Abstract
Key points
This study explores the nature of the cis retinol that Müller cells in the retina provide to cones for the regeneration of their visual pigment.
We report that the retina visual cycle provides cones exclusively with 11‐cis chromophore in both salamander and mouse and show that this selectivity is dependent on the 11‐cis‐specific cellular retinaldehyde binding protein (CRALBP) present in Müller cells.
Even though salamander blue cones and green rods share the same visual pigment, only blue cones but not green rods are able to dark‐adapt in the retina following a bleach and to use exogenous 9‐cis retinol for pigment regeneration, suggesting that access to the retina visual cycle is cone‐specific and pigment‐independent.
Our results show that the retina produces 11‐cis retinol that can be oxidized and used for pigment regeneration and dark adaptation selectively in cones and not in rods.
Abstract
Chromophore supply by the retinal Müller cells (retina visual cycle) supports the efficient pigment regeneration required for cone photoreceptor function in bright light. Surprisingly, a large fraction of the chromophore produced by dihydroceramide desaturase‐1, the putative all‐trans retinol isomerase in Müller cells, appears to be 9‐cis retinol. In contrast, the canonical retinal pigment epithelium (RPE) visual cycle produces exclusively 11‐cis retinal. Here, we used the different absorption spectra of 9‐cis and 11‐cis pigments to identify the isoform of the chromophore produced by the visual cycle of the intact retina. We found that the spectral sensitivity of salamander and mouse cones dark‐adapted in the isolated retina (with only the retina visual cycle) was similar to that of cones dark‐adapted in the intact eye (with both the RPE and retina visual cycles) and consistent with pure 11‐cis pigment composition. However, in mice lacking the cellular retinaldehyde binding protein (CRALBP), cone spectral sensitivity contained a substantial 9‐cis component. Thus, the retina visual cycle provides cones exclusively with 11‐cis chromophore and this process is mediated by the 11‐cis selective CRALBP in Müller cells. Finally, despite sharing the same pigment, salamander blue cones, but not green rods, recovered their sensitivity in the isolated retina. Exogenous 9‐cis retinol produced robust sensitivity recovery in bleached red and blue cones but not in red and green rods, suggesting that cis retinol oxidation restricts access to the retina visual cycle to cones.
Keywords: cone photoreceptors, CRALBP, dark adaptation, retina, retinol dehydrogenase, visual cycle
Key points
This study explores the nature of the cis retinol that Müller cells in the retina provide to cones for the regeneration of their visual pigment.
We report that the retina visual cycle provides cones exclusively with 11‐cis chromophore in both salamander and mouse and show that this selectivity is dependent on the 11‐cis‐specific cellular retinaldehyde binding protein (CRALBP) present in Müller cells.
Even though salamander blue cones and green rods share the same visual pigment, only blue cones but not green rods are able to dark‐adapt in the retina following a bleach and to use exogenous 9‐cis retinol for pigment regeneration, suggesting that access to the retina visual cycle is cone‐specific and pigment‐independent.
Our results show that the retina produces 11‐cis retinol that can be oxidized and used for pigment regeneration and dark adaptation selectively in cones and not in rods.
Abbreviations
- CRALBP
cellular retinaldehyde binding protein
- DES1
dihydroceramide desaturase‐1
- ERG
electroretinography
- Gnat1
rod transducin α‐subunit
- LED
light‐emitting diode
- λmax
wavelength of maximum absorbance
- M‐cone
middle wavelength‐sensitive cone
- RDH
retinol dehydrogenase
- RPE
retinal pigment epithelium
Introduction
The vertebrate retina has two distinct classes of ciliary photoreceptors, rods and cones, for dim light and bright light vision, respectively (Baylor, 1987). Both photoreceptors have visual pigments (rhodopsin in rods and cone pigment in cones) composed of protein moiety, opsin and chromophore, typically 11‐cis retinal. Upon absorbing light, visual pigment is activated to trigger the phototransduction cascade and subsequently decays into apo‐opsin and photoisomerization product, all‐trans retinal (Ebrey & Koutalos, 2001). Thus, regeneration of the pigment is required for continuous light perception. This is achieved by a biochemical process known as the visual cycle (or retinoid cycle) that recycles all‐trans chromophore back into cis form in retinal pigment epithelium (RPE visual cycle; for both rods and cones) or in retinal glial Müller cells (retina visual cycle; only for cones) (Wang & Kefalov, 2011; Saari, 2012). The retina visual cycle has been suggested to enable cones to adapt to darkness rapidly and to function under bright light by providing cis retinal exclusively to cones faster than the RPE pathway (Mata et al. 2002; Miyazono et al. 2008; Kolesnikov et al. 2011; Xue et al. 2015). In this pathway, all‐trans retinal released from the visual pigment upon its decay is reduced to all‐trans retinol in cones (Ala‐Laurila et al. 2006; Miyazono et al. 2008) and transported to adjacent Müller cells. There, all‐trans retinol is isomerized to cis retinol (Kaylor et al. 2013) and returned to cones where it is oxidized to cis retinal to regenerate visual pigments (Jones et al. 1989; Miyazono et al. 2008; Wang et al. 2009; Wang & Kefalov, 2009).
Recently, dihydroceramide desaturase‐1 (DES1) was identified as the putative retinol isomerase in Müller cells that converts all‐trans retinol to cis retinol isomers (Kaylor et al. 2013). Surprisingly, 9‐cis retinol represents a larger fraction of the DES1 isomerization products than 11‐cis retinol. In addition, purified carp cones have both 11‐cis and 9‐cis retinol oxidation activity (Sato et al. 2013), and rod and cone opsins readily bind 9‐cis retinal to form photosensitive pigment (Pepperberg et al. 1976; Fukada et al. 1990; Makino et al. 1999). Therefore, the 9‐cis isomer may be a physiologically relevant chromophore supplied by the retina visual cycle. Consistent with this notion, 9‐cis retinoids have been detected in dark‐adapted mouse and chicken retinas, with 9‐cis retinal 26‐fold more abundant in the cone‐dominant chicken retina compared to the rod‐dominant mouse retina (Kaylor et al. 2013). Dark‐reared mice with deficient RPE visual cycle also slowly accumulate 9‐cis retinal in rods (Fan et al. 2003). However, the possibility that Müller cells provide 9‐cis chromophore to cones has not been investigated in the intact retina and its significance for the function of cones remains unknown.
The mechanisms regulating access to the retina visual cycle are not well understood. Considering that cis retinol is the probable product of the retina visual cycle (Mata et al. 2002), it would be expected that only photoreceptors capable of oxidizing cis retinol to cis retinal will be able to use it for pigment regeneration (Wang & Kefalov, 2009; Wang et al. 2014). However, investigating the oxidation of 11‐cis retinol in intact photoreceptors has been hampered by the difficulty of obtaining this retinoid and by its low stability (Parker et al. 2011). Thus, identifying a stable and readily available alternative to 11‐cis retinol, such as 9‐cis retinol, would be of substantial value for investigating the molecular mechanisms regulating the retina visual cycle.
Here, we first addressed the possible involvement of 9‐cis retinol in the retina visual cycle by analysing the chromophore content of salamander and mouse cones dark‐adapted with chromophore provided by the Müller cells in the isolated retina. Next, we examined which of four types of salamander photoreceptors, red and blue cones, and red and green rods, can access the retina visual cycle and whether such access requires the ability to utilize cis retinol for pigment regeneration. Our findings extend our understanding of the molecular mechanisms that regulate the regeneration of pigment and the dark adaptation of cone photoreceptors.
Methods
Ethical approval
All experiments were performed in accordance with the principles of UK regulations as described by Grundy (2015) and were approved by the Washington University Animal Studies Committee.
Single‐cell suction recordings
Single‐cell recordings were carried out as previously described (Wang et al. 2009). Briefly, larval tiger salamanders (Ambystoma tigrinum, Charles D. Sullivan Co. Inc., Nashville, TN, USA) were dark‐adapted overnight in darkness, decapitated and double‐pithed. Their eyes were then enucleated under dim red light and subsequently hemisected under infrared illumination. The retina was peeled off from the eyecup in Ringer solution containing 110 mm NaCl, 2.5 mm KCl, 1.6 mm MgCl2, 1.0 mm CaCl2, 100 mg l−1 BSA, 10 mm glucose and 10 mm Hepes (pH 7.8). Visible residual pigment epithelium was removed with forceps. The retina was chopped with a razor blade and photoreceptors were dissociated mechanically by trituration with a wide‐bore Pasteur pipette. When necessary, the isolated intact retina or dissociated photoreceptors thus prepared were fully bleached by white light (150 W halogen lamp for 40 s) and then kept in darkness for 1–2 h prior to the recordings.
Retinoid solutions were prepared by dissolving 300 μg of dry retinoid (11‐cis retinal: a generous gift from the National Eye Institute and Rosalie Crouch; 9‐cis retinoids: Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) into 4 μl ethanol and then gradually adding 4 ml Ringer solution or reference electrode solution (110 mm NaCl, 2.5 mm KCl, 1.6 mm MgCl2, 1.0 mm CaCl2 and 10 mm Hepes, pH 7.8). When treating with retinoid, the retinoid solution was added at a final concentration of ∼60 μm to a Petri dish containing bleached dissociated photoreceptor cells and then incubated in darkness for 1–2 h before the recordings.
A small aliquot of dissociated photoreceptors was placed on a recording chamber and perfused with Ringer solution. The outer segment or inner segment of a single photoreceptor was drawn into a tight‐fitting glass pipette and stimulated with calibrated test flashes generated by a white light‐emitting diode (LED; SR‐01‐WC120, Quadica Developments Inc., Brantford, Ontario, Canada) or a halogen lamp (FCR 12 V 100 W, Ushio, Cypress, CA, USA). In the LED system, flash duration and intensity were controlled by an LED driver (LDC210C, Thorlabs, Newton, NJ, USA). In the halogen lamp system, they were controlled by a set of calibrated natural density filters and a computer‐controlled shutter. In both cases, light wavelength was controlled by an interference filter. The light sources were calibrated after each experiment. The signals generated by the photoreceptor were amplified with an amplifier (Axopatch 200B, Axon Instruments, Inc., Union City, CA, USA) and tunable active filter (Model 3382, Krohn‐Hite Corporation, Brockton, MA, USA), low‐pass filtered at 30 Hz (8‐pole Bessel, Model 3382, Krohn‐Hite Corporation), digitized at 1 kHz (Digidata 1322A, Axon Instruments, Inc.) and stored on a computer using pClamp9 software (Molecular Devices, Sunnyvale, CA, USA). Rod and cone subtypes were identified based on their morphology and characteristic spectral sensitivities.
Transretinal electroretinography (ERG) recordings
Transretinal ERG recordings from mouse middle wavelength‐sensitive cones (M‐cones) were performed as previously described (Vinberg et al. 2014) with rod transducin a‐subunit knock‐out [Gnat1−/−) mice (BALB/c background, Leu450 variant of RPE‐specific protein 65 kDa (RPE65)] that lack rod signalling (Calvert et al. 2000). Young adult animals of either sex (1.5–3 months old) were used in all experiments. Animals were provided with standard chow (LabDiet 5053; LabDiet, Purina Mills, St Louis, MO, USA) and maintained under a 12 h light (10–20 Lux)/12 h dark cycle. The mice were dark‐adapted overnight prior to the experiments and were killed by asphyxiation with a rising concentration of CO2. The eyes were enucleated and hemisected as described above. The retina was dissected in Locke's solution [112.5 mm NaCl, 3.6 mm KCl, 2.4 mm MgCl2, 1.2 mm CaCl2, 10 mm Hepes, 20 mm NaHCO3, 3 mm sodium succinate, 0.5 mm sodium glutamate, 0.02 mm EDTA, 10 mm glucose, 0.1% minimum essential media vitamins and 0.2% minimum essential media amino acids, pH 7.4]. When treating with 9‐cis retinal, dissected retinas were pre‐incubated in a Petri dish containing Locke's solution supplemented with 100 mm 9‐cis retinal and 1% (w/v) BSA for 10 min and then the endogenous pigments were fully bleached by strong light (1.7 × 107 photons μm−2 s−1, 500 nm, 51 s) prior to the recordings. The retina was placed photoreceptor‐side up onto the specimen holder (OcuScience, Henderson, NV, USA) modified with a piece of black filter paper (HABG01300, Millipore, Billerica, MA, USA) on the top of the holder dome. The retina was perfused at 1–2 ml min−1 with Locke's solution bubbled with 95% O2/5% CO2 and supplemented with 40 μm dl‐AP4 (3699, Tocris Bioscience, Ellisville, MO, USA), 2 mm l‐aspartate and 100 μm BaCl2 to isolate the photoreceptor component of the ERG signal (a‐wave). Before the recordings, the retina was allowed to stabilize for 15 min. The temperature of the retina was maintained at 33–36°C during the recordings by heating the perfusion tubing located immediately before the specimen holder with a ceramic heater (Shi et al. 2007). Bleaching and light stimulation were applied with calibrated light from the LED system (see above). For bleaching 90% of cone visual pigments, the retina was exposed to a 2.56 s step of 505 nm light. Bleaching light duration was determined from the relation F = 1 − exp(−IPt), where F is the fraction of pigment to be bleached (0.9), t is the duration of light exposure, I is the light intensity (1.2 × 108 photons μm−2 s−1), and P is the photosensitivity of mouse cones at the wavelength of peak absorbance (7.5 × 10−9 μm2), adopted from Nikonov et al. (2006). The photoresponse signal after light stimulation was amplified by a differential amplifier (DP‐311, Warner Instruments) and tunable active filter (Model 3382, Krohn‐Hite Corporation), low‐pass filtered at 300 Hz (8‐pole Bessel; model 3382, Krohn‐Hite Corporation), digitized at 1 kHz (Digidata 1322A, Molecular Devices, LLC, Hamden, CT, USA) and stored on a computer using pClamp9 software (Molecular Devices).
Data analysis
Data were analysed using pClamp9 (Molecular Devices) and Origin 7.5 (OriginLab, Northampton, MA, USA). Flash sensitivities were determined from response amplitudes below 30% of the maximum recorded from a photoreceptor or retina, and are plotted as the ratio of response amplitude and flash intensity. For salamander photoreceptors, spectral sensitivity plots thus obtained were fitted with a mixed 11‐cis retinal (A1) and 11‐cis 3,4‐didehydroretinal (A2) template (Govardovskii et al. 2000) and, if necessary, A1 9‐cis template with fixed wavelength of maximum absorbance (λmax) values (Makino et al. 1999). The ratio of templates in the fitting was obtained by least‐squares analysis fit to the spectral sensitivity data, with their ratio set as a free parameter, and used as the ratio of 11‐cis A1, A2 and 9‐cis visual pigments. For mouse retinas, plots were fitted with the A1 11‐cis template with λmax set as a free parameter.
Statistical analysis
For all experiments, data are expressed as means ± SEM. Two‐tailed unpaired Student's t‐test was used to test for the significance of differences in the mean values of two sample groups, with an accepted significance level of P < 0.05.
Results
Isoform of the chromophore supplied to cones by the retina visual cycle
The recently identified putative retinol isomerase in Müller cells, DES1, produces preferably 9‐cis retinol over 11‐cis retinol (Kaylor et al. 2013). However, the nature of the chromophore supplied by Müller cells to cones in the intact retina has not been investigated. We addressed this issue by using spectral sensitivity measurements from salamander and mouse cones to identify the fraction of their pigment associated with 9‐cis chromophore. We started by comparing salamander red cones that were (1) dark‐adapted in vivo (with retinoid supplied by both the RPE and the retina visual cycles), (2) dissociated and fully bleached (without access to retinoid supply) and (3) dark‐adapted in the isolated retina after a full bleach (with retinoid supplied only by the retina visual cycle). Response families of these red cones were collected by single‐cell suction recordings (Fig. 1 A–C). As previously reported (Wang et al. 2009), compared with cones dark‐adapted in vivo (Fig. 1 A), bleached dissociated cones were substantially desensitized (Fig. 1 B), whereas cones in the isolated retina partially recovered their sensitivity after the bleach (Fig. 1 C; see also Table 1). To examine the fraction of 9‐cis analogue regenerated pigment, we determined the sensitivity of cones at six different wavelengths and compared their action spectra (Fig. 2 A–C). Salamander red cones with 9‐cis analogue pigments have substantially blue‐shifted spectral sensitivity (20 nm shift for A1, 38 nm shift for A2) (Makino et al. 1999). Thus, if 9‐cis isomer is provided by the retina visual cycle, cones dark‐adapted in the retina would be expected to have a blue‐shifted λmax. However, cones dark‐adapted in the retina (Fig. 2 C) showed spectral sensitivity identical to that from cones dark‐adapted in vivo (Fig. 2 A) or bleached without access to chromophore (Fig. 2 B), with respective λmax values of 586, 584 and 585 nm (Table 2). In addition, the spectral sensitivities in all three cases could be fit well by mixed A1 and A2 11‐cis visual pigment templates (Govardovskii et al. 2000), without need of a 9‐cis component. The A1/A2 ratios were estimated at ∼40:60 in all three cases, showing no preference for one subtype of chromophore by the retina visual cycle. Together, these results demonstrate that, similar to the canonical RPE visual cycle, the retina visual cycle in salamander supplies cones exclusively with the 11‐cis isomer chromophore.
Figure 1. Single‐cell suction electrode recordings from salamander red cones .
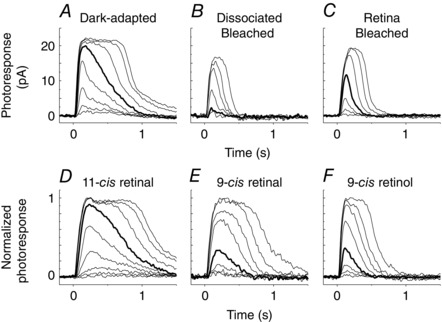
A–C, representative flash response families from cones dark‐adapted in vivo (A), dissociated and bleached (B), or bleached and dark‐adapted in the retina isolated from the RPE (C). D–E, normalized flash response families from bleached dissociated red cones incubated with 11‐cis retinal (D), 9‐cis retinal (E) or 9‐cis retinol (F). Test flashes at 620 nm and 20 ms duration of intensity increasing with a step of ∼0.5 log units were delivered at time 0. Each trace is the averaged response from 2–10 flash trials. Bold traces represent responses to a flash of 5400–5700 photons μm−2.
Table 1.
Estimated flash sensitivity (pA photons−1 μm2) at λmax in single‐cell suction recordings
| Dark‐adapted | Dissociated Bleached | Retina Bleached | 11‐cis retinal | 9‐cis retinal | 9‐cis retinol | |
|---|---|---|---|---|---|---|
| Red cone | 1.8 × 10−2 *** | 5.6 × 10−4 | 3.1 × 10−3 * | 1.7 × 10−2 *** | 4.1 × 10−3 * | 4.1 × 10−3 ** |
| (100%, n = 18) | (3.2%, n = 16) | (17%, n = 20) | (98%, n = 5) | (23%, n = 7) | (23%, n = 10) | |
| Red rod | 6.1 × 100 *** | 1.7 × 10−2 | 1.5 × 10−2 | ND | ND | 2.5 × 10−2 |
| (100%, n = 7) | (0.28%, n = 9) | (0.25%, n = 5) | (0.41%, n = 11) | |||
| Blue cone | 8.8 × 10−2 *** | 3.0 × 10−3 | 2.0 × 10−2 * | ND | ND | 1.8 × 10−2 * |
| (100%, n = 8) | (3.4%, n = 8) | (22%, n = 10) | (21%, n = 8) | |||
| Green rod | 1.1 × 100 ** | 5.0 × 10−3 | 8.6 × 10−3 | ND | ND | 9.1 × 10−4 |
| (100%, n = 20) | (0.44%, n = 10) | (0.77%, n = 7) | (0.081%, n = 5) |
Percentage values are relative sensitivity to that of cells dark‐adapted in vivo (Dark‐adapted). * P < 0.05, ** P < 0.01, *** P < 0.005 by two‐tailed t‐test versus measured sensitivity of dissociated and bleached cells (Dissociated Bleached) at 600 nm (red cones), 500 nm (red rods) or 440 nm (blue cones and green rods). ND, not determined.
Figure 2. Spectral sensitivity of salamander red cones .
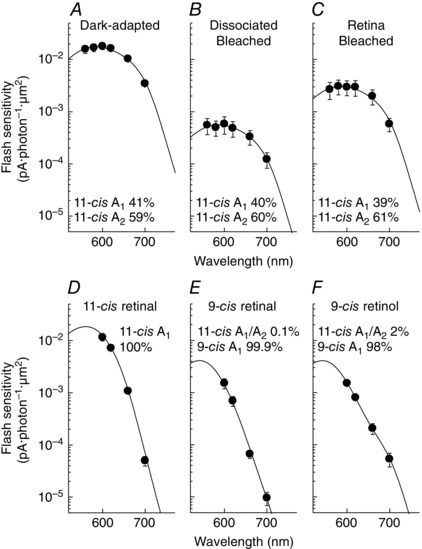
A–C, averaged spectral sensitivities of cones dark‐adapted in vivo (A, n = 18), dissociated and bleached (B, n = 16) or bleached and dark‐adapted in the retina isolated from the RPE (C, n = 20). D–F, averaged spectral sensitivities of dissociated red cones bleached and incubated with 11‐cis retinal (D, n = 5), 9‐cis retinal (E, n = 7) or 9‐cis retinol (F, n = 10). The fits to the data are sums of A1 and A2 11‐cis spectral templates (A–D, A1/A2 ratios indicated in each panel), and A1 9‐cis spectral template (E, F). See Methods for details. Values show the fraction of each pigment template used in the fitting. Here and in all subsequent figures and tables, data are shown as mean ± SEM.
Table 2.
Estimated λmax (nm) of action spectra
| Dark‐adapted | Dissociated Bleached | Retina Bleached | 11‐cis retinal | 9‐cis retinal | 9‐cis retinol | |
|---|---|---|---|---|---|---|
| Red cone | 584 (18) | 585 (16) | 586 (20) | 562 (5) | 542 (7) | 542 (10) |
| Red rod | 516 (7) | 516 (9) | 521 (5) | 497 (11) | ||
| Blue cone | 438 (8)* | 438 (8)* | 438 (10)* | 422 (8) | ||
| Green rod | 440 (20) | 440 (10) | 440 (7) | ND |
Values were estimated by fitting spectral sensitivity plots with visual pigment templates except for those marked with an asterisk. Estimated endogenous A1/A2 11‐cis chromophore ratios were different among red cones (40:60), red rods (18:82 or 0:100) and green rods (0:100) in cells not treated with exogenous chromophore (Dark‐adapted, Dissociated Bleached and Retina Bleached), probably due to individual differences among salamanders. Numbers of cells are shown in parentheses. *Estimated from pigment templates with A1/A2 11‐cis chromophore ratios in red rods dark‐adapted in vivo (18:82) with a fixed λmax at 438 nm (Makino et al. 1999). ND, not determined.
As some of the biochemical experiments with DES1 have been performed in mouse tissue (Kaylor et al. 2013), we also examined the nature of the chromophore provided by Müller cells to cones in mice. We determined the spectral sensitivity of mouse M‐cones by ex vivo transretinal recordings (Vinberg et al. 2014), for simplicity using isolated Gnat1− / − retinas that lack rod signalling (Calvert et al. 2000). The ERG a‐wave (cone response) component was isolated by suppressing pharmacologically all post‐photoreceptor ERG components (see Methods for details). Response families were recorded from mouse cones dark‐adapted in vivo (Fig. 3 A) and from cones that were bleached by 90% and then dark‐adapted in the isolated retina (Fig. 3 B). As expected, M‐cones largely recovered from the bleach but remained slightly desensitized compared to dark‐adapted cones due to the absence of RPE (Kolesnikov et al. 2011). Importantly, cones in the two conditions showed similar spectral sensitivity (Fig. 3 D). In both cases the cone action spectrum could be fit well with 100% A1 11‐cis visual pigment template (Govardovskii et al. 2000), yielding an estimated λmax of 518 nm for both dark‐adapted and bleached cones. Interestingly, these values are about 10 nm longer than those previously determined by in vivo ERG (511 nm; Jacobs et al. 1991) or with recombinant visual pigment (508 nm; Sun et al. 1997). The reason for this difference is unclear although it is worth pointing out that in our case the test flash stimulation was applied to the retina from the photoreceptor side, opposite to its pathway in vivo. Thus, it is possible that the optical properties of the retina and photoreceptors might affect the effective spectral sensitivity of cones. Despite this issue, the essentially identical spectral sensitivities of M‐cones dark‐adapted in vivo and in an isolated retina suggest that, as in salamander, the retina visual cycle in mouse also provides 11‐cis, and not 9‐cis, chromophore to cones.
Figure 3. Transretinal recordings and spectral sensitivity of mouse M‐cones .
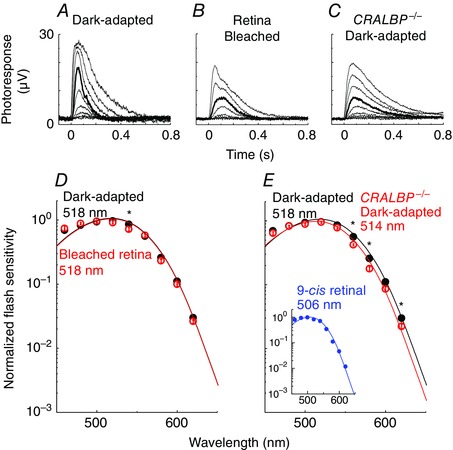
A–C, representative flash response families from cones in Gnat1 −/− retina dark‐adapted in vivo (A) or bleached and dark‐adapted in the isolated retina (B), as well as in CRALBP−/− Gnat1 −/− retina dark‐adapted in vivo (C). Test flashes at 505 nm and 2 or 20 ms duration of intensity increasing with a step of ∼0.5 log units were delivered at time 0. Each trace is the averaged response from 2–10 flash trials. Bold traces represent responses to a flash of 32,000 photons μm−2. D and E, averaged and normalized spectral sensitivities of cones in Gnat1− / − retina dark‐adapted in vivo (filled circles, n = 16), cones in isolated Gnat1− / − retina after a 90% bleach (D; open circles, n = 9), and cones in CRALBP− / − Gnat1− / − retina dark‐adapted in vivo (E; open circles, n = 13). The inset in E shows a spectrum obtained from cones in Gnat1− / − retina incubated with 9‐cis retinal after a >99% bleach. The data were fit with the A1 11‐cis pigment template with λmax as a free parameter. *Statistically significant difference, P < 0.05.
Next, to begin addressing the molecular mechanism of this 11‐cis selectivity, we tested mice lacking the cellular retinaldehyde binding protein (CRALBP). CRALBP is a water‐soluble 36 kDa protein expressed in Müller cells (Saari et al. 2001) that binds 11‐cis retinoids with higher affinity than their 9‐cis analogues (Saari & Bredberg, 1987; Golovleva et al. 2003) and plays an important role in the retina visual cycle (Collery et al. 2008; Fleisch et al. 2008; Xue et al. 2015). To determine the potential role of CRALBP in the 11‐cis selectivity of the retina visual cycle, we compared the cone action spectra in dark‐adapted control (Gnat1− / −) and CRALBP‐deficient (CRALBP− / − Gnat1− / −) retinas. As shown in Fig. 3 E, the deletion of CRALBP induced a shift in the cone spectrum λmax from 518 nm in control Gnat1− / − retina to 514 nm in CRALBP− / − Gnat1− / − retina. Such a blue spectral shift suggests the presence of 9‐cis chromophore in cones from retinas missing CRALBP. Consistent with this notion, treating bleached control mouse M‐cones with exogenous 9‐cis retinal resulted in a 12 nm blue shift in their action spectrum, with a corresponding λmax of 506 nm (Fig. 3 E, inset). Spectral templates for mouse cone pigments with 9‐cis retinal have not been reported. Thus, to estimate the fraction of 9‐cis pigment in CRALBP‐deficient cones based on their spectral sensitivity, we used the salamander red rod templates (Makino et al. 1999) which undergo a 12 nm blue shift upon replacing 11‐cis retinal with 9‐cis retinal, identical to the 12 nm blue spectral shift observed empirically by us in mouse M‐cones upon bleaching and regenerating their pigment with 9‐cis retinal. We found that a 4 nm blue shift in the action spectrum corresponds to the replacement of the majority (60%) of the 11‐cis pigment with 9‐cis pigment. Thus, the 4 nm blue shift in the action spectrum of cones from CRALBP‐deficient mice indicates the presence of a substantial amount (probably >50%) of 9‐cis cone pigment.
Sensitivity recovery with 9‐cis retinol in salamander red cones
The lack of detectable 9‐cis visual pigment in cones dark‐adapted in the isolated retina indicates that no 9‐cis retinal was made available to the cones. However, this result could be due either to the lack of production of 9‐cis retinol by the Müller cells, or, alternatively, to the inability of cones to oxidize 9‐cis retinol to the 9‐cis retinal needed for pigment regeneration. To distinguish between these two possibilities, we examined directly the ability of cones to use exogenous 9‐cis retinol for pigment regeneration. To do that, we measured the absolute and spectral sensitivity of dissociated red cones after a full bleach and 2 h of dark incubation in the presence of exogenous A1 9‐cis retinol (Figs 1 F and 2 F). A1 11‐cis retinal and A1 9‐cis retinal were used as controls to confirm the increased sensitivity upon pigment regeneration and the blue shift induced by 9‐cis pigment formation (Figs 1 D, E and 2 D, E). Spectral sensitivity plots were fitted with a mixed A1 and A2 11‐cis spectral template (Govardovskii et al. 2000) for the unbleached pigment with native chromophores, and the A1 11‐cis retinal or A1 9‐cis retinal templates (Makino et al. 1999) for the fraction of pigment regenerated with exogenous chromophore. In the fittings of cones with exogenous 9‐cis isomer, the A1/A2 ratio of 11‐cis templates was fixed at the 40:60 ratio estimated in cones without exogenous retinoids (Fig. 2 A–C). Shiff‐base formation between the exogenous retinal and proteins other than opsins was not likely to be a factor in our spectral sensitivity measurements as the stimulating light was orthogonal to the long axis of the cells (perpendicular to the outer segment), ruling out any masking effects due to absorption of light in the inner segment of the cell. Consistent with this notion, the action spectra could be fit well with a combination of 9‐cis and 11‐cis spectral templates for salamander red cone pigment (see below).
Compared to bleached cones (Fig. 2 B), cones incubated with 11‐cis retinal, 9‐cis retinal or 9‐cis retinol showed a respective 30‐ (Fig. 2 D), 7.2‐ (Fig. 2 E) and 7.2‐ (Fig. 2 F) fold sensitivity recovery at their estimated peak wavelength. The absolute sensitivities (Table 1) and λmax (Table 2) of red cones with 9‐cis retinol (4.1 × 10−3 pA photons−1 μm2, 543 nm) were essentially identical to those with 9‐cis retinal (4.1 × 10−3 pA photons−1 μm2, 542 nm), unlike those with 11‐cis retinal (1.7 × 10−2 pA photons−1 μm2, 562 nm). These results demonstrate that 9‐cis retinol was oxidized in red cones into 9‐cis retinal and utilized for efficient pigment regeneration. The 4‐fold lower sensitivity of cones with 9‐cis isomers could be largely explained by the 3.3‐fold lower quantum yield of 9‐cis pigment vs. 11‐cis pigment (Hubbard & Kropf, 1958). In contrast to the substantial recovery of sensitivity of bleached red cones, treatment of red rods with 9‐cis retinol failed to promote an increase in their sensitivity (Fig. 4 D and H). Together, these results demonstrate that 9‐cis retinol is able to promote pigment regeneration and recovery of sensitivity following a bleach in red cones but not in red rods.
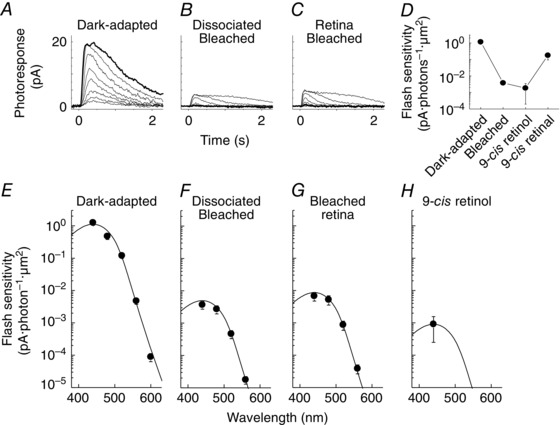
Figure 4. Single‐cell suction electrode recordings and spectral sensitivity of salamander red rods .
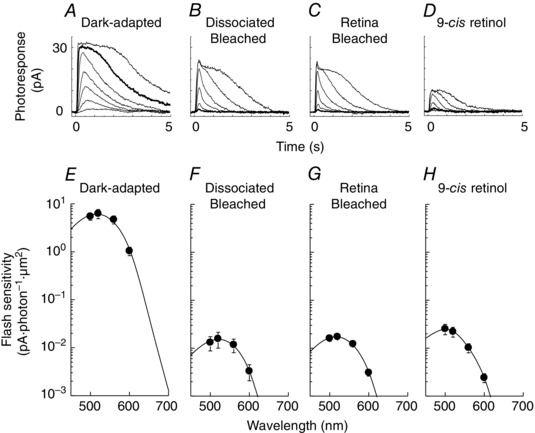
A–D, representative 500 nm flash response families from rods dark‐adapted in vivo (A), dissociated and bleached (B), bleached and dark‐adapted in the isolated retina (C), and dissociated, bleached and incubated with 9‐cis retinol (D). Bold traces represent responses to a flash of 52 photons μm−2. E–G, averaged spectral sensitivities of salamander red rods dark‐adapted in vivo (E, n = 7), dissociated and bleached (F, n = 9), bleached and dark‐adapted in the isolated retina (G, n = 5), and dissociated, bleached and incubated with 9‐cis retinol (H, n = 11). Chromophore compositions of A1 11‐cis, A2 11‐cis and A1 9‐cis (for H) were estimated as 18:82 (E), 18:82 (F), 0:100 (G) and 5:20:75 (H).
Interestingly, red cones treated with 9‐cis retinol retained the small amount of unbleached native 11‐cis pigment (Fig. 2 F), whereas incubation with 9‐cis retinal resulted in complete shift to 9‐cis pigment with essentially no residual 11‐cis pigment (Fig. 2 E). This result is consistent with the relatively open chromophore binding pocket of red cone pigment which causes the gradual replacement of the native 11‐cis chromophore in red salamander cones when treated with excess of exogenous 9‐cis retinal (Matsumoto et al. 1975; Kefalov et al. 2005). Indeed, the incubation of bleached red cones with A1 11‐cis retinal resulted in a similar loss of the residual native A2 11‐cis component of the pigment (Fig. 2 D).
Access to the retina visual cycle is independent of the pigment type
The salamander retina presents a unique opportunity to examine whether the nature of the visual pigment plays a role in regulating access to the retina visual cycle. In an unusual arrangement, blue cones and green rods in the salamander share the same visual pigment (Ma et al. 2001), providing a way to address this question. Thus, we next compared the dark adaptation in isolated salamander retina of its different rod and cone types. As previously shown (Wang et al. 2009), bleached red cones could utilize chromophore supplied by the Müller cells and as a result largely recovered their sensitivity even in the absence of RPE in the isolated retina (compare Fig. 2 A–C). In contrast, salamander red rods could not recover their sensitivity when bleached in isolated retina (Fig. 4 A–C, see also Fig. 4 E–G) confirming the inability of these photoreceptors to regenerate their visual pigment by accessing the retina visual cycle. We then examined the recovery of blue cones from a bleach in the isolated retina. Similar to red cones, blue cones also recovered their sensitivity in the isolated retina (Fig. 5 A–C, see also Fig. 5 E–G). Thus, similar to red cones, blue cones were able to utilize chromophore from the retina visual cycle for pigment regeneration and dark adaptation. In contrast, despite using the same visual pigment as blue cones, green rods were unable to recover their sensitivity following a bleach in the isolated retina (Fig. 6 A–C, see also Fig. 6 E–G) and remained permanently desensitized. We did not study the possible blue shift in the action spectrum of these cells because the expected spectral shift with 9‐cis chromophore is relatively small (14 nm for A1 and 18 nm for A2; Makino et al. 1999) in blue cones and green rods compared with that in red cones (20 nm for A1 and 38 nm for A2; Makino et al. 1999). Together, these results demonstrate that despite sharing the same visual pigment, blue cones in the salamander can utilize chromophore from the retina visual cycle to regenerate their visual pigment and dark‐adapt, whereas green rods cannot. Thus, the ability to access the retina visual cycle is restricted to cones and it is independent of the type of pigment expressed in photoreceptors.
Figure 5. Single‐cell suction electrode recordings and spectral sensitivity of salamander blue cones .
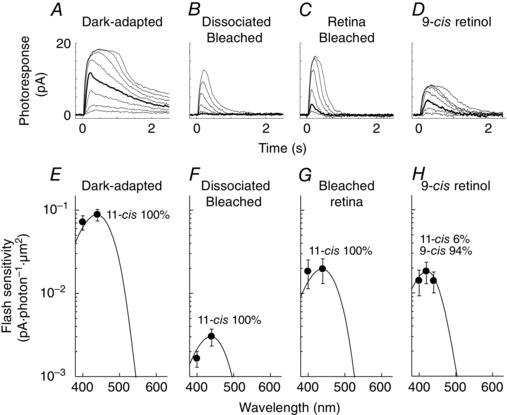
A–D, representative 440 nm flash response families from cones dark‐adapted in vivo (A), dissociated and bleached (B), bleached and dark‐adapted in the isolated retina (C), and dissociated, bleached and incubated with 9‐cis retinol (D). Bold traces represent responses to a flash of 200 photons μm−2. E–H, averaged spectral sensitivities of salamander blue cones dark‐adapted in vivo (E, n = 8), dissociated and bleached (F, n = 8), bleached and dark‐adapted in the isolated retina (G, n = 10), and dissociated, bleached and incubated with 9‐cis retinol (H, n = 8). Curves in E–G were obtained by fitting each 440 nm point with a combined 18% A1 and 82% A2 11‐cis pigment template as estimated from red rods dark‐adapted in vivo (Fig. 4 E). The curve in H was obtained by fitting the 420 nm data point with a combined A1/A2 11‐cis template (F) and the A1 9‐cis blue cone pigment template.
Figure 6. Single‐cell suction electrode recordings and spectral sensitivity of salamander green rods .
A–C, representative 440 nm flash response families from rods dark‐adapted in vivo (A), dissociated and bleached (B), and bleached and dark‐adapted in the isolated retina (C). Bold traces represent responses to a flash of 150 photons μm−2. D, change in flash sensitivity of green rods at 440 nm after dark adaptation in vivo (Dark‐adapted), full bleach (Dissociated Bleached), incubation with 9‐cis retinol (9cROL) and incubation with 9‐cis retinal after washing out 9‐cis retinol (9cRAL) (n = 2 for all conditions). E–H, averaged spectral sensitivities of salamander green rods dark‐adapted in vivo (E, n = 20), dissociated and bleached (F, n = 10), bleached and dark‐adapted in the isolated retina (G, n = 7), and dissociated, bleached and incubated with 9‐cis retinol (H, n = 5). Based on spectral templates fit, chromophore composition was estimated as 100% A2 11‐cis for E–G and the same template was used to fit the data in H.
Finally, we also investigated whether salamander blue cones and green rods can utilize 9‐cis retinol for pigment regeneration. Coincident with their ability to access the retina visual cycle, blue cones also showed substantial sensitivity recovery with 9‐cis retinol (Fig. 5 D and H). Similar to the case of red cones, the recovery of sensitivity of blue cones treated with 9‐cis retinol was only partial, probably due to the lower quantum yield of the resulting 9‐cis pigment compared to the native 11‐cis pigment. In contrast, 9‐cis retinol was unable to promote pigment regeneration and dark adaptation in green rods (Fig. 6 H). Indeed, the sensitivity of green rods treated with 9‐cis retinol was lower than in their pre‐treatment bleached state, consistent with increasing the rod opsin activity upon the non‐covalent binding of chromophore analogues (Kefalov et al. 1999; Isayama et al. 2006) (but see also Ala‐Laurila et al. 2009). The dark current of 9‐cis retinol‐treated green rods was also substantially reduced (Table 3), precluding us from obtaining reliable response family recordings. We confirmed the viability of these green rods by a subsequent treatment with 9‐cis retinal, which resulted in substantial recovery of their sensitivity (Fig. 6 D). Together, these results demonstrate that 9‐cis retinol is able to promote pigment regeneration and recovery of sensitivity following a bleach in blue cones but not in green rods. Combined with our results from red cones and red rods above, these findings suggest that the oxidation of cis‐retinol is restricted only to cones and is independent of the type of opsin expressed in the photoreceptors.
Table 3.
Dark current (pA), measured from saturated photoresponses in single‐cell suction electrode recordings
| Dark‐adapted | Dissociated Bleached | Retina Bleached | 11‐cis retinal | 9‐cis retinal | 9‐cis retinol | |
|---|---|---|---|---|---|---|
| Red cone | 21 ± 2 (18) | 17 ± 3 (11) | 21 ± 2 (12) | 9.1 ± 1.5 (5) | 15 ± 2 (5) | 12 ± 1 (9) |
| Red rod | 30 ± 3 (7) | 15 ± 2 (9) | 17 ± 3 (5) | 7.5 ± 0.9 (11) | ||
| Blue cone | 12 ± 2 (8) | 12 ± 1 (8) | 15 ± 1 (9) | 10 ± 2 (7) | ||
| Green rod | 24 ± 2 (20) | 5.6 ± 1.0 (14) | 8.2 ± 1.8 (7) | 1.5 ± 0.5 (4) |
Numbers of cells are shown in parentheses.
Discussion
Nature of the chromophore supplied by Müller cells to cones
The nature of the chromophore provided by Müller cells to cones as part of the retina visual cycle has been unclear. Biochemical studies have suggested that the putative Müller cell isomerase DES1 has a strong preference for 9‐cis retinol over 11‐cis retinol (Kaylor et al. 2013). Consistent with this possibility, 9‐cis retinoid has been found in an array of species and conditions (Fan et al. 2003, Kaylor et al. 2013). On the other hand a recent study found that cultured rat Müller cells release 11‐cis retinol (Betts‐Obregon et al. 2014). We addressed this question directly by investigating the nature of the chromophore driving the regeneration of cone visual pigment in isolated salamander and mouse retinas, which is driven by the retina visual cycle. Our results demonstrate that retinal Müller cells provide cones exclusively with 11‐cis and not with 9‐cis chromophore in both salamander (Fig. 2 A–C) and mouse (Fig. 3 D). Furthermore, our results show that CRALBP in Müller cells plays a role in this process (Fig. 3 E). As CRALBP binding favours 11‐cis over 9‐cis retinoids (Saari & Bredberg, 1987; Golovleva et al. 2003), the most likely explanation is that it enhances the conversion of all‐trans retinol to 11‐cis retinol by mass action law: as the DES1‐catalysed isomerization of all‐trans retinol produces a mix of retinoid isomers (Kaylor et al. 2013), CRALBP would bind to and remove selectively 11‐cis retinol, thus promoting the production of 11‐cis retinol. It is possible that in the absence of CRALBP a small fraction of 9‐cis retinal is generated from all‐trans retinal by dihydroflavin‐catalysed reactions (Futterman & Rollins, 1973). However, as these reactions are slow and the levels of 9‐cis retinal generated are miniscule, we believe that their contribution under our experimental conditions is negligible. Finally, a potential 11‐cis selection mechanism that is yet to be explored in vivo involves the recently proposed 11‐cis‐specific retinyl ester synthase in Müller cells, multifunctional O‐acyltransferase (Kaylor et al. 2014). The selective esterification of 11‐cis retinol to 11‐cis retinyl ester would also drive by mass action the production specifically of 11‐cis retinoid in Müller cells. Such a scenario gives rise to the testable hypothesis that deletion of multifunctional O‐acyltransferase will disinhibit the production of 9‐cis retinol, causing accumulation of 9‐cis retinal‐based cone visual pigment.
Cell type selectivity of the retina visual cycle
Our results demonstrate that not all photoreceptors can access the retina visual cycle. As previously shown, we found that salamander red cones (Figs 1 and 2) but not red rods (Fig. 4) can regenerate a substantial fraction of their pigment and largely recover their sensitivity following a bleach in the isolated retina. The ability of salamander blue cones and green rods to access the retina visual cycle had not been investigated. Whereas blue cones have cone‐like morphology, their opsin, also expressed in green rods (Ma et al. 2001), has some rhodopsin‐like properties so that binding of chromophore analogues, such as beta‐ionone to blue cone opsin results in activation of the downstream phototransduction cascade (Isayama et al. 2006). This activation is typically observed for the opsin of red rods (Kefalov et al. 1999, 2003) but not red cones (Jin et al. 1993; Ala‐Laurila et al. 2009). Thus, it was not clear whether blue cones will be able to regenerate their pigment via the retina visual cycle similar to red cones, or will be blocked from accessing this pathway similar to red rods. We found that blue cones undergo substantial recovery of their sensitivity following a bleach in the isolated retina (Fig. 5 C and G). In contrast, green rods, despite using the same visual pigment, were unable to recover with the help of the retina visual cycle following a bleach (Fig. 6 C and G). Thus, the type of the photoreceptor plays a critical role in regulating access to the retina visual cycle so that red and blue cones can readily regenerate their visual pigment in the isolated retina, whereas red and green rods cannot.
One of the mechanisms that possibly regulate access to the retina visual cycle is the ability to oxidize 11‐cis retinol, the putative retinoid produced by the Müller cells (Jones et al. 1989; Wang & Kefalov, 2009; Wang et al. 2014). Considering that our results demonstrate that only cones but not rods can utilize the retina visual cycle for pigment regeneration, one would expect that oxidation of cis retinol will also be restricted to cones. Thus, we also investigated the ability of salamander photoreceptors to oxidize cis retinol and use it for pigment regeneration. As 11‐cis retinol is difficult to obtain and unstable (Parker et al. 2011), we sought to determine whether its commercially available 9‐cis analogue is a viable substitute. We found that 9‐cis retinol produces robust sensitivity recovery comparable with that of 9‐cis retinal in red cones (Fig. 2 F and E) and blue cones (Fig. 5 H), but not in red rods (Fig. 4 H) or green rods (Fig. 6 H). These results are consistent with the previous finding in a recent study that 11‐cis retinol promotes pigment regeneration in salamander red and blue cones but not in red and green rods (Ala‐Laurila et al. 2009). Together, our results demonstrate that only photoreceptors able to access the retina visual cycle can oxidize cis retinol and use it for pigment regeneration. Thus, this reaction appears to be one of the requirements for use of the retina visual cycle. The ability of 9‐cis retinol to substitute for 11‐cis retinol renders it an excellent experimental tool for investigating the mechanism driving the oxidation of cis retinol in cone photoreceptors and eventually identifying the enzyme responsible for this reaction. Indeed, 9‐cis and 11‐cis retinol oxidation are shown to be catalysed by the same enzyme in carp cones (Sato et al. 2015).
Candidate enzymes for the oxidation of cis retinol in cones
Our results presented here suggest that the oxidation of cis retinol is cone‐specific. However, the identity of the enzyme driving this reaction remains unknown. The list of potential candidates includes retinol dehydrogenase 8 (RDH8), RDH13L (and its functional homologue in mice, RDH14) and RDH12. Recently, the deletion of RDH8 was found to block the 9‐cis retinol‐dependent sensitivity recovery in mouse M‐cones (Kolesnikov et al. 2015). However, RDH8 is expressed in the outer segment of both rods and cones (Maeda et al. 2005; Miyazono et al. 2008) and it appears to be predominantly an all‐trans retinal reductase (Rattner et al. 2000; see also Palczewski et al. 1994). RDH13L was identified as an 11‐cis/9‐cis RDH expressed in carp cone inner segments but not in rods (Sato et al. 2015). Although RDH13L is not present in amphibians and mammals, its functional homologue in mice, RDH14 (Sato et al. 2015), is conserved among vertebrates and appears to be expressed in mouse and bovine rods and cones (Haeseleer et al. 2002; Kanan et al. 2008) as well as in human retina (Zhang et al. 2015). RDH12 belongs to the same RDH subfamily as RDH14 (Haeseleer et al. 2002) and catalyses both the reduction and the oxidation of all‐trans/11‐cis/9‐cis retinoid (Belyaeva et al. 2005). RDH12 is expressed in the inner segments of both rods and cones in mouse retina (Maeda et al. 2006) and recombinant human RDH12 shows ∼2000‐fold greater affinity for NADP(H) than NAD(H), implying its role predominantly as a retinal/aldehyde reductase (Belyaeva et al. 2005). In fact, RDH12 accounts for 30% of all‐trans retinal reductase activity in mouse eye (Maeda et al. 2007).
Our results presented here demonstrate that in salamander retina access to the retina visual cycle and oxidation of cis retinol are restricted to red and blue cones and exclude red and green rods. In the case of mouse, access to the retina visual cycle is also restricted to cones and excludes rods (Wang & Kefalov, 2009). However, the hybrid rods in rd7 mice that express a subset of cone genes (Corbo & Cepko, 2005) are able to regenerate a substantial fraction of their rhodopsin without assistance from the RPE (Wang et al. 2014). Together, these findings point to a future differential expression analysis in salamander rods versus cones and wild type versus rd7 rods as a viable approach for identifying the elusive cis retinol dehydrogenase of the retina visual cycle.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
Conception and design of the experiments: S.S. and V.J.K. Collection, assembly, analysis and interpretation of data: S.S. and V.J.K. Writing the article: S.S. and V.J.K. Both authors approved the final version of the manuscript.
Funding
This work was supported by NIH grants EY019312 and EY21126 (V.J.K.), EY002687 to the Department of Ophthalmology and Visual Sciences at Washington University, and by Research to Prevent Blindness.
Acknowledgements
We are grateful to Alexander Kolesnikov and Frans Vinberg from the Kefalov lab and to Rosalie Crouch from the Medical University of South Carolina for their comments on the manuscript and to the National Eye Institute and Rosalie Crouch for the 11‐cis retinal.
This is an Editor's Choice article from the 15 November 2016 issue.
References
- Ala‐Laurila P, Cornwall MC, Crouch RK & Kono M (2009). The action of 11‐cis‐retinol on cone opsins and intact cone photoreceptors. J Biol Chem 284, 1649–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala‐Laurila P, Kolesnikov AV, Crouch RK, Tsina E, Shukolyukov SA, Govardovskii VI, Koutalos Y, Wiggert B, Estevez ME & Cornwall MC (2006). Visual cycle: dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol 128, 15–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA (1987). Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci 28, 3–9. [PubMed] [Google Scholar]
- Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS & Kedishvili NY (2005). Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol‐binding protein type I (CRBPI) and cellular retinaldehyde‐binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry 44, 703–047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts‐Obregon BS, Gonzalez‐Fernandez F & Tsin AT (2014). Interphotoreceptor retinoid‐binding protein (IRBP) promotes retinol uptake and release by rat Muller cells (rMC‐1) in vitro: implications for the cone visual cycle. Invest Ophthalmol Vis Sci 55, 626–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN Jr, Makino CL & Lem J (2000). Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha‐subunit. Proc Natl Acad Sci USA 97, 1391–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collery R, McLoughlin S, Vendrell V, Finnegan J, Crabb JW, Saari JC & Kennedy BN (2008). Duplication and divergence of zebrafish CRALBP genes uncovers novel role for RPE‐ and Muller‐CRALBP in cone vision. Invest Ophthalmol Vis Sci 49, 381–820. [DOI] [PubMed] [Google Scholar]
- Corbo JC & Cepko CL (2005). A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S‐cone syndrome. PLoS Genet 1, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T & Koutalos Y (2001). Vertebrate photoreceptors. Prog Retin Eye Res 20, 4–4. [DOI] [PubMed] [Google Scholar]
- Fan J, Rohrer B, Moiseyev G, Ma JX & Crouch RK (2003). Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock‐out mice. Proc Natl Acad Sci USA 100, 1366–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch VC, Schonthaler HB, von Lintig J & Neuhauss SC (2008). Subfunctionalization of a retinoid‐binding protein provides evidence for two parallel visual cycles in the cone‐dominant zebrafish retina. J Neurosci 28, 820–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y, Okano T, Shichida Y, Yoshizawa T, Trehan A, Mead D, Denny M, Asato AE & Liu RS (1990). Comparative study on the chromophore binding sites of rod and red‐sensitive cone visual pigments by use of synthetic retinal isomers and analogues. Biochemistry 29, 313–140. [DOI] [PubMed] [Google Scholar]
- Futterman S & Rollins MH (1973). The catalytic isomerization of all‐trans‐retinal to 9‐cis‐retinal and 13‐cis‐retinal. J Biol Chem 248, 777–779. [PubMed] [Google Scholar]
- Golovleva I, Bhattacharya S, Wu Z, Shaw N, Yang Y, Andrabi K, West KA, Burstedt MS, Forsman K, Holmgren G, Sandgren O, Noy N, Qin J & Crabb JW (2003). Disease‐causing mutations in the cellular retinaldehyde binding protein tighten and abolish ligand interactions. J Biol Chem 278, 1239–2402. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG & Donner K (2000). In search of the visual pigment template. Vis Neurosci 17, 50–28. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 254–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS & Palczewski K (2002). Dual‐substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem 277, 4553–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R & Kropf A (1958). The action of light on rhodopsin. Proc Natl Acad Sci USA 44, 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayama T, Chen Y, Kono M, Degrip WJ, Ma JX, Crouch RK & Makino CL (2006). Differences in the pharmacological activation of visual opsins. Vis Neurosci 23, 89–08. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J & Deegan JF 2nd (1991). Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353, 65–56. [DOI] [PubMed] [Google Scholar]
- Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF & Cornwall MC (1993). Noncovalent occupancy of the retinal‐binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron 11, 51–22. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC & Chader GJ (1989). Retinoid requirements for recovery of sensitivity after visual‐pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA 86, 960–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Kasus‐Jacobi A, Moiseyev G, Sawyer K, Ma JX & Al‐Ubaidi MR (2008). Retinoid processing in cone and Muller cell lines. Exp Eye Res 86, 34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylor JJ, Cook JD, Makshanoff J, Bischoff N, Yong J & Travis GH (2014). Identification of the 11‐cis‐specific retinyl‐ester synthase in retinal Muller cells as multifunctional O‐acyltransferase (MFAT). Proc Natl Acad Sci USA 111, 730–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylor JJ, Yuan Q, Cook J, Sarfare S, Makshanoff J, Miu A, Kim A, Kim P, Habib S, Roybal CN, Xu T, Nusinowitz S & Travis GH (2013). Identification of DES1 as a vitamin A isomerase in Muller glial cells of the retina. Nat Chem Biol 9, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov V, Fu Y, Marsh‐Armstrong N & Yau KW (2003). Role of visual pigment properties in rod and cone phototransduction. Nature 425, 52–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Carter Cornwall M & Crouch RK (1999). Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol 113, 49–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC & Yau KW (2005). Breaking the covalent bond – a pigment property that contributes to desensitization in cones. Neuron 46, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Maeda A, Tang PH, Imanishi Y, Palczewski K & Kefalov VJ (2015). Retinol dehydrogenase 8 and ATP‐binding cassette transporter 4 modulate dark adaptation of M‐cones in mammalian retina. J Physiol 593, 492–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Tang PH, Parker RO, Crouch RK & Kefalov VJ (2011). The mammalian cone visual cycle promotes rapid M/L‐cone pigment regeneration independently of the interphotoreceptor retinoid‐binding protein. J Neurosci 31, 790–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL & Crouch RK (2001). A visual pigment expressed in both rod and cone photoreceptors. Neuron 32, 45–61. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W & Palczewski K (2005). Role of photoreceptor‐specific retinol dehydrogenase in the retinoid cycle in vivo . J Biol Chem 280, 1882–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA & Palczewski K (2006). Retinol dehydrogenase (RDH12) protects photoreceptors from light‐induced degeneration in mice. J Biol Chem 281, 3769–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Sun W, Zhang H, Baehr W & Palczewski K (2007). Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci USA 104, 1956–9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Groesbeek M, Lugtenburg J & Baylor DA (1999). Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys J 77, 102–035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC & Travis GH (2002). Isomerization and oxidation of vitamin A in cone‐dominant retinas: a novel pathway for visual‐pigment regeneration in daylight. Neuron 36, 6–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Tokunaga F & Yoshizawa T (1975). Accessibility of the iodopsin chromophore. Biochim Biophys Acta 404, 30–08. [DOI] [PubMed] [Google Scholar]
- Miyazono S, Shimauchi‐Matsukawa Y, Tachibanaki S & Kawamura S (2008). Highly efficient retinal metabolism in cones. Proc Natl Acad Sci USA 105, 1605–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J & Pugh EN Jr (2006). Physiological features of the S‐ and M‐cone photoreceptors of wild‐type mice from single‐cell recordings. J Gen Physiol 127, 35–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson‐Batres MA & Saari JC (1994). Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry 33, 1374–3750. [DOI] [PubMed] [Google Scholar]
- Parker R, Wang JS, Kefalov VJ & Crouch RK (2011). Interphotoreceptor retinoid‐binding protein as the physiologically relevant carrier of 11‐cis‐retinol in the cone visual cycle. J Neurosci 31, 471–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, Lurie M, Brown PK & Dowling JE (1976). Visual adaptation: effects of externally applied retinal on the light‐adapted, isolated skate retina. Science 191, 39–96. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM & Nathans J (2000). Identification and characterization of all‐trans‐retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all‐trans‐retinal to all‐trans‐retinol. J Biol Chem 275, 1103–1043. [DOI] [PubMed] [Google Scholar]
- Saari JC (2012). Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 32, 12–45. [DOI] [PubMed] [Google Scholar]
- Saari JC & Bredberg DL (1987). Photochemistry and stereoselectivity of cellular retinaldehyde‐binding protein from bovine retina. J Biol Chem 262, 761–622. [PubMed] [Google Scholar]
- Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, Huang J, Possin DE & Crabb JW (2001). Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 73–48. [DOI] [PubMed] [Google Scholar]
- Sato S, Fukagawa T, Tachibanaki S, Yamano Y, Wada A & Kawamura S (2013). Substrate specificity and subcellular localization of the aldehyde‐alcohol redox‐coupling reaction in carp cones. J Biol Chem 288, 3658–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Miyazono S, Tachibanaki S & Kawamura S (2015). RDH13L, an enzyme responsible for the aldehyde‐alcohol redox coupling reaction (AL‐OL coupling reaction) to supply 11‐cis retinal in the carp cone retinoid cycle. J Biol Chem 290, 298–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Yau KW, Chen J & Kefalov VJ (2007). Signaling properties of a short‐wave cone visual pigment and its role in phototransduction. J Neurosci 27, 1008–0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Macke JP & Nathans J (1997). Mechanisms of spectral tuning in the mouse green cone pigment. Proc Natl Acad Sci USA 94, 886–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg F, Kolesnikov AV & Kefalov VJ (2014). Ex vivo ERG analysis of photoreceptors using an in vivo ERG system. Vision Res 101, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC & Kefalov VJ (2009). Intra‐retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci 12, 29–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS & Kefalov VJ (2009). An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19, 166–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS & Kefalov VJ (2011). The cone‐specific visual cycle. Prog Retin Eye Res 30, 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Nymark S, Frederiksen R, Estevez ME, Shen SQ, Corbo JC, Cornwall MC & Kefalov VJ (2014). Chromophore supply rate‐limits mammalian photoreceptor dark adaptation. J Neurosci 34, 1121–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Shen SQ, Jui J, Rupp AC, Byrne LC, Hattar S, Flannery JG, Corbo JC & Kefalov VJ (2015). CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest 125, 72–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Dufresne C, Turner R, Ferri S, Venkatraman V, Karani R, Lutty GA, Van Eyk JE & Semba RD (2015). The proteome of human retina. Proteomics 15, 83–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


