Abstract
AIM
To investigate the association between hepatitis C virus (HCV) infection and risk of renal cell carcinoma (RCC).
METHODS
A literature search was performed from inception until February 2016. Studies that reported relative risks, odd ratios, hazard ratios or standardized incidence ratio comparing the risk of RCC among HCV-infected participants vs those without HCV infection were included. Participants without HCV infection were used as comparators. Pooled odds ratios and 95%CI were calculated using a random-effect, generic inverse variance method.
RESULTS
Seven observational studies were with 196826 patients were included in the analysis to assess the risk of RCC in patients with HCV. A significantly increased risk of RCC among participants with HCV infection was found with a pooled RR of 1.86 (95%CI: 1.11-3.11). The association between RCC and HCV was marginally insignificant after a sensitivity analysis limited only to studies with adjusted analysis, with a pooled RR of 1.50 (95%CI: 0.93-2.42).
CONCLUSION
Our study demonstrated a potential association between HCV infection and RCC. Further studies of RCC surveillance in patients with HCV are required.
Keywords: Hepatitis C virus, Renal cancer, Kidney cancer, Systematic review, Meta-analysis
Core tip: Hepatitis C virus (HCV) is a leading cause of cirrhosis in the United States with a steadily increasing prevalence over the past two decades. Interestingly, HCV infection may also be associated with an increased risk of renal cell carcinoma (RCC) as observed in several epidemiologic studies. To further investigate this possible association, we conducted this systematic review and meta-analysis of observational studies reporting the risk of RCC among HCV-infected patients. We found a significantly increased risk of RCC among participants with HCV infection with the pooled risk ratio of 1.86 (95%CI: 1.11-3.11).
INTRODUCTION
Hepatitis C virus (HCV) remains the most common cause of chronic liver disease and cirrhosis worldwide and is one of the leading causes of chronic hepatitis and cirrhosis in the United States with a steadily increasing prevalence over the past two decades[1,2]. While commonly associated with hepatocellular carcinoma[2], hepatitis C infection is associated with extrahepatic malignancies including cholangiocarcinoma, non-Hodgkin’s lymphoma and possibly myeloma[3-5]. The oncogenic properties of hepatitis C are hypothesized to be secondary to chronic antigenic stimulation of the immune system, promotion of a chronic inflammatory state or direct oxidative stress[6]. Besides, chronic HCV infection has also been linked to a myriad of extrahepatic diseases including increasing the risk of renal disease and increasing the prevalence of chronic kidney disease up to 40% higher compared to non-infected patients[7,8].
Renal cell carcinoma (RCC), arising from the renal cortex is responsible for 80% of all renal malignancies and accounts for approximately 14000 deaths each year in the United States[9]. The risk factors for RCC such as acquired cystic kidney disease, smoking, kidney stones, obesity, hereditary factors have been described[10-14]. The epidemiological studies have demonstrated an increasing incidence of RCC, particularly in African Americans[15].
Secondary to the oncogenic nature of Hepatitis C, several studies have linked chronic infection with an increased risk for development of RCC[16-22]. However, the findings from these studies were contradictory. Thus, we performed this meta-analysis to examine the risk of RCC in HCV-infected patients.
MATERIALS AND METHODS
Literature search
Two investigators (Karn Wijarnpreecha and Wisit Cheungpasitporn) independently reviewed published studies indexed in MEDLINE, EMBASE, and the Cochrane database from their inception to February 2016 using the search strategy that included the terms for “hepatitis” and “renal cancer” as described in Item S1 in online supplementary data. No limitation on language was applied. A manual search for additional studies using references of selected retrieved articles was also performed. Three investigators (Karn Wijarnpreecha, Charat Thongprayoon and Wisit Cheungpasitporn) independently reviewed the titles and abstracts of the studies identified in the search based on inclusion and exclusion criteria. The full text of the included studies from the first phase was reviewed independently to ascertain whether or not they matched the inclusion criteria. We also performed a manual search of conference proceedings from major gastroenterology and hepatology meetings for additional abstracts on the topic. When additional information was needed, we contacted the corresponding investigators of eligible studies.
Study selection
The inclusion criteria were as follows: (1) observational studies assessing the association between hepatitis C and RCC; (2) odds ratios, relative risks or hazard ratios with 95%CI were provided; and (3) individuals without HCV infection were used as comparators in cohort studies while individuals without RCC were applied as comparators in the cross-sectional and case-control studies.
Study acceptability was individually defined by the three investigators mentioned above. Disagreements in the ascertainment of study eligibility were settled by joint agreement. Also, the quality of each study was individually appraised by each investigator. We used the validated Newcastle-Ottawa quality assessment scale for cohort and case-control studies[23] and modified Newcastle-Ottawa scale[24] for the cross-sectional study.
Data extraction
A data collection report was utilized to derive the information from each study including name of title and the first author, year of study and publication, country, demographic data of the participants, number of participants, method used to diagnose the HCV infection and RCC, effect estimates (odds ratios, relative risks or hazard ratios) with 95%CI, and factors adjusted in the multivariate analysis. To assure the certainty, this data extraction process was reviewed by all investigators.
Statistical analysis
Review Manager (RevMan) 5.3 software from the Cochrane Collaboration was utilized for meta-analysis. Generic inverse variance (DerSimonian and Laird) method[25] was employed to combined adjusted point estimates and standard errors from each study. We used a random-effect model due to the high likelihood of between-study variance from different study designs and populations. Cochran’s Q test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0%-25%, 25%-50%, 50%-75%, and greater than 75% embodied insignificant, low, moderate and high heterogeneity, respectively[26].
RESULTS
Of 5778 potentially relevant articles, 5582 articles were excluded by the title and abstract not fulfilling inclusion criteria due to the type of article, study design, population, or outcome of interest. Additionally, 189 articles were excluded (35 articles were not observational studies, and 154 articles did not describe the outcomes of interest, Macleod et al[27]’s study did not contain data on specific viral hepatitis{Macleod, 2013 #83}). Finally, seven observational studies (4 cohort and 3 case-controlled studies) with 196826 patients were included in the meta-analysis[16-22]. Item S2 describes the study selection flow. The characteristics and quality appraisal of the included studies of HCV and RCC are shown in Table 1. Four studies were conducted in Europe, 2 in the United States, and 1 in Australia.
Table 1.
Main characteristics of the studies included in this meta-analysis
| Ref. | Country | Study design | Year | Total number | Study sample | Exposure definition | Exposure measurement | Outcome definition | Outcome ascertainment | Adjusted OR | Confounder adjustment | Quality assessment (Newcastle-Ottawa scale) |
| Amin et al[16] | Australia | Cohort study | 2006 | 75834 in HCV patients | People notified with HCV infection to the New South Wales Health Department’s Notifiable Diseases Database between 1 January 1990 and 31 December 2002 (HCV group) and NSW population (control group) | HCV infection | Detection of anti-HCV antibody or HCV RNA | Renal cancer or kidney cancer | The NSW Central Cancer Registry with ICD-10 code C64 for kidney cancer and C65 for renal cancer | Kidney cancer 0.9 (0.6–1.4) | Age, sex and calendar year | Selection: 4 Comparability: 1 Outcome: 3 |
| Malagu arnera et al[21] | Italy | Case control study | 2006 | 315 (15 case and 300 control) | Elderly kidney cancer patients attending geriatric department (case) and elderly volunteers (control) | Positive Anti-HCV | Detection of Anti-HCV antibody using enzyme-linked immunosorbent assay. Assay positive samples were confirmed by immunoblotting | Kidney cancer | N/A | 10.29 (3.49-30.36) | None | Selection: 3 Comparability: 0 Outcome: 2 |
| Omland et al[22] | Denmark | Cohort study | 2010 | 4204 in HCV patients | Acute or chronic HCV infected patients listed in the Danish National Hospital Registry between 1994 and 2003 without previous diagnosis of cancer (HCV) and general population (control) | Acute or chronic HCV infection | ICD-10 code (B17.1 and B18.2) from the Danish National Hospital Registry | Kidney cancer | The Danish Cancer Registry with ICD-7 code 180 | 3.60 (0.98–9.22) | Age, sex and year of diagnosis | Selection: 4 Comparability: 1 Outcome: 2 |
| Gordon et al[19] | United States | Cohort study | 2010 | 67063 | HCV-tested patients (both positive and negative) between 1997 and 2006 from administrative data from Henry Ford hospital | HCV infection | Positive anti-HCV test using enzyme-linked immunosorbent assay, confirmed by a documented positive molecular assay for HCV RNA | Renal cell carcinoma | Health system cancer registry, confirmed by medical record and pathology report review | 1.77 (1.05-2.98) | Age, race, gender, chronic kidney disease | Selection: 4 Comparability: 1 Outcome: 3 |
| Budakoğlu et al[17] | Turkey | Case-control study | 2011 | 6170 (903 case and 5267 control) | Patients who had histologically proven renal cell carcinoma diagnosis between 2005 to 2010 from six tertiary cancer centers (case) and healthy people who were living in the same geographic regions (control) | Positive anti-HCV | Positive anti-HCV test using enzyme-linked immunosorbent assay | Renal cell carcinoma | Histopathology report | 1.08 (0.62-1.88) | None | Selection: 3 Comparability: 0 Outcome: 3 |
| Hofmann et al[20] | Sweden | Cohort study | 2011 | 43000 in HCV patients | All Swedish residents diagnosed with HCV infection between 1990 and 2006 (HCV group) and general population (control) | Chronic HCV infection | The national surveillance database at the Swedish Institute for Infectious Disease Control | Kidney cancer | The national Cancer register with ICD-7 code 180.0 and 180.9 and histologic confirmation | 1.2 (0.8–1.7) | Age, sex and calendar year | Selection: 4 Comparability: 1 Outcome: 3 |
| Gonzalez et al[18] | United States | Case-control study | 2015 | 240 (140 case and 100 control) | Newly diagnosed renal cell carcinoma (case) and newly diagnosed colon cancer patients (control) | Chronic HCV infection | Detection of anti-HCV antibody or HCV RNA | Renal cell carcinoma | Histopathology report | Positive HCV RNA 24.20 (2.4 - > 999.9) | Sex, age, race, BMI, smoking, alcohol abuse, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, chronic kidney disease, cirrhosis | Selection: 3 Comparability: 2 Outcome: 3 |
BMI: Body mass index; DNHR: Danish National Hospital Registry; ELISA: Enzyme-linked immunosorbent assay; HCV: Hepatitis C virus; N/A: Not available; NSW: New South Wales; RCC: Renal cell carcinoma; SIR: Standardized incidence ratio.
The risk of renal cell carcinoma in patients with hepatitis C virus infection
Among individuals with HCV infection, there was a significantly increased risk of RCC with the pooled risk ratio (RR) of 1.86 (95%CI: 1.11-3.11, I2 =77%), as demonstrated in Figure 1. The statistical heterogeneity was high with an I2 of 77%. The association between RCC and HCV was marginally insignificant after the sensitivity analysis including only studies with confounder adjustment[16,18-20,22] with a pooled RR of 1.50 (95%CI: 0.93-2.42, I2 =64%), as shown in Figure 2.
Figure 1.
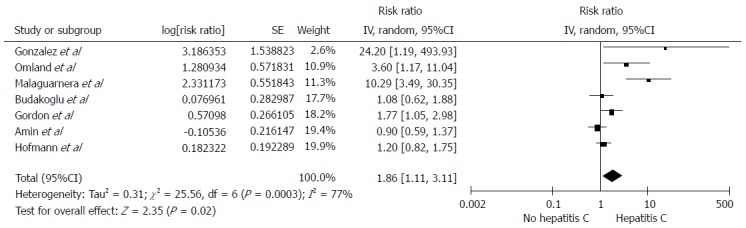
Forest plot of all included studies of the association between hepatitis C infection and renal cell carcinoma. Square data markers represent risk ratios (RRs); horizontal lines, the 95%CI with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95%CI for the outcome of interest.
Figure 2.
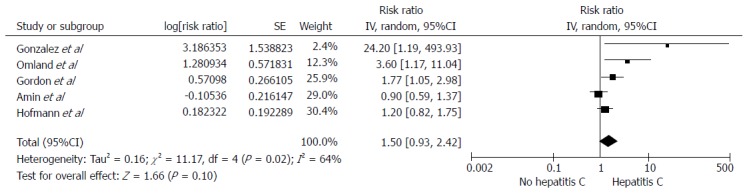
Forrest plot of all included studies in sensitivity analysis of the association between hepatitis C infection and renal cell carcinoma. Square data markers represent risk ratios (RRs); horizontal lines, the 95%CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95%CI for the outcome of interest.
Evaluation for publication bias
Two authors (Karn Wijarnpreecha and Wisit Cheungpasitporn) independently performed the assessment of the risk of bias of the included studies. Only minor disagreements between 2 reviewers were present and were resolved by discussion and consensus. A funnel plot was constructed to assess publication bias for the risk of RCC in HCV-infected patients (Figure S1). The funnel plot was suggestive of a small publication bias toward studies with a positive correlation between HCV infection and RCC.
DISCUSSION
This meta-analysis was conducted to summarize all presently available data on the association between HCV infection and RCC. Our study demonstrated a 1.86-fold increased risk of RCC among participants who had HCV infection compared to those without HCV infection. Our analysis also illustrates that that RCC patients with HCV were significantly younger than RCC patients without HCV. The results of our study reinforce the hypothesis that HCV may accelerate the risk of developing RCC.
Although the nature in which HCV induces RCC is not entirely understood, several hypotheses exist. A recent bioinformatics study demonstrated a plausible biological relationship between HCV infection and the development of RCC via the NY-REN-54 protein. The NY-REN-54 protein which is an altered ubiquitin-related protein that plays a role in the disturbance impairs the autophagic response via to the ubiquitin-protein ligase-related self-regulatory mechanism, which in turn promotes oncogenesis[28]. Additionally, inhibition of cytotoxic T-lymphocyte-dependent apoptosis by the hepatitis virus secondarily leads to a disturbance in host immunity and normal tissue homeostasis leading to carcinoma formation[29]. Lukkonen et al[30] demonstrated an increased expression of serine protease inhibitor Kazal (SPIK), a cellular protein that inhibits serine protease-related apoptosis, in RCC tissue samples as an additional mechanism for HCV-induced RCC.
There are several limitations in our study. Firstly, there was a high amount of statistical heterogeneity present in the completed analysis. The potential source of this heterogeneity includes variation in confounder-adjusted methods (e.g., age, sex, ethnicity, and chronic kidney disease), exposure measurement, outcome ascertainments, and follow-up duration. Secondly, it should be noted that there was a potential small publication bias with a positive association between HCV infection and RCC. The possibility of selection bias could play a role in chart-reviewed population base study. Finally, this meta-analysis of observational studies could only show an association. It cannot establish causality as an unknown number of confounders could play a role in the association between HCV and RCC.
In summary, this study demonstrates a potential association between HCV infection and RCC. In the new era of treatment of HCV infection, direct-acting antiviral agents have been demonstrated to be effective therapy and increasingly used[31]. These agents may potentially decrease the incidence of RCC in HCV patients in the long term.
COMMENTS
Background
Hepatitis C virus (HCV) is one of the leading causes of cirrhosis as the prevalence of HCV has been steadily increasing over the past two decades in the United States. Extrahepatic manifestations of HCV are common. Interestingly, HCV infection could also increase the risk of renal cell carcinoma (RCC) as observed in several epidemiologic studies.
Research frontiers
The results of those epidemiologic studies were inconsistent. To further investigate this possible association of HCV and RCC, the authors conducted this systematic review and meta-analysis of observational studies reporting the risk of RCC among HCV-infected patients.
Innovations and breakthroughs
The authors found a significantly increased risk of RCC among participants with HCV infection with the pooled risk ratio (RR) of 1.86 (95%CI: 1.11-3.11). The sensitivity analysis including only studies with confounder adjustment also demonstrated increased risk of RCC among participants with HCV infection with the pooled RR of 1.50 (95%CI: 0.93-2.42) even though without reaching statistical significance.
Applications
This study demonstrated a potential association between HCV infection and RCC. This finding suggests that a history of HCV is potentially associated with RCC and may impact clinical management and cancer surveillance.
Peer-review
The manuscript is an interesting meta-analysis about the correlation of HCV infection and RCC development. The aim of the meta-analysis is clearly stated and methods are well-described.
Footnotes
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 8, 2016
First decision: May 19, 2016
Article in press: August 18, 2016
P- Reviewer: Levinson A, Malnick S, Procopio G S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
References
- 1.Udompap P, Mannalithara A, Heo NY, Kim D, Kim WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol. 2016;64:1027–1032. doi: 10.1016/j.jhep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveria Andrade LJ, D’Oliveira A, Melo RC, De Souza EC, Costa Silva CA, Paraná R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 5.Hartridge-Lambert SK, Stein EM, Markowitz AJ, Portlock CS. Hepatitis C and non-Hodgkin lymphoma: the clinical perspective. Hepatology. 2012;55:634–641. doi: 10.1002/hep.25499. [DOI] [PubMed] [Google Scholar]
- 6.Vlaar AP, Rietdijk ST, Zeerleder SS, Boerman T, Beuers U. Malignancies associated with chronic hepatitis C: case report and review of the literature. Neth J Med. 2011;69:211–215. [PubMed] [Google Scholar]
- 7.Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V, Yamamoto AM, Camproux AC, Hausfater P, Musset L, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore) 2000;79:47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C Virus Infection Increases the Risk of Developing Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2015;60:3801–3813. doi: 10.1007/s10620-015-3801-y. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, Chow WH. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008;168:268–277. doi: 10.1093/aje/kwn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, Erickson SB. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM. 2015;108:205–212. doi: 10.1093/qjmed/hcu195. [DOI] [PubMed] [Google Scholar]
- 12.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur Urol. 2016;70:458–466. doi: 10.1016/j.eururo.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 13.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Wiklund F, Tretli S, Choueiri TK, Signoretti S, Fall K, Adami HO. Risk of bilateral renal cell cancer. J Clin Oncol. 2009;27:3737–3741. doi: 10.1200/JCO.2008.20.6524. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353–2358. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 16.Amin J, Dore GJ, O’Connell DL, Bartlett M, Tracey E, Kaldor JM, Law MG. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol. 2006;45:197–203. doi: 10.1016/j.jhep.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Budakoğlu B, Aksoy S, Arslan Ç, Üyetürk Ü, Babacan NA, Özcan MF, Yıldız R, Öven BB, Özdemir NY, Dizdar Ö, Büyükberber S, Akıncı MB, Türker I, Öksüzoğlu B, Altundag K, Zengin N. Frequency of HCV infection in renal cell carcinoma patients. Med Oncol. 2012;29:1892–1895. doi: 10.1007/s12032-011-9928-6. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez HC, Lamerato L, Rogers CG, Gordon SC. Chronic hepatitis C infection as a risk factor for renal cell carcinoma. Dig Dis Sci. 2015;60:1820–1824. doi: 10.1007/s10620-015-3521-3. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SC, Moonka D, Brown KA, Rogers C, Huang MA, Bhatt N, Lamerato L. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev. 2010;19:1066–1073. doi: 10.1158/1055-9965.EPI-09-1275. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann JN, Törner A, Chow WH, Ye W, Purdue MP, Duberg AS. Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: a nationwide register-based cohort study in Sweden. Eur J Cancer Prev. 2011;20:326–330. doi: 10.1097/CEJ.0b013e32834572fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malaguarnera M, Gargante MP, Risino C, Ranno S, Berretta M, Cannizzaro MA, Costanzo M, Fricia T, Rampello E, Romano M. Hepatitis C virus in elderly cancer patients. Eur J Intern Med. 2006;17:325–329. doi: 10.1016/j.ejim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Omland LH, Farkas DK, Jepsen P, Obel N, Pedersen L. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol. 2010;2:179–186. doi: 10.2147/clep.s10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, White E. Risk factors for renal cell carcinoma in the VITAL study. J Urol. 2013;190:1657–1661. doi: 10.1016/j.juro.2013.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiwanitkit V. Renal cell carcinoma and hepatitis C virus infection: is there any cause-outcome relationship? J Cancer Res Ther. 2011;7:226–227. doi: 10.4103/0973-1482.82931. [DOI] [PubMed] [Google Scholar]
- 29.Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukkonen A, Lintula S, von Boguslawski K, Carpén O, Ljungberg B, Landberg G, Stenman UH. Tumor-associated trypsin inhibitor in normal and malignant renal tissue and in serum of renal-cell carcinoma patients. Int J Cancer. 1999;83:486–490. doi: 10.1002/(sici)1097-0215(19991112)83:4<486::aid-ijc9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Kalaghatgi P, Sikorski AM, Knops E, Rupp D, Sierra S, Heger E, Neumann-Fraune M, Beggel B, Walker A, Timm J, et al. Geno2pheno[HCV] - A Web-based Interpretation System to Support Hepatitis C Treatment Decisions in the Era of Direct-Acting Antiviral Agents. PLoS One. 2016;11:e0155869. doi: 10.1371/journal.pone.0155869. [DOI] [PMC free article] [PubMed] [Google Scholar]


