Abstract
Cells in the mammalian hippocampal formation subserve neuronal representations of environmental location and support navigation in familiar environments. Grid cells constitute one of the main cell types in the hippocampal formation and are widely believed to represent a universal metric of space independent of external stimuli. Recent evidence showing that grid symmetry is distorted in non‐symmetrical environments suggests that a re‐examination of this hypothesis is warranted. In this review we will discuss behavioural and physiological evidence for how environmental shape and in particular enclosure boundaries influence grid cell firing properties. We propose that grid cells encode the geometric layout of enclosures.
Introduction
The invention of geometry started as a practical enterprise concerned with measuring ‘distances and limits of the earth’ in ancient Egypt around 4000 years ago to provide a basis for reconstructing property entitlements following the annual flooding of the Nile (Heilbron, 2000). Later geometrical concepts were translated into a more abstract ‘language’ describing the angular and metric relation between connected lines. Until quite recently the ability to construct and analyse geometric properties seemed to be a high level cognitive activity unique to humans. However, in the past 30 years an accumulating body of evidence has suggested that vertebrates as diverse as non‐human primates (Gouteux et al. 2001), birds (Kelly et al. 1998; Vargas et al. 2004) and fish (Sovrano et al. 2007) can use geometric cues (i.e. angular and length relationship of surrounding boundaries). It is still unclear how and where in the brain this information is processed and how it is integrated with featural information from specific modalities such as vision, olfaction and touch. Here we propose that geometric information could be encoded in the medial entorhinal cortex (mEC) by an interacting network of grid, boundary, and place cells (Hartley et al. 2000; Moser et al. 2008; Krupic et al. 2014) where it is also combined with the information derived from sensory cues.
Can animals ‘understand’ geometry?
For the purposes of this paper, the ‘geometry’ of an enclosure is defined as the angular and length relationships of the boundaries of the enclosure. These tend to be the walls or edges of the environment but may also include internal landmarks especially if they are large and immobile. These cues can be contrasted with local featural cues such as the colour, texture or smell of the walls and floor. There is ample evidence suggesting that vertebrates as diverse as rats (Cheng, 1986; Jones et al. 2007), pigeons (Kelly et al. 1998; Vargas et al. 2004), fish (Sovrano et al. 2007) and humans (Hermer & Spelke, 1994; Hartley et al. 2004) use ‘geometrical cues’ to find their whereabouts. For instance, it has been shown that animals could successfully find a reward location when the only information available was the shape of the box. In this case the limiting factor on their ability was the ambiguity introduced by the symmetry of the enclosure. Thus, if a reward is placed in a top left corner of a rectangular enclosure, the disoriented animal will tend to search with an equal probability either in the left top or bottom right corners indicating it is using the length relations of the sides to identify the goal (Fig. 1 A). If the same experiment was carried out in a square enclosure the animal would search in all four corners with equal probabilities (Fig. 1 B). On the other hand, this behaviour could also be observed if the animal relied on viewpoint‐specific ‘snapshots’ of the visual scene in order to navigate (Collett, 1996; see also Pearce et al. 2004). However, while it is possible that animals are using a scene‐matching strategy under certain circumstances, there is some evidence that they are also using more abstract geometric information. In particular, both humans and rats estimated the reward location using the fixed ratios of the distances between opposing walls in rescaled and transformed enclosures (Tommasi & Thinus‐Blanc, 2004; Hartley et al. 2004). When rats were pre‐trained to search for a reward hidden in the centre of a square, they could generalize this ‘knowledge’ and tended to search in the middle of novel rectangular as well as triangular enclosures (Tommasi & Thinus‐Blanc, 2004), all of which corresponded to very different viewpoint‐specific ‘snapshots’ at the reward location (Fig. 1 C–F). However, it must be noted that the results of this potentially important experiment need to be treated with caution since the final behavioural measurements of positions in the different enclosures were made using a fixed overhead camera centred over each box which might have been used as a common beacon by the animals.
Figure 1. Rats use geometric cues to navigate .
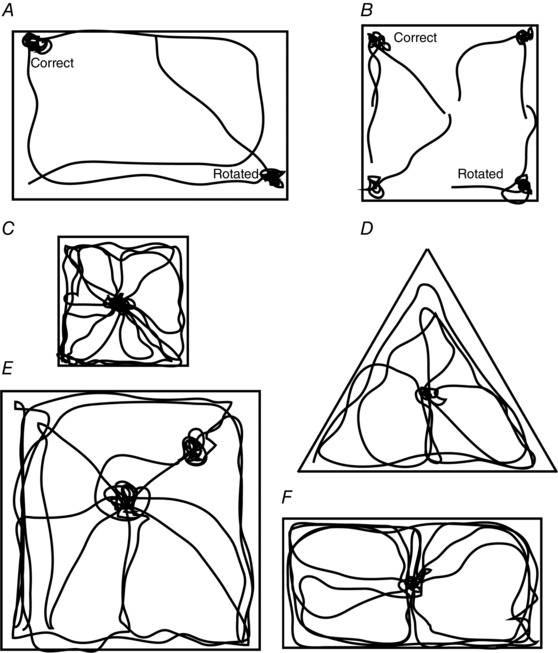
Schematic representations of search trajectories of a rat in rectangular (A) and square (B) enclosures. Rat trajectory is represented with a black line. ‘Correct’ indicates a corner with a hidden reward and ‘rotated’ a corner corresponding to the 180 deg rotation of the correct corner. Rats can use the asymmetry of the rectangle to identify the correct and rotated corners but lack this information in the square. C, schematic representation of the search trajectory of a typical rat trained to search for a hidden pellet in the centre of a small square. D–F, rats could generalize their knowledge of a hidden food location in triangular, larger square and rectangular enclosures. Note that in the large square rats tended to search at a fixed distances from nearby walls as well as in the centre of the enclosure. A and B, after Cheng, 1986; Hermer‐Vazquez et al. 2001, C–F, after Tommasi & Thinus‐Blanc, 2004.
More generally, it has been shown that an animal can use both the angular relationship of the enclosing boundaries as well as the relationship between their lengths (shorter wall vs. longer wall). Interestingly when these two properties are put in conflict in parallelogram‐shaped environments, the animal tends to choose the corner defined by the angular relationships if it is located near an acute‐angled corner and the relative wall lengths near an obtuse‐angled corner (Tommasi & Polli, 2004).
Some studies report that geometrical cues can overshadow sensory information (Cheng, 1986; Hermer & Spelke, 1994; Hayward et al. 2004). However, in general the results on the overall dominance of geometric information are strongly mixed, suggesting that different types of information are used depending on demand, reliability and salience (Kelly et al. 2009). Differences between studies may also be due to species, gender (males tend to rely more on geometric cues than females (Williams et al. 1990; Jones & Healy, 2006)) and previous experience, i.e. reared in the laboratory conditions vs. wild‐caught (Gray et al. 2005). For example, human adults primarily use featural cues (visual cues and objects in particular; Hermer & Spelke, 1994) unless required to simultaneously perform a demanding verbal task in which case they rely more on geometric cues (Hermer‐Vazquez et al. 1999).
Additional information can either potentiate (Graham et al. 2006) or overshadow the geometrical cues (Gray et al. 2005). Animals can ‘add’ (Pearce et al. 2006; Graham et al. 2006) different type of cues to improve their performance. For instance, when two adjacent coloured walls are added to a kite‐shaped environment they help the animal to find the correct location. In an extreme case, prior training to use the colour of the walls in a square led to blocking of the use of environmental layout when the animals were subsequently trained in a rectangle containing the same coloured walls (Pearce et al. 2006).
Thus current experimental studies strongly suggest that many vertebrate species possess the abstract notion of geometry and naturally use geometric cues together with other featural information to locate themselves in the environment. However, the outstanding questions are where in the brain these various types of information are processed, what is the site of their interaction and under what circumstances one type dominates over the other.
Neural representation of geometry
How is ‘geometry’ represented in the brain? Is there a single brain area where it is processed and perhaps a dedicated class of neurons which would ‘encode’ geometry? The first clue came in the seventies when it was shown that the hippocampal formation played a key role in spatial navigation (O'Keefe & Dostrovsky, 1971; Morris et al. 1982), hinting that it could be essential for processing geometric information. The majority of cells in the hippocampus proper (called place cells) are active when the animal visits a restricted portion of the environment called the place field (Fig. 2 A). Different place cells are active in different portions of the environment providing the brain with a ‘cognitive map’ of space (O'Keefe & Nadel, 1978). The first clear evidence of place cell response to geometric properties of the enclosure came from the work of Muller and Kubie (1987) where they showed that ‘when the apparatus floor plan was changed from circular to rectangular, the firing pattern of a [place] cell in an apparatus of one shape could not be predicted from a knowledge of the firing pattern in the other shape’ – the phenomenon which was called ‘place cell remapping’ (see also Lever et al. 2002; Wills et al. 2005; Leutgeb et al. 2005). But random remapping alone would not allow the brain to infer the geometric identity of the enclosure. Rather it would indicate that the change of enclosure was noted and that different enclosures are represented by different cognitive maps. Importantly, if the animal was placed in two enclosures of identical shape but located in different experimental rooms place cells would still remap even though the geometry did not change (Leutgeb et al. 2005).
Figure 2. Main spatial neurons in the hippocampal formation .
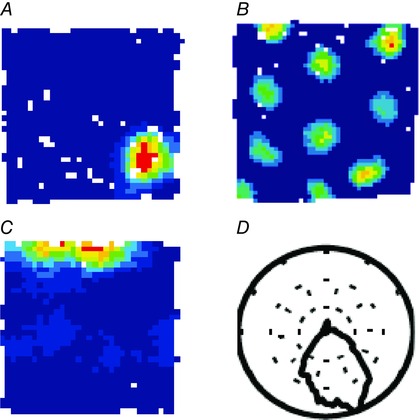
Rate maps of a typical place cell (A), grid cell (B), and boundary cell (C). Warm colours represent higher firing rates. Preferred direction of a head direction cell (D) shown by a polar plot.
Interestingly when the shape of the environment was re‐scaled (Muller & Kubie, 1987; O'Keefe & Burgess, 1996) place cells re‐scaled with it in a coherent and homogeneous manner, i.e. the rescaling was similar across the entire enclosure and it was proportional to the rescaling of the arena. The rescaling occurred even when the transformation of the enclosure resulted in a new shape: from a square to a rectangle as in O'Keefe & Burgess (1996). The authors interpreted these findings as implying that the place cells represented the geometry of the enclosure (O'Keefe & Burgess, 1996). But a coherent rescaling of a number of place cells with the deformation of a shape could also imply that the animal ‘corrected’ for change in shape and possibly did not register it. In other words, if the place response determines an animal's perceived location as some evidence previously suggested (O'Keefe & Speakman, 1987; Zinyuk et al. 2000; Fenton et al. 2010), rescaling a perceived location proportionally to the rescaling of the enclosure would imply that the place cells could not convey the information about the changes made to the environment. Alternatively place cell remapping as a mechanism for representing shape could be viable if combined with categorical representation (Freedman et al. 2001), when in addition to randomly changed place cell activity a constant subset of place cells was activated which would be unique for every shape. Currently there is no experimental data supporting the existence of categorical representation in the hippocampus proper. In contrast some experimental observations show that a similar ensemble of cells is active when a rat is navigating in circular or square enclosures positioned in the same absolute space within a room, whereas a completely different groups of place cells are active when the animal is foraging in identical square enclosures placed in different experimental rooms (Leutgeb et al. 2005). Perhaps instead place cells might better be viewed as a very large set of building blocks of a spatial cognitive map, whose distance and angular relations must be defined elsewhere in order to get a coherent map and it is this coherence which represents the concept of the ‘geometry’ of an enclosure.
geo‐Metric System
In order to ‘understand’ the geometry of an environment one has to be able to measure different boundaries of the enclosure by either comparing their lengths in respect to each other (which would give an ordinal metric) or by measuring each of them against something like an ‘internal ruler’ (which would give a ratio metric). In addition one has to have the means to measure angular relationship between connected segments. Similar to lengths, angles could be measured either by comparing various angles to each other or by having an ‘internal protractor’ against which each is compared. Grid cells located in the medial entorhinal cortex could in principle carry out such operations (Fig. 2 B). When an animal forages in a highly symmetrical two dimensional enclosures (e.g. square or circular enclosures), grid cells are active in multiple fields covering the entire enclosure and arranged in a hexagonally symmetrical pattern (Hafting et al. 2005). Grid cells are anatomically arranged along the dorsoventral axis of layer II medial entorhinal cortex in discrete modules each with a distinct scale defined by the average distance from the six nearest fields (Barry et al. 2007; Stensola et al. 2012). Different grid modules may have different orientations relative to the reference frame of the enclosure (Stensola et al. 2012). However, overall grid orientations across all the modules tend to align to each other or be 30 deg apart (Krupic et al. 2015). They align to the walls of the enclosure with a slight 7.5–8.8 deg offset (Krupic et al. 2014, 2015; Stensola et al. 2015; Sun et al. 2015). Hence the grid cell system has the capacity to orient the animal based on the geometric layout of the enclosure as well as to measure the distances between different walls. Importantly grid structure becomes non‐homogeneous in more complex shapes such as trapezoids (Krupic et al. 2015). It is expanded and rotated in the narrower end of the trapezoid compared to the wider end, suggesting that estimated distances in the narrower end might appear to be shorter than in the wider end – space could be perceived as shrinking towards the narrow end (i.e. if an average distance between the two adjacent firing fields on the right side (the wide part of the trapezoid) was equal to 40 cm, the distance on the left (the narrow part of the trapezoid) would generally be larger, say 50 cm, because the grid expanded. This implies that 40 cm on the right may be perceived as equal to 50 cm on the left as indicated by rat's internal ruler). Interestingly in behavioural studies animals find it more difficult to orient themselves in more complex enclosures such as kite‐shaped ones compared to more symmetrical enclosures such as rectangles (Pearce et al. 2006; Jones et al. 2007).
Local vs. global grid
The non‐homogeneity of grid cell firing suggests that the grid cell system is not a universal metric system as previously suggested (Hafting et al. 2005; Moser & Moser, 2008; Buzsáki & Moser, 2013; Moser et al. 2014) but instead reflects the local spatial configuration of the enclosure. Indeed it has been shown that in some situations rats use local rather than global geometric features of the enclosure to find a reward (Pearce et al. 2004). However, this is not to say that various corners of the enclosure should be seen as separate independent entities. Instead it could be that the local configuration influences an otherwise continuous grid whereby local distortions propagate through the entire grid as seen in the rate maps of grid cells recorded in trapezoids (Krupic et al. 2015) and even in the larger square enclosures (Stensola et al. 2015). In the trapezoid, the grid structure is disproportionately distorted in the wider more‐rectangular end as well as in the narrower triangular end (Fig. 3).
Figure 3. Grid cells in different shape enclosures .
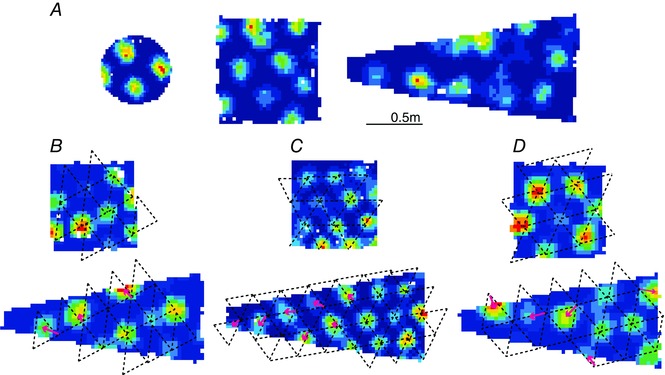
A, typical grid cells recorded from the same rat in a circle (left), square (middle) and trapezoid (right). The same grid cell was recorded in the square and trapezoid. B–D, grid cells from three different rats recorded in a square (top) and trapezoid (bottom). Positions of grid fields in the square were well approximated by the nodes of a regular superimposed grid (dashed black line) whereas the same grid (rotated by 10 deg counterclockwise (C) and 90 deg clockwise (D)) superimposed on a trapezoid approximated grid fields on the right side but not on the left of the enclosure. Pink arrows show how far the grid fields were displaced from the nodes of the regular grid.
The representation of an environment may initially be local and become more global with experience. Rats presented with identical inter‐connected compartments at first found it difficult to discriminate between them as they searched for reward in similar places relative to the walls in both compartments (Grieves et al. 2015). In line with this, both place cells and grid cells initially had identical firing patterns within each compartment (Skaggs & McNaughton, 1998; Derdikman et al. 2009; Spiers et al. 2015; Carpenter et al. 2015). But with experience the grid cell pattern becomes more global suggesting that separate enclosures are integrated into a single continuous frame (Carpenter et al. 2015). The same tendency of the grid to become more coherent with experience is seen in polarized continuous enclosures (Krupic et al. 2015), but the time it takes for a grid to become regular and continuous seems to be rather long, on the order of days (Barry et al. 2012; Krupic et al. 2015; Carpenter et al. 2015), and depends on the complexity of the enclosure shape: it takes 5 days for a grid to converge in a trapezoid compared to 3 days in a square of equal area. It is also conceivable that grid responses to geometric features can be task dependent and forcing a rat to rely more on geometric cues may make the grid system more responsive to the shape of an enclosure.
Interaction between geometric and featural cues
A number of studies have suggested that geometric information is processed in a dedicated ‘geometric module’, a dedicated brain region not accessible by other (sensory) modalities (Cheng, 1986; Gallistel, 1993; Wall et al. 2004, 2004; Cheng & Newcombe, 2005), an idea more generally proposed by Fodor (1983). The modularity idea was supported by the observation that often different types of information are not combined to calculate location (Cheng, 1986; Hermer & Spelke, 1994). However, more recently a substantial body of evidence has accumulated which strongly suggests that different types of information do interact under certain circumstances (Tommasi & Thinus‐Blanc, 2004; Cheng & Newcombe, 2005; Gray et al. 2005; Pearce et al. 2006). Hence it is possible that different types of information are processed by different brain areas but are integrated in a ‘receiver’ area where the location estimation occurs. For instance, the ‘receiver’ area for self‐location read‐out could be the hippocampus proper (O'Keefe & Conway, 1978). Anderson & Jeffery (2003) studied the interaction of geometric and feature cues on place cell activity by recording hippocampal place cells while rats foraged in four different square enclosures: black colour with lemon smell, black with vanilla, white with lemon and black with lemon. Some place cells ignored the feature cues and responded the same in all four square boxes but most responded in complex ways to the interaction of shape with colour and odour.
The medial entorhinal cortex, one of the major hippocampal input region, could be essential for processing information about the geometry of the enclosure. It is not clear which brain areas convey information about featural cues (such as visual, tactile and olfactory cues). One of the strong candidates is the lateral entorhinal cortex, another major input area to the hippocampus, which may be encoding the information about discrete objects and sensory cues, such as sounds and smells (Knierim et al. 2014). One of the strongest projections from visual areas terminates in the postrhinal cortex which has only meagre direct projections to the hippocampus but instead strongly projects to the medial entorhinal cortex (Furtak et al. 2007). Thus it is likely that information on visual as well as geometric cues is integrated within the medial entorhinal cortex. However, it is still possible that processing of these various types of information is done separately (and independently) by different (functional) cell types.
One cell type in the medial entorhinal cortex which might contribute to the representation of geometry is the boundary cell (Fig. 2 C), also called ‘border cell’, which is usually active whenever the animal is at the border of the enclosure (Savelli et al. 2008; Solstad et al. 2008; see also Barry et al. 2006; Lever et al. 2009 for boundary vector cells in subiculum). Different boundary cells prefer different orientations of the wall usually with a firing range of 90 deg, e.g. one‐quarter of a circular environment or one side of a square. However, there must be a ‘metric system’ which combines information from different boundary cells to create a representation of the geometric layout of the enclosure. Otherwise shapes like squares and rectangles would look the same from the point of view of boundary cells, but they are clearly distinguished by the animal as shown by its ability to orient itself in a rectangle: in a rectangle correct and rotated corners are chosen most frequently, whereas all four corners are selected at similar frequencies in a square (Fig. 1 A; Cheng, 1986; Hermer‐Vazquez et al. 2001). Hence there must be another cell type which integrates information about borders, and their angular and distance relations. We suggest that this could be accomplished by grid cells.
Recent findings suggest that grid cells are strongly affected by the geometry of the enclosure (Krupic et al. 2015; Stensola et al. 2015). For example, it has been shown that the grid pattern is rotated by rotating a polarized enclosure even when many stable visual and other featural cues are available to the animal (Krupic et al. 2015). The grid pattern is also transformed in response to transformation of a familiar enclosure (Barry et al. 2007) and may lose its hexagonality in more complex shapes such as trapezoids (Krupic et al. 2015). These observations strongly suggest that the grid cell encodes or reflects the shape of the enclosure. However, the grid cell pattern can also be rotated by rotating a large polarising visual cue card when the animal is exploring a circular enclosure (Hafting et al. 2005), demonstrating that grid orientation can be affected by the prominent distal visual landmarks as well. It is likely that the rotation of a prominent distal cue primarily affects the head direction cells (Fig. 2 D), cells which are active when the animal is facing specific directions in an allocentric reference frame (Ranck, 1984; Taube et al. 1990 a,b), which in turn rotate all other spatial cell types (Knierim et al. 1995; Solstad et al. 2008; Bush et al. 2014). When muscimol was injected into the hippocampus, both place cells and grid cells lost their spatial firing properties, whereas head direction cells as well as boundary cells remained intact (Bonnevie et al. 2013). Furthermore, many grid cells instead showed head direction preference and inactivation of head direction cells in the anterodorsal thalamic nucleus completely degraded the grid cell pattern (Winter et al. 2015), further corroborating the idea that head direction input has a strong direct effect on its directional firing properties of grid cells.
Thus in principle the grid pattern is affected by both types of information. As a result, a geometric representation is possibly conveyed by the grid generation mechanism (instead of a dedicated cell type) where the places are connected to each other and confined by borders and controlled by other sensory input (mostly visual cues possibly via head direction cells).
Constructing the grid
It is widely believed that grid cells represent a path integration system (Hafting et al. 2005; McNaughton et al. 2006; Moser & Moser, 2008; Buzsáki & Moser, 2013; Moser et al. 2014). According to this view, the grid cell signal is generated by integrating a velocity signal (McNaughton et al. 2006; Fuhs & Touretzky, 2006; Burgess et al. 2007, 2008; Fiete et al. 2008; Hasselmo, 2008). Path integration is subject to cumulative errors and it has been suggested that grid cells receive inputs from boundary cells to correct for this (Bush et al. 2014; Cheung, 2014; Hardcastle et al. 2015).
Another way to view a grid is as a system that arranges places into a topographical order. We (Krupic et al. 2014) and others (Kropff & Treves, 2008) have proposed that grid cells may be generated by the inputs from place units. We suggested that place inputs could be viewed as competing place cells. We also suggested that boundary cells could contribute to place unit interaction by ‘pushing’ place fields away from the boundaries (Krupic et al. 2014). Such interaction would give rise to two important properties: firstly, the grid orientation should tend to align to the walls; secondly, grids should have a tendency to be more irregular and elliptical in more polarized environments such as trapezoids. Both of these properties were verified experimentally (Krupic et al. 2015; Stensola et al. 2015). If the fundamental building blocks of a grid were indeed place and not periodic band inputs (Burgess et al. 2007, 2008; Hasselmo, 2008; Mhatre et al. 2012; see also Welday et al. 2011; Krupic et al. 2012) for some experimental evidence, or travelling bumps of activity (Fuhs & Touretzky, 2006; Guanella et al. 2007; Fiete et al. 2008; Pastoll et al. 2013; Couey et al. 2013; see Bonnevie et al. 2013; Schmidt‐Hieber & Häusser, 2013; Domnisoru et al. 2013) for some experimental evidence, it would be conceivable that path integration (measuring distance and angles) is computed somewhere else: perhaps in the medial septum and thalamic nuclei where strong rhythmic and directional signals are located (Welday et al. 2011; Fuhrmann et al. 2015). Further evidence in support of this hypothesis comes from observations that lesions of the anterodorsal thalamus (Frohardt et al. 2006) did not completely impair path integration capability, whereas the same authors showed that these manipulations disrupted the grid cell firing pattern (Winter et al. 2015). In contrast, lesions to the hippocampus proper induced a complete inability of an animal to rely on path integration (Maaswinkel et al. 1999; Wallace & Whishaw, 2003; but see Alyan & McNaughton, 1999), suggesting that place cells play a key role in path integration.
Interestingly, it has recently been shown that lesions to the medial entorhinal cortex reduce an animal's ability to navigate to a goal (Hales et al. 2014) while sparing place cell activity. One possible explanation of this deficit is that the impairment might be due to an inability to associate places with each other (resulting in impaired navigational capability), but once the goal is reached the animal is able to recognize it.
How to dissociate path integration from the topographical arrangement of places? It is a difficult task, which requires the inactivation of place cells with grid cells left intact. So far this experiment has not been possible (Bonnevie et al. 2013), unlike the reversed paradigm, where place cells were spared but grid cells were disrupted (Koenig et al. 2011; Brandon et al. 2014). A possible indirect test could be to test an animal's ability to plan a route to the goal in the absence of grid cells (but with place cells spared): we predict that route planning should be impaired whereas place recognition ability should be preserved. Another indirect test could include using geometry to orient. We predict that an animal's ability to use geometric cues for navigation might also be compromised.
Conclusions
There is strong evidence from both behavioural studies as well as neural recordings that vertebrates can ‘understand’ the geometry of an environment and use angular and length relations of the walls to locate themselves. They can do so by both estimating the fixed distances to the walls or by estimating the fixed ratios of the distances to the opposing walls (Tommasi & Thinus‐Blanc, 2004; Hartley et al. 2004). While the former navigational strategy could be explained by viewpoint matching, the latter must be a true example of geometric cue based navigation.
On the whole, space does not have a shape so it is intriguing that animals are able to ‘perform’ geometric calculations. Unlike lab rooms and experimental enclosures, from the evolutionary point of view wild rats lived in fields and burrows which had obstacles, landmarks (probably a natural site closest to the confined enclosure is a flat hilltop). There was a suggestion (Benhamou & Poucet, 1998) that the perception of geometry can be created by arranging local objects in a particular geometrical configuration. However other studies suggest that this is not the case (Skov‐Rackette & Shettleworth, 2005). It has been shown that the rotation of local objects arranged in a triangular layout had little control over place fields (Cressant et al. 1997), suggesting that such object arrangements are coded differently than the geometry of the enclosure. Perhaps the distinction between boundaries and objects reduces to the idea that small objects only occupy one or two place fields but a boundary occupies several place fields in a particular configuration, e.g. a straight line. Grid cells might detect such a configuration. In other words if a group of disconnected objects was large enough and moved coherently, they would control the location of place cells. This view is partially supported by the observation of Cressant and colleagues (Cressant et al. 1997,1999) who showed that co‐recorded place cells acted differently: one of them actually followed the rotation of the objects. It is yet to be determined whether this happened by chance or there was some systematic response: for example the ones closest to the rotated objects respond to their movements/rotations). There are still no reports of similar studies carried out to look at grid cell response to such object manipulations.
What do we mean by ‘encoding the geometry’? Generally it would mean that the shape of the enclosure could be decoded from grid cell activity. At the moment there is no good evidence in support of this hypothesis. For example, it does not seem to be possible to distinguish a circular from a square enclosure based on grid cell activity alone. However, it would be possible to do it based on the activity of boundary cells. On the other hand, boundary cells alone could not distinguish a square from the rectangle unless combined with information about their lengths which could in principle be provided by grid cells. Thus it could be that grid interaction with boundary cells, place cells and head direction cells (Fig. 2 D), possibly by the way grid cells are generated (Krupic et al. 2014), enables encoding of geometric information. The nature of such interaction could depend on animals’ experience, task at hand, cue reliability and salience, size of enclosure (Learmonth et al. 2002) and possibly even the way the animal enters the enclosure (Jones et al. 2007). Currently very little is known about how grid cell symmetry is affected by the strategy the animal is using to navigate.
Additional information
Competing interests
The authors declare no conflicts of interest.
Author contributions
All authors wrote the manuscript. All authors approved the final version of the manuscript and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
J.K. is a Sir Henry Wellcome Fellow; M.B., S.B. and J.O'K. are supported by the Wellcome Trust and Gatsby Charitable Foundation.
Biographies
Julija Krupic is a Sir Henry Wellcome Fellow. Her main interest is concerned with finding the underlying mechanism and functional role of spatial cells in the hippocampal formation, in particular grid cells, place cells and boundary cells. She is also interested in unravelling how place cell activity contributes to an animal's perception of location.

Marius Bauza is a research associate. His main work interests include understanding the development and underlying mechanisms of spatial memory system in the hippocampal formation.
Stephen Burton is a research associate studying spatial memory and its representation in the hippocampal formation, with particular focus on place cells, grid cells and spatial cells in the dentate gyrus.
John O'Keefe is interested in spatial cells in the hippocampal formation and how they form a cognitive map. He is currently the director of the Sainsbury Wellcome Centre for Neural Circuits and Behaviour.
This review was presented at the symposium “Knowing where you are: circuit mechanisms for estimating location”, which took place at the BNA 2015 Festival of Neuroscience, between 12–15 April 2015.
References
- Alyan S & McNaughton BL (1999). Hippocampectomized rats are capable of homing by path integration. Behav Neurosci 113, 19–31. [DOI] [PubMed] [Google Scholar]
- Anderson MI & Jeffery KJ (2003). Heterogeneous modulation of place cell firing by changes in context. J Neurosci 23, 8827–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Ginzberg LL, O'Keefe J & Burgess N (2012). Grid cell firing patterns signal environmental novelty by expansion. Proc Natl Acad Sci USA 109, 17687–17692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Hayman R, Burgess N & Jeffery KJ (2007). Experience‐dependent rescaling of entorhinal grids. Nat Neurosci 10, 682–684. [DOI] [PubMed] [Google Scholar]
- Barry C, Lever C, Hayman R, Hartley T, Burton S, O'Keefe J, Jeffery K & Burgess N (2006). The boundary vector cell model of place cell firing and spatial memory. Rev Neurosci 17, 71–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou S & Poucet B (1998). Landmark use by navigating rats (Rattus norvegicus) contrasting geometric and featural information. J Comp Psychol 112, 317–322. [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI & Moser M‐B (2013). Grid cells require excitatory drive from the hippocampus. Nat Neurosci 16, 309–317. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK & Leutgeb S (2014). New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron 82, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N (2008). Grid cells and theta as oscillatory interference: Theory and predictions. Hippocampus 18, 1157–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Barry C & O'Keefe J (2007). An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, Barry C & Burgess N (2014). What do grid cells contribute to place cell firing? Trends Neurosci 37, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G & Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal–entorhinal system. Nat Neurosci 16, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F, Manson D, Jeffery K, Burgess N & Barry C (2015). Grid cells form a global representation of connected environments. Curr Biol 25, 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K (1986). A purely geometric module in the rat's spatial representation. Cognition 23, 149–178. [DOI] [PubMed] [Google Scholar]
- Cheng K & Newcombe N (2005). Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev 12, 1–23. [DOI] [PubMed] [Google Scholar]
- Cheung A (2014). Estimating location without external cues. PLoS Comput Biol 10, e1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett TS (1996). Insect navigation en route to the goal: multiple strategies for the use of landmarks. J Exp Biol 199, 227–235. [DOI] [PubMed] [Google Scholar]
- Couey JJ, Witoelar A, Zhang S‐J, Zheng K, Ye J, Dunn B, Czajkowski R, Moser M‐B, Moser EI, Roudi Y & Witter MP (2013). Recurrent inhibitory circuitry as a mechanism for grid formation. Nat Neurosci 16, 318–324. [DOI] [PubMed] [Google Scholar]
- Cressant A, Muller RU & Poucet B (1997). Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci 17, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Muller RU & Poucet B (1999). Further study of the control of place cell firing by intra‐apparatus objects. Hippocampus 9, 423–431. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser M‐B & Moser EI (2009). Fragmentation of grid cell maps in a multicompartment environment. Nat Neurosci 12, 1325–1332. [DOI] [PubMed] [Google Scholar]
- Domnisoru C, Kinkhabwala AA & Tank DW (2013). Membrane potential dynamics of grid cells. Nature 495, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck‐Santini P‐P, Zinyuk LE, Š Kubík, J Bureš, Poucet B, Muller RU & Olypher AV (2010). Attention‐like modulation of hippocampus place cell discharge. J Neurosci 30, 4613–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Burak Y & Brookings T (2008). What grid cells convey about rat location. J Neurosci 28, 6858–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA (1983). Modularity of Mind. MIT Press, Cambridge, MA, USA. [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T & Miller EK (2001). Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Bassett JP & Taube JS (2006). Path integration and lesions within the head direction cell circuit: comparison between the roles of the anterodorsal thalamus and dorsal tegmental nucleus. Behav Neurosci 120, 135–149. [DOI] [PubMed] [Google Scholar]
- Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M & Remy S (2015). Locomotion, theta oscillations, and the speed‐correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 86, 1253–1264. [DOI] [PubMed] [Google Scholar]
- Fuhs MC & Touretzky DS (2006). A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci 26, 4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei S‐M, Agster KL & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17, 709–722. [DOI] [PubMed] [Google Scholar]
- Gallistel C (1993). The Organization of Learning. MIT Press, Cambridge, MA, USA. [Google Scholar]
- Gouteux S, Thinus‐Blanc C & Vauclair J (2001). Rhesus monkeys use geometric and nongeometric information during a reorientation task. J Exp Psychol Gen 130, 505. [DOI] [PubMed] [Google Scholar]
- Graham M, Good MA, McGregor A & Pearce JM (2006). Spatial learning based on the shape of the environment is influenced by properties of the objects forming the shape. J Exp Psychol Anim Behav Process 32, 44–59. [DOI] [PubMed] [Google Scholar]
- Gray ER, Bloomfield LL, Ferrey A, Spetch ML & Sturdy CB (2005). Spatial encoding in mountain chickadees: features overshadow geometry. Biol Lett 1, 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieves RM, Jenkins BW, Harland B, Wood ER & Dudchenko PA (2015). Place field repetition and spatial learning in a multicompartment environment. Hippocampus 26, 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanella A, Kiper D & Verschure P (2007). A model of grid cells based on a twisted torus topology. Int J Neural Syst 17, 231–240. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M‐B & Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S & Clark RE (2014). Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus‐dependent place memory. Cell Rep 9, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle K, Ganguli S & Giocomo LM (2015). Environmental boundaries as an error correction mechanism for grid cells. Neuron 86, 827–839. [DOI] [PubMed] [Google Scholar]
- Hartley T, Burgess N, Lever C, Cacucci F & O'Keefe J (2000). Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379. [DOI] [PubMed] [Google Scholar]
- Hartley T, Trinkler I & Burgess N (2004). Geometric determinants of human spatial memory. Cognition 94, 39–75. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (2008). Grid cell mechanisms and function: Contributions of entorhinal persistent spiking and phase resetting. Hippocampus 18, 1213–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A, Good MA & Pearce JM (2004). Failure of a landmark to restrict spatial learning based on the shape of the environment. Q J Exp Psychol B 57, 289–314. [DOI] [PubMed] [Google Scholar]
- Heilbron JL (2000). Geometry Civilized: History, Culture, and Technique. Clarendon Press, Clarendon, Oxford. [Google Scholar]
- Hermer L & Spelke ES (1994). A geometric process for spatial reorientation in young children. Nature 370, 57–59. [DOI] [PubMed] [Google Scholar]
- Hermer‐Vazquez L, Moffet A & Munkholm P (2001). Language, space, and the development of cognitive flexibility in humans: the case of two spatial memory tasks. Cognition 79, 263–299. [DOI] [PubMed] [Google Scholar]
- Hermer‐Vazquez L, Spelke ES & Katsnelson AS (1999). Sources of flexibility in human cognition: dual‐task studies of space and language. Cognit Psychol 39, 3–36. [DOI] [PubMed] [Google Scholar]
- Jones CM & Healy SD (2006). Differences in cue use and spatial memory in men and women. Proc R Soc B Biol Sci 273, 2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Pearce JM, Davies VJ, Good MA & McGregor A (2007). Impaired processing of local geometric features during navigation in a water maze following hippocampal lesions in rats. Behav Neurosci 121, 1258–1271. [DOI] [PubMed] [Google Scholar]
- Kelly D, Spetch ML & Heth C (1998). Pigeons’ (Columba livia) encoding of geometric and featural properties of a spatial environment. J Comp Psychol 112, 359–369. [Google Scholar]
- Kelly JW, McNamara TP, Bodenheimer B, Carr TH & Rieser JJ (2009). Individual differences in using geometric and featural cues to maintain spatial orientation: Cue quantity and cue ambiguity are more important than cue type. Psychon Bull Rev 16, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS & McNaughton BL (1995). Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15, 1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP & Deshmukh SS (2014). Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local – global reference frames. Philos Trans R Soc Lond B Biol Sci 369, 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK & Leutgeb S (2011). The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595. [DOI] [PubMed] [Google Scholar]
- Kropff E & Treves A (2008). The emergence of grid cells: Intelligent design or just adaptation? Hippocampus 18, 1256–1269. [DOI] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, Barry C & O'Keefe J (2015). Grid cell symmetry is shaped by environmental geometry. Nature 518, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, Lever C & O'Keefe J (2014). How environment geometry affects grid cell symmetry and what we can learn from it. Philos Trans R Soc B Biol Sci 369, 20130188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J, Burgess N & O'Keefe J (2012). Neural Representations of location composed of spatially periodic bands. Science 337, 853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth AE, Nadel L & Newcombe NS (2002). Children's use of landmarks: implications for modularity theory. Psychol Sci 13, 337–341. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL & Moser M‐B (2005). Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309, 619–623. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O'Keefe J & Burgess N (2009). Boundary Vector Cells in the subiculum of the hippocampal formation. J Neurosci 29, 9771–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N & O'Keefe J (2002). Long‐term plasticity in hippocampal place‐cell representation of environmental geometry. Nature 416, 90–94. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Jarrard LE & Whishaw IQ (1999). Hippocampectomized rats are impaired in homing by path integration. Hippocampus 9, 553–561. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI & Moser M‐B (2006). Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci 7, 663–678. [DOI] [PubMed] [Google Scholar]
- Mhatre H, Gorchetchnikov A & Grossberg S (2012). Grid cell hexagonal patterns formed by fast self‐organized learning within entorhinal cortex. Hippocampus 22, 320–334. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP & O'Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E & Moser M‐B (2008). Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci 31, 69–89. [DOI] [PubMed] [Google Scholar]
- Moser EI & Moser M‐B (2008). A metric for space. Hippocampus 18, 1142–1156. [DOI] [PubMed] [Google Scholar]
- Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T & Moser M‐B (2014). Grid cells and cortical representation. Nat Rev Neurosci 15, 466–481. [DOI] [PubMed] [Google Scholar]
- Muller RU & Kubie JL (1987). The effects of changes in the environment on the spatial firing of hippocampal complex‐spike cells. J Neurosci 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J & Burgess N (1996). Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. [DOI] [PubMed] [Google Scholar]
- O'Keefe J & Conway DH (1978). Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res 31, 573–590. [DOI] [PubMed] [Google Scholar]
- O'Keefe J & Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Res 34, 171–175. [DOI] [PubMed] [Google Scholar]
- O'Keefe J & Nadel L (1978). The Hippocampus as a Cognitive Map. Oxford University Press, UK. [Google Scholar]
- O'Keefe J & Speakman A (1987). Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res 68, 1–27. [DOI] [PubMed] [Google Scholar]
- Pastoll H, Solanka L, van Rossum MCW & Nolan MF (2013). Feedback inhibition enables θ‐nested γ oscillations and grid firing fields. Neuron 77, 141–154. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Good MA, Jones PM & McGregor A (2004). Transfer of spatial behavior between different environments: implications for theories of spatial learning and for the role of the hippocampus in spatial learning. J Exp Psychol Anim Behav Process 30, 135–147. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Graham M, Good MA, Jones PM & McGregor A (2006). Potentiation, overshadowing, and blocking of spatial learning based on the shape of the environment. J Exp Psychol Anim Behav Process 32, 201–214. [DOI] [PubMed] [Google Scholar]
- Ranck JB Jr (1984). Head‐direction cells in the deep cell layers of dorsal presubiculum in freely moving rats. Soc Neurosci Abstr. [Google Scholar]
- Savelli F, Yoganarasimha D & Knierim JJ (2008). Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 18, 1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Hieber C & Häusser M (2013). Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci 16, 325–331. [DOI] [PubMed] [Google Scholar]
- Skaggs WE & McNaughton BL (1998). Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci 18, 8455–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov‐Rackette SI & Shettleworth SJ (2005). What do rats learn about the geometry of object arrays? Tests with exploratory behavior. J Exp Psychol Anim Behav Process 31, 142–154. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M‐B & Moser EI (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Bisazza A & Vallortigara G (2007). How fish do geometry in large and in small spaces. Anim Cogn 10, 47–54. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Hayman RMA, Jovalekic A, Marozzi E & Jeffery KJ (2015). Place field repetition and purely local remapping in a multicompartment environment. Cereb Cortex N Y N 1991 25, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Frøland K, Moser M‐B & Moser EI (2012). The entorhinal grid map is discretized. Nature 492, 72–78. [DOI] [PubMed] [Google Scholar]
- Stensola T, Stensola H, Moser M‐B & Moser EI (2015). Shearing‐induced asymmetry in entorhinal grid cells. Nature 518, 207–212. [DOI] [PubMed] [Google Scholar]
- Sun C, Kitamura T, Yamamoto J, Martin J, Pignatelli M, Kitch LJ, Schnitzer MJ & Tonegawa S (2015). Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci USA 112, 9466–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU & Ranck JB Jr (1990. a). Head‐direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU & Ranck JB Jr (1990. b). Head‐direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi L & Polli C (2004). Representation of two geometric features of the environment in the domestic chick (Gallus gallus). Anim Cogn 7, 53–59. [DOI] [PubMed] [Google Scholar]
- Tommasi L & Thinus‐Blanc C (2004). Generalization in place learning and geometry knowledge in rats. Learn Mem 11, 153–161. [DOI] [PubMed] [Google Scholar]
- Vargas JP, Petruso EJ & Bingman VP (2004). Hippocampal formation is required for geometric navigation in pigeons. Eur J Neurosci 20, 1937–1944. [DOI] [PubMed] [Google Scholar]
- Wall PL, Botly LCP, Black CK & Shettleworth SJ (2004). The geometric module in the rat: independence of shape and feature learning in a food finding task. Anim Learn Behav 32, 289–298. [DOI] [PubMed] [Google Scholar]
- Wallace DG & Whishaw IQ (2003). NMDA lesions of Ammon's horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur J Neurosci 18, 513–523. [DOI] [PubMed] [Google Scholar]
- Welday AC, Shlifer IG, Bloom ML, Zhang K & Blair HT (2011). Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J Neurosci 31, 16157–16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Barnett AM & Meck WH (1990). Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci 104, 84–97. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N & O'Keefe J (2005). Attractor dynamics in the hippocampal representation of the local environment. Science 308, 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Clark BJ & Taube JS (2015). Spatial navigation. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347, 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA & Bures J (2000). Understanding hippocampal activity by using purposeful behavior: Place navigation induces place cell discharge in both task‐relevant and task‐irrelevant spatial reference frames. Proc Natl Acad Sci USA 97, 3771–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]


