Abstract
In the previous studies, the cytotoxicities of anthranilate sulfonamides were investigated. Herein, the bioactivities of 4-substituted (X = NO2, OCH3, CH3, Cl) benzenesulfonamides of anthranilic acid (5-8) are reported. The results revealed that all sulfonamides selectively exerted antifungal activity (25-50 % inhibition) against C. albicans at 4 μg/mL. Furthermore, compounds 6 and 8 show antioxidative (SOD) activity. These sulfonamides, except for 6, selectively display cytotoxic effects toward MOLT-3 cells. It is interesting to note that sulfonamides with electron withdrawing substituent (5, X = NO2) exhibited the highest cytotoxicity. This study provided preliminary structure-activity relationship of the anthranilic sulfonamides that is useful for further in-depth investigation.
Keywords: sulfonamides, anthranilic acid, antimicrobials, antioxidants, cytotoxicity
Introduction
Sulfonamides (1, R = heterocyclic rings, Figure 1(Fig. 1)) are compounds constituting diverse medicinal applications, widely used as antimicrobial (Genç et al., 2008[6]; Ozbek et al., 2007[11]), anticancer (Ghorab et al., 2009[7]; El-Sayed et al., 2011[5]), antiinflammatory (Borne et al., 1974[1]) and antiviral agents as well as HIV protease inhibitors (De Clercq, 2001[2]). Sulfonamide (1, R =H) is well recognized as an antimetabolite (Mengelers et al., 1997[10]). It has a similar structure to p-aminobenzoic acid (PABA, 2) which is an essential compound for the synthesis of tetrahydrofolate in bacteria (Mengelers et al., 1997[10]). Anthranilic acid (o-aminobenzoic acid, 3), an isomeric form of PABA, has been shown to be an interesting pharmacophore essential for biological activities. Its derivatives such as anthranilate sulfonamides have been recently reported to be cytotoxic (Shahlaei et al., 2010[16]) acting as methionine aminopeptidase-2 (MetAP-2) inhibitors. Previously, anthranilate-hydroxamic acid sulfonamides were reported to act as matrix metalloproteinase (MMP) inhibitors. These compounds were potent inhibitors of MMP-9 and MMP-13 (Levin et al., 2001[9]). MMP-9 was shown to be potential inhibitors of tumor metastasis, while MMP-3 provided protection against the cartilage degradation associated with osteoarthritis.
Figure 1. Chemical structures of sulfonamides and aminobenzoic acids.
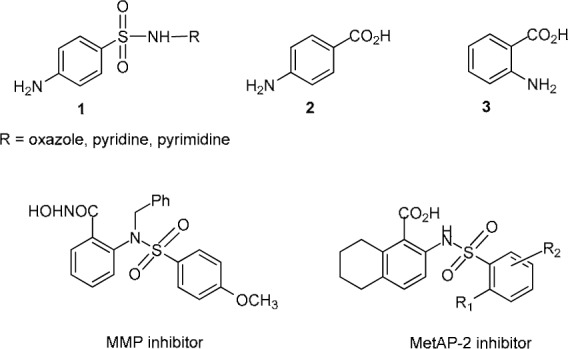
Considering the molecular structures of anthranilates, the MMP and MetAP-2 inhibitors, therefore, simple and small molecules of sulfonamide derivatives of anthranilic acid 5-8 (Figure 2(Fig. 2)) are interesting compounds for this investigation. Based on the literature, some bioactivities of these sulfonamides (5-8), namely antiinflammatory and aldose reductase inhibitory activities have been previously reported (Borne et al., 1974[1]; DeRuiter et al., 1989[4]; Wydysh et al., 2009[19]). The present study reports antimicrobial, antioxidative and cytotoxic effects of the sulfonamides 5-8.
Figure 2. Chemical structures of sulfonamides 5-8.
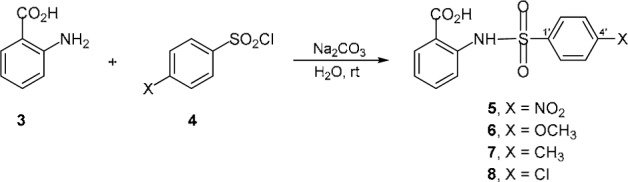
In general, sulfonamides are prepared from the reaction of amines and sulfonyl halides in organic solvents. In this study, an environmentally friendly method using water as the solvent, was conducted for the synthesis of sulfonamides (Figure 2(Fig. 2)).
Materials and Methods
General
1H-NMR spectra were recorded on a Bruker AVANCE 300 NMR spectrometer (operating at 300 MHz for 1H). Infrared spectra (IR) were obtained on a Perkin Elmer System 2000 FTIR. Melting points were determined on an Electrothermal melting point apparatus (Electrothermal 9100) and are uncorrected. Column chromatography was carried out using silica gel 60 (0.063-0.200 mm). Analytical thin layer chromatography (TLC) was performed on silica gel 60 PF254 aluminium sheets (cat. No. 7747 E., Merck). Solvents were distilled prior to use. Reagents for cell culture were as follows: RPMI-1640 (Rosewell Park Memorial Institute medium, Gibco and Hyclone laboratories, USA), HEPES (N-2-hydroxyethyl-piperazine-N'-2-ethanesulfonic acid), L-glutamine, penicillin, streptomycin, sodium pyruvate and glucose (Sigma, USA), Ham's/F12 (Nutrient mixture F-12), DMEM (Dulbecco's Modified Eagle's Medium) and FBS (fetal bovine serum, Hyclone laboratories, USA), gentamicin sulfate (Government Pharmaceutical Organization, Thailand), MTT (3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide, Sigma Aldrich, USA). Vitamin E, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and bovine erythrocyte superoxide dismutase (SOD) were obtained from Sigma Chemical Co. (USA). DMSO was purchased from Fluka.
Tested sulfonamides
Sulfonamides (5-8) were prepared using the modified method (Kamal et al., 2008[8]). A general procedure for the sulfonylation of amine (3) by benzenesulfonyl chlorides (4, X = NO2, OCH3, CH3, Cl) is as follow.
A mixture of anthranilic acid (3, 10 mmol) and substituted arenesulfonyl chloride (4, 30 mmol) was suspended in water (20 mL). The suspension was basified (pH 8) with saturated Na2CO3 and further stirred at room temperature. The reaction was monitored by TLC chromatograms. The completed reaction was added conc. HCl, precipitates were collected by filtration and washed with 0.1 M HCl and cold water. Purification by silica gel column chromatography (hexane:EtOAc; 9:1-6:4) gave the desired sulfonamides 5-8. Their IR and 1H-NMR spectral data and melting points were performed. The compounds are listed as follow.
2-(4'-Nitrophenylsulfonamido)benzoic acid (5); pale-yellow powder (95.70 %), m.p. 209-212 °C (lit. m.p. 215-218 °C; Borne et al., 1974[1]).
2-(4'-Methoxyphenylsulfonamido) benzoic acid (6); pale-yellow powder (98.07 %), m.p. 171-173 °C.
2-(4'-Methylphenylsulfonamido) benzoic acid (7); pale-yellow powder (95.86 %), m.p. 186-188 °C (lit. m.p. 229-231 °C; Borne et al., 1974[1]; lit. m.p. 212 °C; Peifer et al., 2007[12]).
2-(4'-Chlorophenylsulfonamido) benzoic acid (8); pale-yellow powder (80.49 %), m.p. 177-180 °C (lit. m.p. 201-203 °C; Borne et al., 1974[1]).
Bioactivities
Antimicrobial assay
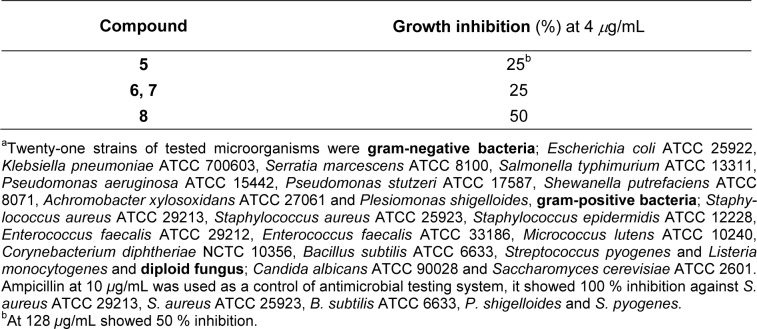
Antimicrobial activity was performed using the agar dilution method as previously described (Prachayasittikul et al., 2011[15]). The compounds dissolved in DMSO were individually mixed with Müller Hinton (MH) broth. The solution was then transferred to the MH agar solution to yield the final concentrations of 256-2 μg/mL. Twenty one strains of microorganisms, cultured in MH broth at 37 °C for 24 h, were diluted with 0.9 % normal saline solution to adjust the cell density of 1×108 cell/mL. The organisms were inoculated onto each plate and further incubated at 37 °C for 24-48 h. Compounds which possessed high efficacy to inhibit bacterial cell growth were analyzed. The microorganisms used for the activity testing are shown in Table 1(Tab. 1).
Table 1. Antimicrobial activitya (C. albicans) of sulfonamides 5-8.
Radical scavenging: DPPH assay
Reaction between DPPH and tested compounds was investigated via spectrophotometric method (Prachayasittikul et al., 2010[14]). The DPPH assay was initiated by adding 1 mL of 0.1 mM solution of DPPH in methanol to a sample solution (0.45 mL, 1 mg/mL dissolved in DMSO). The reaction mixture was incubated for 30 min in a dark room. The absorbance at 517 nm (UV-1610, Shimadzu) was measured and the percentage of radical scavenging activity (RSA) was calculated from the following equation:
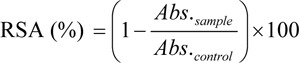
where Abs.control is the absorbance of control without compounds and Abs.sample is the absorbance of tested compounds. Vitamin E was used as a standard.
Superoxide scavenging: SOD assay
Inhibition of the photoreduction of nitro blue tetrazolium (NBT) was performed to measure the SOD activity (Suksrichavalit et al., 2009[17]) The photochemically excited riboflavin was first reduced by methionine into a semiquinone, which donated an electron to oxygen to form a superoxide source. The superoxide readily converted the NBT into a purple formazan product. As a result, the SOD activity was inversely related to the amount of formazan formation. Purified SOD from bovine erythrocytes was used as a control.
Cytotoxic assay
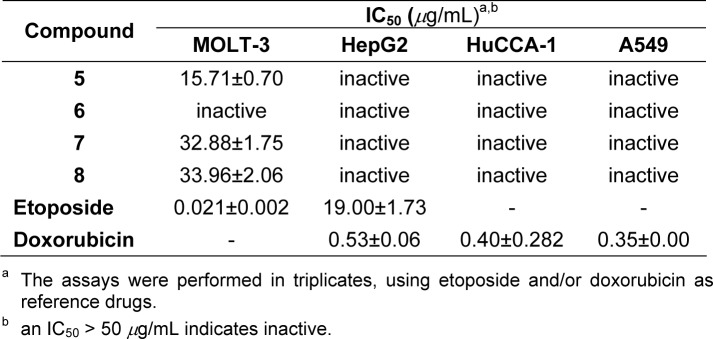
Cytotoxic assay was evaluated as described (Tengchaisri et al., 1998[18]). Cancer cells were grown in Ham's/F12 medium containing 2 mM L-glutamine supplemented with 100 U/mL penicillin, streptomycin and 10 % FBS, except for HepG2 cell which was grown in DMEM. Briefly, cell lines suspended in RPMI-1640 containing 10 % FBS were seeded with 1×104 cells (100 μL) per well in a 96-well plate. The incubation was performed at 37 °C under humidified atmosphere (95 % air, 5 % CO2) for 24 h. Additional medium (100 μL) containing the test compound and vehicle was added to a final concentration of 50 μg/mL, 0.2 % DMSO and further incubated for 3 days. Cells were fixed with 95 % EtOH, stained with crystal violet solution and lysed with a solution of 0.1 N HCl in MeOH. The absorbance was measured at 550 nm. On the other hand, HuCCA-1, A549 and HepG2 cells were stained with MTT. IC50 values were determined as the drug and sample concentrations at 50 % inhibition of the cell growth. The tested cell lines were T-lymphoblast (MOLT-3, acute lymphoblastic leukemia), human hepatocellular carcinoma cell line (HepG2), human cholangiocarcinoma cancer cell (HuCCA-1) and human lung carcinoma cell line (A549).
Results and Discussion
Chemistry
Sulfonamides 5-8 were successfully prepared in high yields (80-98 %) using the base catalyzed sulfonylation reaction of anthranilic acid with 4-substituted benzenesulfonyl chlorides (4) under the modified environmentally friendly method (Kamal et al., 2008[8]). Their structures were confirmed by 1H-NMR and IR spectra. Although, the synthesis of sulfonamides 5-8 were previously reported (Borne et al., 1974[1]; Wydysh et al., 2009[19]; Deng and Mani, 2006[3]), in this study relatively higher yield products were achieved.
Biological activities
Antimicrobial activity
Compounds 5-8 were tested for their antimicrobial action using the agar dilution method against twenty-one strains of microorganisms (gram-positive and gram-negative bacteria and diploid fungus). It was found (Table 1(Tab. 1)) that the tested compounds selectively displayed growth inhibition (25-50 %) against C. albicans ATCC 90028 at 4 μg/mL. When tested at higher concentration (128 μg/mL), the sulfonamide 5 exhibited 50 % inhibition against the C. albicans. The tested sulfonamides were found to be inactive antibacterials.
It was reported that the degree of ionization of sulfonamido group (-SO2NH-C6H4-Y) which gave anionic N-atom (-SO2N̅-C6H4-Y), was important for antibacterial activity (Genç et al., 2008[6]; Mengelers et al., 1997[10]). The anionic form enhanced its solubility and caused ionic interaction with receptors e. g. dihydropteroate synthetase or dihydrofolate synthetases (Mengelers et al., 1997[10]). In this respect the sulfonamide requires an electron withdrawing substitutent (Y) on the phenyl ring, particularly where Y = NO2 at p-position provided the compound with high antibacterial activity as compared to the m-position (Genç et al., 2008[6]). On the other hand, the hydrophobic effect was not important for the activity (Mengelers et al., 1997[10]). The sulfonamides 5-8 were shown to be inactive antibacterials, which could be due to their sulfonamido moieties (-SO2NH-C6H4-CO2H-ortho) that requires the electron withdrawing group at the p-position of the phenyl ring (C6H4-CO2H).
Antioxidative activities
Studies were performed to investigate the superoxide scavenging (SOD) and radical scavenging (DPPH) activities of the sulfonamides. Compounds 6 and 8 (Table 2(Tab. 2)) exhibited weak SOD activity with 15.7 and 6.1 % NBT inhibition at 300 μg/mL. Whereas sulfonamides 5 and 7 were inactive superoxide scavengers. On the other hand, all compounds showed no radical scavenging activity (DPPH). So far, antioxidant properties of these anthranilic sulfonamides were not found in the literature. Study of the molecular structures revealed that the SOD activity depended on the asymmetric charge localization of the compound, where high asymmetric charge afforded the compound with high SOD activity (Prachayasittikul et al., 2010[13]).
Table 2. Antioxidative activities of sulfonamides 5-8.
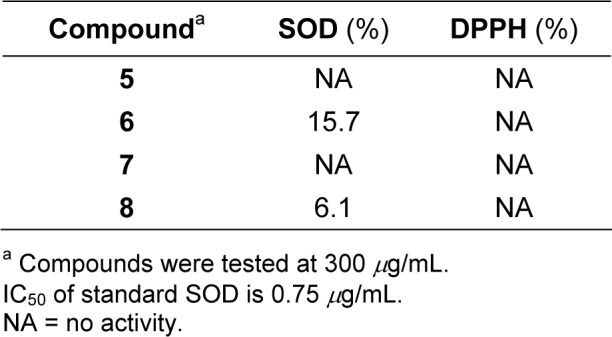
The sulfonamide 6, with OCH3 group at C-4' displayed the highest SOD activity when compared to the compound 8 bearing Cl at C-4'. This could presumably explain that the stroger electron donating effect of OCH3 contributed asymmetric charge on 4'-methoxybenzenesulfonyl moiety of the molecule (6).
Cytotoxicity
Cytotoxicity was evaluated against four cell lines (Table 3(Tab. 3)) using etoposide and/or doxorubicin as the reference drugs. Results showed that the cytotoxic activity was observed for compounds 5, 7 and 8 toward MOLT-3 cell with IC50 values of 15.71, 32.88 and 33.96 μg/mL, respectively. Interestingly, the sulfonamide 5 with NO2 substituted at C-4' position exerted the highest cytotoxicity when compared to compounds 7 and 8 with CH3 and Cl groups at C-4', respectively. Both compounds 7 and 8 had comparable cytotoxic effect, but with the IC50 values of approximately two folds higher than the compound 5. On the other hand, sulfonamide 6 bearing OCH3 group at C-4' was shown to be inactive compound. However, all sulfonamides were inactive cytotoxics when tested with HuCCA-1, A549 and HepG2 cells. The results suggested that the substituents at C-4' position of compounds 5-8 governed their cytotoxicities. It is evident that an electron withdrawing group (NO2) at C-4' exhibited the highest cytotoxicity, whereas electron releasing groups; CH3 and Cl provided the compounds with lower cytotoxicity. In case of the stronger electron releasing group, OCH3 which exerted no cytotoxicity as noted for the sulfonamide 6.
Table 3. Cytotoxicity of sulfonamides 5-8.
Conclusion
4-Substituted (X) benzenesulfonamides of anthranilic acid (5-8) were prepared in high yields. Bioactivity testings revealed that all sulfonamides selectively exert antifungal action (25-50 % inhibition) against C. albicans with low concentration at 4 μg/mL. Some of the tested compounds (6 and 8) show SOD activity, whereas 6 is shown to be the highest antioxidant. These sulfonamides, except for 6, selectively display cytotoxic effect toward MOLT-3 cells. The sulfonamide with electron withdrawing substituent (5, X = NO2) exhibited the highest cytotoxicity, but with no antioxidant property. This study provided preliminary structure-activity relationship of the anthranilic sulfonamides useful for further in-depth investigation.
Notes
Supaluk Prachayasittikul and Virapong Prachayasittikul (Department of Clinical Microbiology and Applied Technology, Faculty of MedicalTechnology, Mahidol University, Bangkok 10700, Thailand; Telephone: 662-441-4376, Fax: 662-441-4380, Email: mtvpr@mahidol.ac.th) contributed equally as corresponding authors.
Acknowledgements
This work was supported in part by the research grant of Srinakharinwirot University (B.E. 2554). This project is supported by the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. We thank the Chulabhorn Research Institute for the cytotoxic assay.
References
- 1.Borne RF, Peden RL, Waters IW, Weiner M, Jordan R, Coats EA. Anti-inflammatory activity of para-substituted N-benzenesulfonyl derivatives of anthranilic acid. J Pharm Sci. 1974;63:615–617. doi: 10.1002/jps.2600630428. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. New developments in anti-HIV chemotherapy. Curr Med Chem. 2001;8:1543–1572. doi: 10.2174/0929867013371842. [DOI] [PubMed] [Google Scholar]
- 3.Deng X, Mani NS. A facile, environmentally benign sulfonamide synthesis in water. Green Chem. 2006;8:835–838. [Google Scholar]
- 4.DeRuiter J, Borne RF, Mayfield CA. N- and 2-substituted N-(phenylsulfonyl)-glycines as inhibitors of rat lens aldose reductase. J Med Chem. 1989;32:145–151. doi: 10.1021/jm00121a027. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed NS, El-Bendary ER, El-Ashry SM, El-Kerdawy MM. Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo[3,2-a]pyrimidines. Eur J Med Chem. 2011;46:3714–3720. doi: 10.1016/j.ejmech.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob. 2008;7:17–22. doi: 10.1186/1476-0711-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghorab MM, Ragab FA, Hamed MM. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem. 2009;44:4211–4217. doi: 10.1016/j.ejmech.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Kamal A, Reddy JS, Bharathi EV, Dastagiri D. Base-free monosulfonylation of amines using tosyl or mesyl chloride in water. Tetrahedron Lett. 2008;49:348–353. [Google Scholar]
- 9.Levin JI, Du MT, DiJoseph JF, Killar LM, Sung A, Walter T, et al. The discovery of anthranilic acid-based MMP inhibitors. Part 1: SAR of the 3-position. Bioorg Med Chem Lett. 2001;11:235–238. doi: 10.1016/s0960-894x(00)00642-9. [DOI] [PubMed] [Google Scholar]
- 10.Mengelers MJB, Hougee PE, Janssen LHM, Van Miert ASJPAM. Structure-activity relationships between antibacterial activities and physicochemical properties of sulfonamides. J Vet Pharmacol Ther. 1997;20:276–283. doi: 10.1046/j.1365-2885.1997.00063.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozbek N, Katircioğlu H, Karacan N, Baykal T. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorg Med Chem. 2007;15:5105–5109. doi: 10.1016/j.bmc.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Peifer C, Kinkel K, Abadleh M, Schollmeyer D, Laufer S. From five- to six-membered rings: 3,4-diarylquinolinone as lead for novel p38MAP kinase inhibitors. J Med Chem. 2007;50:1213–1221. doi: 10.1021/jm061097o. [DOI] [PubMed] [Google Scholar]
- 13.Prachayasittikul S, Wongsawatkul O, Worachartcheewan A, Nantasenamat C, Ruchirawat S, Prachayasittikul V. Elucidating the structure-activity relationships of the vasorelaxation and antioxidation properties of thionicotinic acid derivatives. Molecules. 2010;15:198–214. doi: 10.3390/molecules15010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prachayasittikul S, Wongsawatkul O, Worachartcheewan A, Ruchirawat S, Prachayasittikul V. Vasorelaxation and superoxide scavenging activities of orotic acid. Int J Pharmacol. 2010;6:375–380. [Google Scholar]
- 15.Prachayasittikul S, Worachartcheewan A, Nantasenamat C, Chinworrungsee M, Sornsongkhram N, Ruchirawat S, et al. Synthesis and structure-activity relationship of 2-thiopyrimidine-4-one analogs as antimicrobial and anticancer agents. Eur J Med Chem. 2011;46:738–742. doi: 10.1016/j.ejmech.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Shahlaei M, Sabet R, Ziari MB, Moeinifard B, Fassihi A, Karbakhsh R. QSAR study of anthranilic acid sulfonamides as inhibitors of methionine aminopeptidase-2 using LS-SVM and GRNN based on principal components. Eur J Med Chem. 2010;45:4499–4508. doi: 10.1016/j.ejmech.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Suksrichavalit T, Prachayasittikul S, Nantasenamat C, Isarankura-Na-Ayudhya C, Prachayasittikul V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur J Med Chem. 2009;44:3259–3265. doi: 10.1016/j.ejmech.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998;133:169–175. doi: 10.1016/s0304-3835(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 19.Wydysh EA, Medghalchi SM, Vadlamudi A, Townsend CA. Design and synthesis of small molecule glycerol 3-phosphate acyltransferase inhibitors. J Med Chem. 2009;52:3317–3327. doi: 10.1021/jm900251a. [DOI] [PMC free article] [PubMed] [Google Scholar]