Erceg S (2014). Perspectives and future directions of human pluripotent stem cell‐based therapies: lessons from Geron's clinical trial for spinal cord injury. Stem Cells Dev 23, 1–4. [DOI] [PubMed] [Google Scholar]
- Mazzanti M & Haydon PG (2003). Astrocytes selectively enhance N‐type calcium current in hippocampal neurons. Glia 41, 128–136. [DOI] [PubMed] [Google Scholar]
- Merz K, Herold S & Lie DC (2011). CREB in adult neurogenesis–master and partner in the development of adult‐born neurons? Eur J Neurosci 33, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon‐Muvdi T & Quinones‐Hinojosa A (2010). Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW & Barres BA (1997). Synaptic efficacy enhanced by glial cells in vitro. Science 277, 1684–1687. [DOI] [PubMed] [Google Scholar]
- Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel‐Morel A, Gonzalez BJ, Vaudry H & Eiden LE (2008). A cAMP‐dependent, protein kinase A‐independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol 73, 1688–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi D & Kemp PJ (2012). The calcium‐sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 74, 271–297. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M & Kaila K (1999). The K+/Cl– co‐transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. [DOI] [PubMed] [Google Scholar]
- Rushton DJ, Mattis VB, Svendsen CN, Allen ND & Kemp PJ (2013). Stimulation of GABA‐induced Ca2+ influx enhances maturation of human induced pluripotent stem cell‐derived neurons. PloS One 8, e81031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J (2014). Function and dysfunction of presenilin. Neurodegener Dis 13, 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Mohamad O, Chen D & Yu SP (2013). Coordinated development of voltage‐gated Na+ and K+ currents regulates functional maturation of forebrain neurons derived from human induced pluripotent stem cells. Stem Cells Dev 22, 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B & McKay R (2000). Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 20, 7377–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K & Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhou L, Wagner AM, Marchetto MC, Muotri AR, Gage FH & Chen G (2013). Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells. Stem Cell Res 11, 743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telezhkin V, Schnell C, Yarova P, Yung S, Cope E, Hughes A, Thompson BA, Sanders P, Geater C, Hancock JM, Joy S, Badder L, Connor‐Robson N, Comella A, Straccia M, Bombau G, Brown JT, Canals JM, Randall AD, Allen ND & Kemp PJ (2016). Forced cell cycle exit and modulation of GABAA, CREB, and GSK3β signaling promote functional maturation of induced pluripotent stem cell‐derived neurons. Am J Physiol Cell Physiol 310, C520–C541. [DOI] [PubMed] [Google Scholar]
- Telezhkin V, Thompson BA, Pardo M, Barriga GG, Brown DA, Canals JM, Allen ND & Kemp PJ (2014). Kv7.2/7.3 channels are enhanced during striatal development and promote neuronal functional maturation of iPS cell‐derived neurons. Biophys J 106 (Suppl 1), 142a. [Google Scholar]
- Telias M, Segal M & Ben‐Yosef D (2014). Electrical maturation of neurons derived from human embryonic stem cells. F1000Res 3, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS & Jones JM (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T, Brodfuehrer J, Choi C, Barvian MR & Fry DW (2005). Discovery of a potent and selective inhibitor of cyclin‐dependent kinase 4/6. J Med Chem 48, 2388–2406. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T & Hisatsune T (2005). GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS & Barres BA (2004). Role for glia in synaptogenesis. Glia 47, 209–216. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS & Barres BA (2001). Control of synapse number by glia. Science 291, 657–661. [DOI] [PubMed] [Google Scholar]
- Vizard TN, O'Keeffe GW, Gutierrez H, Kos CH, Riccardi D & Davies AM (2008). Regulation of axonal and dendritic growth by the extracellular calcium‐sensing receptor. Nat Neurosci 11, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP (2008). Oxygen sensors in context. Biochim Biophys Acta 1777, 1–14. [DOI] [PubMed] [Google Scholar]
- Wiegert JS & Bading H (2011). Activity‐dependent calcium signaling and ERK‐MAP kinases in neurons: A link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium 49, 296–305. [DOI] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ & Fukuda A (2004). Cl– uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol 557, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin M (2016). Cell‐based therapy for degenerative retinal disease. Trends Mol Med 22, 115–134. [DOI] [PubMed] [Google Scholar]


