Abstract
Essential oil from the stem bark of Nigerian species of Psidium guajava of the family Myrtaceae was obtained by hydro-distillation using an all-glass Clavenger apparatus. GC and GC/MS analysis were carried out on the essential oil and was found to contain 62 compounds constituting 99.98 % of the total oil composition. The principal constituents are hydrocarbons, amines, amides and esters with 3,6-dioxa-2,4,5,7-tetraoctane,2,2,4,4,5,5,7,7-octamethyl (11.67 %) and cyclononane (10.66 %) dominating the total essential oil. Brine shrimp lethality test was carried out to determine the toxicity of the oils to living organisms (shrimps). LC50 value (µg/ml) of 1.0009 obtained showed that the essential oil of P. guajava stem bark was toxic. The antioxidant property of essential oil was investigated by measuring the decrease in absorption at 517 nm of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) in a UV/visible spectrophotometer. The oil showed better activity as a radical scavenger than α-tocopherol. The oil activity was 71.83 % at 0.2 mg/ml and the absorption is stoichiometric with respect to the number of electron taken up. Thus, the results of this study showed that the essential oil from P. guajava was not only toxic; it possessed antioxidant activity, which could exert beneficial actions against pathological alterations caused by the presence of highly reactive free radicals. The toxicity of the oil can be taken advantage of in the therapy of diseases involving cell or tumor growth.
Keywords: toxicity; antioxidant; essential oils; Psidium guajava; GC/GC-MS; 1,1-diphenyl-2-picrylhydrazyl
Introduction
Free radicals are chemical species that contain one or more unpaired electrons. They are produced when oxidation reactions occur in cells but excess production starts chain reactions and causes serious damage to biological macromolecules. Antioxidants terminate these chain reactions by removing the excess free radical intermediates, and inhibit other oxidation reactions by being oxidized themselves. Natural antioxidants are substances from plants or animals sources that can delay or terminate the process of oxidation. Natural compounds with anti-oxidative properties include flavonoids, lignans, tannins, essential oils and terpenoids (Gutteridge, 1988[13]; Hochstein and Atallah; 1988[16]; Halliwell, 1989[14]; Potterat, 1997[39]). As a result of prevalence of oxidative stress, studies involving antioxidant chemistry is gaining much relevance in recent times (Feher et al., 1986[12]; Aruoma et al., 1989[5]; Belch et al., 1989[9]; Kehrer, 1993[21]; Sies, 1997[41]; Lee et al., 2001[23]).
Psidium guajava, a common plant with edible fruit has enjoyed many medicinal applications as antibacterial, anti-inflammatory, anti-malaria, haemostatic, antispasmodic, as a tonic, anti-diarrheal, anti-diabetic, anti-rheumatism in traditional medicine (Oliver-Bever, 1986[35]; Iwu, 1993[17]; Burkill, 1997[10]; Olajide et al., 1999[34]). Also much biological work has been carried out on P. guajava and many compounds of medicinal importance have been isolated (Bassols and Demole, 1994[7]; Sen et al., 1995[40]; Kavimani et al., 1997[20]; Nadkarni and Nadkarni, 1999[33]; Tona et al., 1999[42]; Vieira et al., 2001[43]; Paniandy et al., 2000[38]; Abdelrahim et al., 2002[1]; Arima and Danno, 2002[4]; Michael et al., 2002[32]; Lozoya et al., 2002[27]). However, in this present study we report the chemical constituents of essential oil from the stem bark of Nigerian species of P. guajava. The oil was obtained by hydro-distillation and analyzed using Gas Chromatography-Mass Spectrometry (GC-MS). The toxicity of the essential oil was determined by brine shrimp lethality test (BST) which is a bench top bioassay for elementary cyto-toxicity study. P. guajava oil was screened for free-radical scavenging activity by in-vitro assessment on 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). DPPH radical becomes deep violet colour in alcoholic solutions and gives strong absorption at 517 nm in visible spectroscopy but disappears in the presence of a free radical scavenger. The anti-oxidative potential of the oil was compared with known antioxidant standards - ascorbic acid, butylated hydroxylanisole and α-tocopherol.
Material and Methods
Plant materials
Psidium guajava stem bark was collected in August 2009 behind Tedder Hall at the University of Ibadan, Oyo State, Nigeria and authenticated at the Herbarium of the Department of Botany and Microbiology of the institution. The volatile oil was immediately collected from the fresh plant material by hydro-distillation using an all-glass scavenger apparatus.
Reagents
Hexane and methanol (BDH chemicals); 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) were obtained from Sigma Chemical Co (Germany).
Reference standards: Ascorbic acid, Butylated hydroxyanisole (BHA) and α-tocopherol for antioxidant activity.
Major equipments used
UV-visible spectrophotometer (Unico1 200 & Perkin Elmer lambda 25 models), GC-Mass spectrophotometer (Agilent Technologies), Hydro-distiller - Clavenger apparatus.
Isolation of essential oils
The oil was obtained from chopped fresh plant sample (300 g) of P. guajava by hydro-distillation on a Clavenger type apparatus for 4 hours in accordance with the British pharmacopoeia specifications (1980). Small amount of hexane was intermittently added to aid the trapping of the oil during the process. The essential oil was collected and stored at 4 °C in other to prevent loss of oil by volatilization until analysis. The oil yield was calculated relative to the dry matter.
Analysis of the essential oils - Gas chromatography
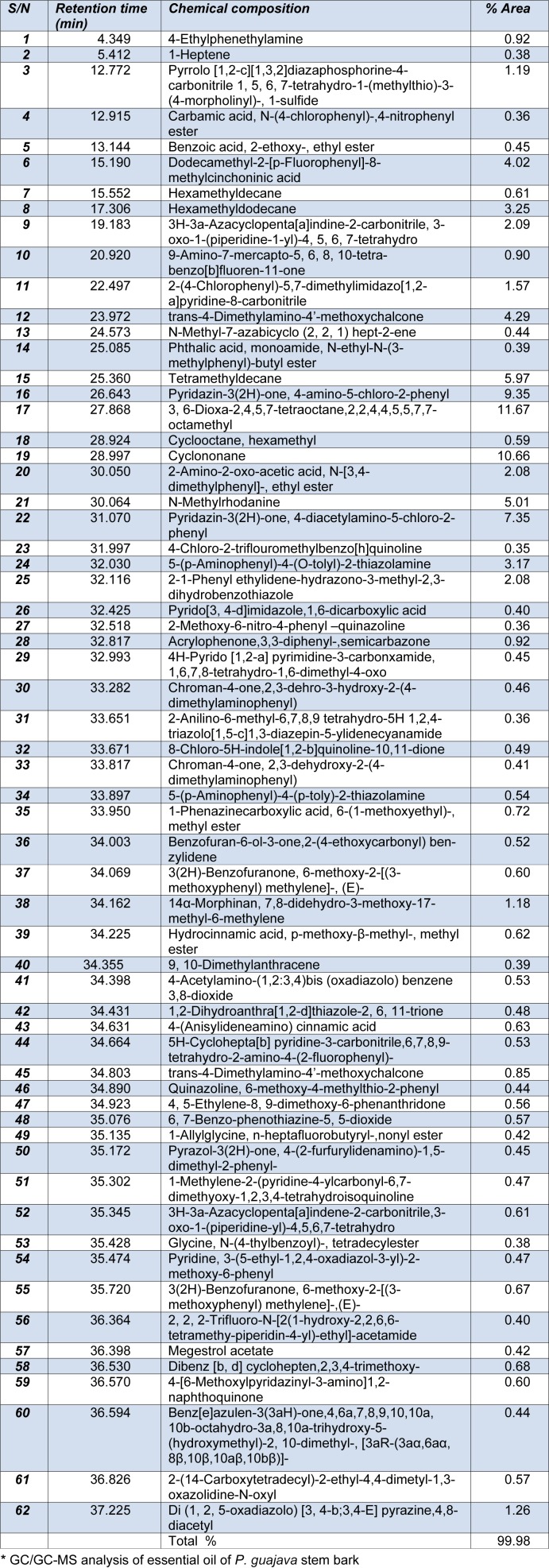
GC-MS analyses of the essential oil was analyzed on an Agilent Technologies 7890A GC system coupled to a 5975C VLMSD mass spectrometer with an injector 7683B series device. An Agilent (9091)-413:325 °C HP-5 column (30 m x 320 µm x 0.25 µm) was used with helium as carrier gas at a flow rate of 3.3245 ml/min. The GC oven temperature was initially programmed at 50 °C (hold for 1 min) and finally at 300 °C (hold for 5 min) at a rate of 80 °C/min while the trial temperature was 37.25 °C. The column heater was set at 250 °C in a split less mode while the pressure was 10.153 psi with an average velocity of 66.45 cm/sec and a hold-up time of 0.75245 min. Mass spectrometry was run in the electron impact mode (EI) at 70eV. The percentage compositions were obtained from electronic integration measurements using flame ionization detector (FID), set at 250 °C. The peak numbers and relative percentages of the characterized components are given in Table 1(Tab. 1).
Table 1. Compounds obtained from GC/GC-MS analysis of P. guajava stem bark essential oil*.
Gas chromatography-mass spectrometry
The essential oils were analysed by GC-MS on an Agilent Technologies 7890A GC system coupled to a 5975C VLMSD mass spectrometer with an injector 7683B series device. An Agilent (9091)-413:325 °C HP-5 column (30 m x 320 µm x 0.25 µm) was used with helium as carrier gas at a flow rate of 3.3245 ml/min. GC oven temperature and conditions were as described above. The injector temperature was at 250 °C. Mass spectra were recorded at 70 eV. Mass range was from m/z 30 to 500.
Identification of components
The individual constituents of the oil were identified on the basis of their retention indices determined with a reference to a homologous series of n-alkanes and by comparison of their mass spectral fragmentation patterns (NIST 08.L database/chemstation data system) with data previously reported in literature (McLafferty and Stauffer, 1989[30]; Adams, 1995[2]; Joulain and König, 1998[19]).
Brine shrimp lethality test
The brine shrimp lethality test (BST) was used to predict the toxicity of the oils and was conducted according to the methods of Meyer et al. (1982[31]), Falope et al. (1993[11]) and Oloyede et al. (2010[36]) using Brine shrimp eggs obtained from Ocean Star International, Inc. Company, USA. The shrimp's eggs were hatched in sea water for 48 h at room temperature. The nauplii (harvested shrimps) were attracted to one side of the vials with a light source. Solutions of the oils were made in DMSO, at varying concentrations (1000, 100 and 10 μg/ml) and incubated in triplicate vials with the brine shrimp larvae. Ten brine shrimp larvae were placed in each of the triplicate vials. Control brine shrimp larvae were placed in a mixture of sea water and DMSO only. After 24h, the vials were examined against a lighted background and the average number of larvae that survived in each vial was determined. The concentration at which fifty percent of the larvae (LC50) were killed was determined using the Finney computer programme.
Free radical scavenging activity - Scavenging effect on DPPH
The free radical scavenging assay has been efficiently used as an adequate parameter for selecting medicinal plants with antioxidant properties. A solution of 0.5 mM 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) in methanol was prepared. The free radical scavenging activity of the oil at 0.1 and 0.2 mg/ml was determined by measuring the decrease in absorption after 10 min of incubation at 517 nm in a UV-visible spectrophotometer. The actual decrease in absorption was measured against that of the control. The same experiment was carried out on ascorbic acid, butylated hydroxyanisole (BHA) and α-tocopherol which are known as antioxidant agents. All test and analysis were run in triplicates and the result obtained was averaged (Koleva et al., 2002[22]; Masuda et al., 2003[29]; Ayoola et al., 2008[6]). The activities were also determined as a function of their % Inhibition which was calculated using the formula;
% Inhibition = {Ac - As / Ac} x 100
Ac = Absorbance of the control
As = Absorbance of the sample.
Statistical analysis
Statistical analysis was carried out with Statistical Package for Social Sciences (SPSS). The data was expressed as the mean ± standard deviation and a probability of less than 0.05 (p < 0.05) was considered to be statistically significant. Graph was drawn using Microsoft Office excel, 2007 software.
Results and Discussion
GC-MS analysis of the essential oils
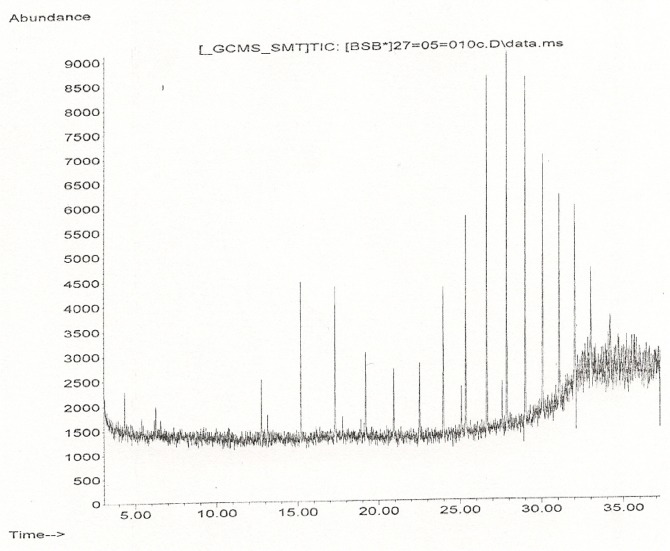
Essential oils from freshly collected stem bark of P. guajava were obtained by means of hydro-distillation. The yield of the 300 g hydro-distilled oil was 0.60 % (w/w). The essential oil, colourless, with characteristic smell was analyzed by GC and GC/MS systems using a polar column. The peaks showing the relative abundance of the chemical components with retention time is presented in Figure 1(Fig. 1).
Figure 1. The peaks of the compounds in Psidium guajava stem bark oil at different retention time from the Gas chromatography-Mass spectrometric (GC-MS) analysis.
Overall, 62 constituents representing 99.98 % of essential oils were identified in the essential oils. Individual constituents of the oil were identified on the basis of their retention indices determined with a reference to a homologous series of n-alkanes and by comparison of their mass spectral fragmentation patterns (NIST 08.L database/chemstation data system) with data previously reported in literature. The results of these analyses are presented in Table 1(Tab. 1). P. guajava stem bark essential oils from the Nigerian soil are hydrocarbons, amines, amides and esters dominated. The following compounds had the highest % peak area, 3,6-dioxa-2,4,5,7-tetraoctane,2,2,4,4, 5,5,7,7-octamethyl (11.67 %), cyclononane (10.66 %), pyridazin-3(2H)-one,4-amino-5-chloro-2-phenyl (9.35 %), pyridazin-3(2H)-one, 4-diacetylamino-5-Chloro-2-phenyl (7.35 %), tetramethyldecane (5.97 %) and N-methylrhodanine (5.01 %) while the remaining compounds (see Table 1(Tab. 1)) appeared in minute quantities. The results obtained from this analysis compare favourably well with compounds early identified in other parts of P. guajava essential oil (Bassols and Demole, 1994[7]; Li et al., 1999[24]; Paniandy et al., 2000[38]; Begum, 2002[8]).
Toxicity analysis
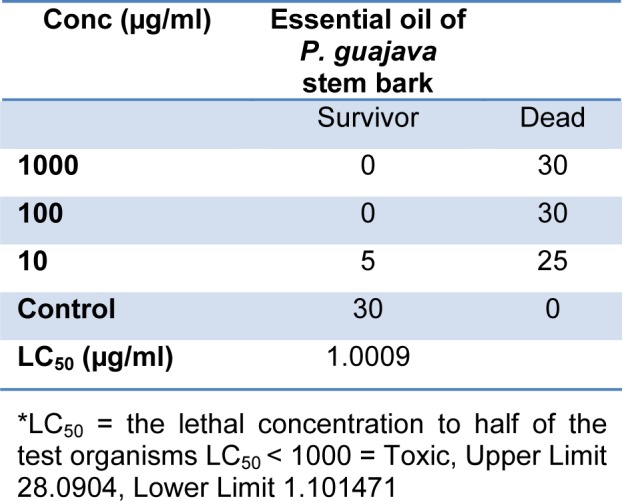
The LC50 value of 1.0009 µg/ml (Table 2(Tab. 2)) obtained from Brine shrimp toxicity test showed that that the essential oil of P. guajava was toxic. The toxicity was determined using brine shrimp larvae (Artermia silina) nauplii which are living organisms with no advanced nervous system. The dominance of hydrocarbons in the essential oil accounted for the toxicity level. Its usage at high concentration should therefore be monitored. The result obtained suggests that P. guajava stem bark essential oil is of relevance in medicine as it has been established by other workers that secondary metabolites from plants which are active medicinally are most times toxic to brine shrimp larvae (Aiyelaagbe et al., 2010[3]; Onocha and Ali, 2010[37]; Oloyede et al., 2010[36]).
Table 2. Brine shrimp lethality test of the essential oil of P. guajava stem bark.
Antioxidant activity
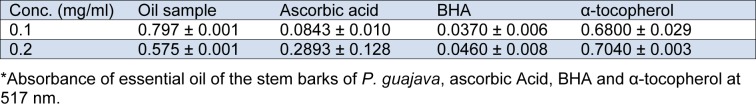
The essential oil samples were screened using 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). The antioxidant activity of the volatile oil was measured in terms of hydrogen donating or radical scavenging ability, using the stable radical DPPH. The volatile oil scavenged DPPH in a concentration-dependent manner as shown in Table 3(Tab. 3). There is decrease in absorbance measurement as the concentration is increased.
Table 3. Scavenging effect of P. guajava essential oils and standards on DPPH.
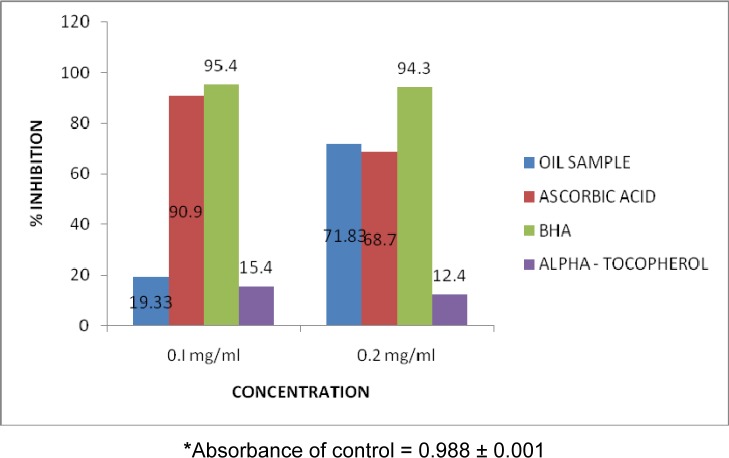
It was observed that the % inhibition of essential oil of P. guajava stem bark at 0.1 and 0.2 mg/ml (19.33 % and 71.83 % respectively) were lower than the % inhibition of two of the standards, ascorbic acid (90.9 % and 68.7 %) and BHA (95.4 % and 94.3 %) but higher than the % inhibition of α-tocopherol (15.4 % and 12.4 %). The % inhibition of the essential oils increased with the increase in the concentration of the oils (Figure 2(Fig. 2)).
Figure 2. % Inhibition of P. guajava essential oil and known antioxidants at absorbance517nm on DPPH*.
This suggests that P. guajava stem bark has weak activity as a proton donor in the reaction involving DPPH but its activity as an OH radical scavenger is promising. One of the most important effective defenses of a living body against diseases is the removal of OH radical. The hydroxyl radical is a very reactive free radical formed in biological systems (Hochstein and Atallah, 1988[16]) and reacts with phospholipids, sugars, amino acids, organic acids and DNA bases found in living cells (Halliwell and Gutteridge, 1984[15]) therefore natural products with antioxidant activity capable of scavenging OH radical might contribute towards the total and partial alleviation damages caused by oxidative reactions (Lin et al., 1995[26]). Thus the result of this study (Table 3(Tab. 3) and Figure 2(Fig. 2)) shows that P. guajava oil possesses antioxidant activity which could exert beneficial actions against pathological alterations caused by the presence of hydroxyl radical. This result agrees favorably with previous reports about the antioxidant potential of Guava fruit (Jimenez-Escrig et al., 2001[18]; Lim et al., 2006[25]; Lugasi et al., 1999[28]).
Conclusions
The stem bark of P. guajava essential oil from the Nigerian soil contains mostly hydrocarbons, amines, amides and esters. The antioxidant activity of the volatile oil was measured in terms of hydrogen donating ability, using the stable radical 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). DPPH is incompatible with oxidizing agents and strong bases. Reduction in absorbance is due to the pairing of the odd electron of the radical, indicating free radical scavenging activity. The oil from guava stem bark was found to have weak activity as a proton donor in the reaction involving DPPH. However, the activity is comparable to α-tocopherol which is known to be a good scavenger of hydroxyl radical. This clearly suggests that essential oil from the stem bark of P. guajava oil has the ability to scavenge OH radical. Also brine shrimp lethality test revealed that the essential from the bark of P. guajava was toxic at varied degrees with reference to 1000 μg/ml. It can therefore be inferred that P. guajava essential oils contain medicinally important secondary plant metabolites.
References
- 1.Abdelrahim SI, Almagboul AZ, Omer ME, Elegami A. Antimicrobial activity of Psidium guajava L. Fitoterapia. 2002;73:713–715. doi: 10.1016/s0367-326x(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 2.Adams RP. Identification of essential oil components by gas chromatography and mass spectroscopy. Carol Stream, IL: Allured Publ; 1995. [Google Scholar]
- 3.Aiyelaagbe OO, Oladosu IA, Olaoluwa OO, Aboaba SA, Oloyede GK, Onah DU. Chemical composition and cytotoxicity of the essential oil of Nigerian Momordica charantia (Hook) Int J Essential Oil Therapeut. 2010;4:32–35. [Google Scholar]
- 4.Arima H, Danno G. Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biosci Biotech Biochem. 2002;66:1727–1730. doi: 10.1271/bbb.66.1727. [DOI] [PubMed] [Google Scholar]
- 5.Aruoma OI, Halliwell B, Laughton MJ, Quinlan JGJ, Gutteridge JMC. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron (ll)–iron(lll) complex. Biochem J. 1989;258:617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoola GA, Coker HAB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennna EC, Atangbayula TO. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res. 2008;7:1019–1024. [Google Scholar]
- 7.Bassols F, Demole EP. The occurrence of pentane-2-thiol in guava fruit. J Essential Oil Res. 1994;6:481–483. [Google Scholar]
- 8.Begum S, Hassan SI, Siddiqui BS. Two new triterpenoids from the fresh leaves of Psidium guajava. Planta Med. 2002;68:1149–1152. doi: 10.1055/s-2002-36353. [DOI] [PubMed] [Google Scholar]
- 9.Belch JJF, Chopra M, Hutchinson S. Free radical pathology in chronic arterial disease. Free Radical Biol Med. 1989;6:375–378. doi: 10.1016/0891-5849(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 10.Burkill HM. The useful plants of West Tropical Africa Vol. 4. Families M-R. 2nd. Royal Botanic Gardens Kew; [Google Scholar]
- 11.Falope MO, Ibrahim H, Takeda Y. Screening of higher plants requested as pesticides using the brine shrimp lethality assay. Int J Pharmacognosy. 1993;37:230–254. [Google Scholar]
- 12.Feher J, Lang I, Deak G. Free radicals in tissue damage in liver disease and therapeutic approach. Tokai J Exp Clin Med. 1986;11:S121–S134. [PubMed] [Google Scholar]
- 13.Gutteridge JMC. Lipid peroxidation;some problems and concepts. In: Halliwell B, editor. Oxygen radicals and tissue injury. Kansas: Allen Press; 1988. pp. 9–19. [Google Scholar]
- 14.Halliwell B. Current status review. Free radicals, reactive oxygen species and human diseases: a critical evaluation with special reference to atheroscelerosis. Brit J Exp Path. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B, Gutteridge MC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochstein P, Atallah AS. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat Res. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 17.Iwu MM. Handbook of African medicinal plants. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 18.Jimenez-Escrig A, Rincon M, Pulido R, Saura-Calixto F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J Agric Food Chem. 2001;49:5489–5493. doi: 10.1021/jf010147p. [DOI] [PubMed] [Google Scholar]
- 19.Joulain D, König WA. The atlas of spectral data of sesquiterpene hydrocarbons. Hamburg: E.B-Verlag; 1998. [Google Scholar]
- 20.Kavimani S, Karpagam RI, Jaykar B. Anti-inflammatory activity of volatile oil of Psidium guajava. Indian J Pharm Sci. 1997;59:142–144. [Google Scholar]
- 21.Kehrer JP. Free radicals as mediator of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 22.Koleva II, van Beek TA, Linssen JP, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Kwon H, Joong H, Lee JW, Lee SY, Baek J, Surh YJ. Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma Lucidum. Phytother Res. 2001;15:245–249. doi: 10.1002/ptr.830. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chen F, Luo J. GC-MS analysis of essential oil from the leaves of Psidium guajava. Zhong Yao Cai. 1999;22:78–80. [PubMed] [Google Scholar]
- 25.Lim YY, Lim TT, Tee JJ. Antioxidant properties of guava fruit: comparison with some local fruits. Sunway Acad J. 2006;3:9–20. [Google Scholar]
- 26.Lin JM, Lin CC, Chen MF, Ujiie T, Takada A. Scavenging effects of Mallotus repandus on active oxygen species. J Ethnopharmacol. 1995;46:175–181. doi: 10.1016/0378-8741(95)01246-a. [DOI] [PubMed] [Google Scholar]
- 27.Lozoya X, Reyes-Morales H, Chavez-Soto MA, Martinez-Garcia M, Del C, Soto-Gonzalez Y, Doubova SV. Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease. J Ethnopharmacol. 2002;83:19–24. doi: 10.1016/s0378-8741(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 28.Lugasi A, Horvahorich P, Dwarschak A. Additional information to the in vitro antioxidant activity of Ginkgo biloba L. Phytother Res. 1999;13:160–162. doi: 10.1002/(SICI)1099-1573(199903)13:2<160::AID-PTR402>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Masuda T, Inaba Y, Maekawa T, Takeda Y, Yamaguchi H, Nakamoto K, Kuninaga H, Nishizato S, Nonaka A. Simple detection method of powerful antiradical compounds in the raw extract of plants and its application for the identification of antiradical plant constituents. J Agric Food Chem. 2003;51:1831–1838. doi: 10.1021/jf026112m. [DOI] [PubMed] [Google Scholar]
- 30.McLafferty FW, Stauffer DB. The Wiley/NBS registry of mass spectral data. New York: Wiley & Sons; 1989. [Google Scholar]
- 31.Meyer BN, Ferrign RN, Putnam JE, Jacobson LB, Nicholas DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica. 1982;45:31–34. [PubMed] [Google Scholar]
- 32.Michael HN, Salib JY, Ishak MS. Acylated flavonol glycoside from Psidium guajava L. seeds. Pharmazie. 2002;57:859–860. [PubMed] [Google Scholar]
- 33.Nadkarni KM, Nadkarni AK. Indian materia medica - with Ayurvedic,UNANI-Tibbi, Siddha, allopathic, homeopathic, naturopathic and home remedies Vol. 1. Bombay: Popular Prakashan Private Ltd; 1999. [Google Scholar]
- 34.Olajide OA, Awe SO, Makinde JM. Pharmacological studies on the leaf of Psidium guajava. Fitoterapia. 1999;70:25–31. [Google Scholar]
- 35.Oliver-Bever B. Medicinal plants in tropical West Africa. Cambridge: Cambridge Univ. Press; 1986. [Google Scholar]
- 36.Oloyede GK, Oladosu IA, Shodia AF. Chemical composition and cytotoxicity of the essential oils of Crinum ornatum (Ait.) Bury. Afr J Pure Appl Chem. 2010;4(3):35–37. [Google Scholar]
- 37.Onocha PA, Ali MS. Antileishmaniasis, phytotoxicity and cytotoxicity of Nigerian Euphorbiaceae Plants 2: Phyllanthus amarus and Phyllanthus muellerianus Extracts. African Sci. 2010;11:85–90. [Google Scholar]
- 38.Paniandy JC, Chane-Ming J, Pieribattesti JC. Chemical composition of the essential oil and headspace solid-phase micro-extraction of the guava fruit (Psidium guajava L.) J Essential Oil Res. 2000;12:153–158. [Google Scholar]
- 39.Potterat O. Antioxidants & free radical scavengers of natural origin. Curr Org Chem. 1997;1:415–440. [Google Scholar]
- 40.Sen T, Nasralla HSH, Chaudhuri AKN. Studies on the anti-inflammatory and related pharmacological activities of Psidium guajava: A preliminary report. Phytother Res. 1995;9:118–122. [Google Scholar]
- 41.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 42.Tona L, Kambu K, Mesia K, Cimanga K, Apers S, Pieters L, Totte J, Vlietinck, AJ, De Bruyne T. Biological screening of traditional preparations from some medicinal plants used as antidiarrhoeal in Kinshasa, Congo. Phytomedicine. 1999;6:59–66. doi: 10.1016/S0944-7113(99)80036-1. [DOI] [PubMed] [Google Scholar]
- 43.Vieira RHS, Dos F, Rodrigues D, Dos P, Goncalves FA, Menezes FGR, de Aragao JS, Sousa OV. Microbicidal effect of medicinal plant extracts (Psidium guajava Linn. and Carica papaya Linn.) upon bacteria isolated from fish muscle and known to induce diarrhea in children. Instituto de Ciencias do Mar-UFC, Ceara, Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2001;43:145–148. doi: 10.1590/s0036-46652001000300005. [DOI] [PubMed] [Google Scholar]