Abstract
The relationship between leptin, insulin resistance and other hormonal parameters in polycystic ovarian syndrome (PCOS) is controversial. We investigated the effect of metformin on leptin levels in women with PCOS. Thirty women with PCOS received metformin 500 mg thrice a day. After two months of metformin treatment the mean leptin levels reduced significantly (p < 0.001). Ovulation was achieved in 28 patients, who also had a significant reduction in leptin levels (p < 0.001). Leptin showed significant positive correlation with weight (p < 0.05) and testosterone (p < 0.01), but no significant correlation with insulin. It is concluded that metformin reduces leptin resistance in PCOS women which induces ovulation. Leptin shows a significant correlation with testosterone and not with insulin.
Keywords: PCOS, leptin, ovulation, metformin, insulin, testosterone
Introduction
Leptin, a 167 amino acid peptide of tumor necrosis factor family of cytokines (Ahima and Flier, 2000[2]), secreted from adipocytes in pulsatile fashion (Caprio et al., 2001[8]), regulates energy homeostasis by regulating food intake and energy balance (Campfield et al., 1995[7]). Leptin levels fall during weight loss and increase the brain activity in areas involved in the control of food intake while restoration of leptin levels maintained weight loss and reversed the changes in the brain activity (Ahima, 2008[1]).
Leptin has also got a permissive role in pathogenesis of reproduction (Kennedy and Mitra, 1963[25]). Abundant leptin receptors have been detected in ovarian granulosa and theca cells (Karlsson et al., 1997[23]). Leptin treatment of these cells in vitro caused significant reduction in steroid output (Spicer and Francisco, 1997[40]). According to Frisch's hypothesis, a critical percent of body fat is required for maintenance of reproductive function (Frisch, 1984[18]). So, in conditions of starvation, when body energy is insufficient, reproductive function takes a back seat to support essential metabolism for survival (Wade et al., 1996[41]). Reduced weight is associated with low leptin levels. However, why overweight patients with hyperleptinemia also have reproductive dysfunction is still intriguing.
A study reports that leptin and insulin receptor deficient mice showed elevated testosterone, infertility and insulin resistance, which are reminiscent of Poly Cystic Ovarian Syndrome (PCOS) in humans (Caro et al., 1996[10]). So, in an attempt to indirectly learn about leptin and ovarian function, PCOS has been targeted for studies. In PCOS, endocrinological dysregulation is manifested as hyperandrogenism and anovulation. However, in the last 15 years, insulin resistance has been identified as a significant contributor to the metabolic and reproductive abnormalities in PCOS (Diamanti-Kandarakis, 2008[13]).
Leptin resistance was introduced in an apparent analogy with that of insulin resistance to explain why hyperleptinemia associated with obesity fails to correct the defect in energy balance and feeding behavior. According to Farooqi et al. (2002[17]), leptin binds to its receptor in hypothalamus and activates JAK-STAT 3 pathway leading to suppression of Neuropeptide Y and Agouti-related protein (peptides which increase food intake) and secretion of Proopiomelanocortin and Corticotropin releasing hormone (peptides which reduce food intake). In obesity, the transport of leptin across the blood brain barrier is diminished and levels of SOCS 3, an inhibitor of leptin signaling is increased in hypothalamus, which leads to leptin resistance (Farooqi et al., 2002[17]; Oral et al., 2002[36]).
Metformin is an antidiabetic drug with anorexigenic properties. Metformin appears to affect ovarian function in a dual mode, through the alleviation of insulin resistance on ovary and through direct effect on ovary. It reduces CYP17 activity in theca cells and reduces steroidogenesis in women with PCOS (Nestler and Jakubowicz, 1996[35]). Metformin also suppresses androstenedione A4 production which increases in an insulin independent action (Attia et al., 2001[4]). It also has modest effect on adipose tissue. A study shows that metformin stimulates catabolism, increases glucose transport and utilization, mitochondrial and peroxismal FA beta oxidation, basal lipolysis and aerobic and anaerobic respiration (Lenhard et al., 1997[32]).
In the present study, leptin levels are evaluated in PCOS women before and after treatment with metformin and they are further correlated with other hormonal parameters.
Material and Methods
This study was performed on 30 PCOS women in reproductive age group (15-35 years). Institutional ethics committee approved the study. Written consent of all the patients was taken before enrollment. The diagnosis of PCOS was based on history of oligomenorrhea, infertility, elevated LH/FSH ratio, elevated androgens and reduced progesterone. All patients were anovular at the time of enrollment, as evidenced by the serum progesterone assays. All the patients had polycystic ovaries (PCO), as seen in ultrasonography (USG). No patient had any other endocrinopathy. They had not been on any hormonal medication for at least 3 months before the study. No patient had cardiac, renal or hepatic disease. At the time of enrollment, all routine investigations were done. Weight, height and BMI were noted. After overnight fasting, fasting blood glucose levels were noted and samples were drawn for serum insulin, serum leptin, serum progesterone and serum testosterone. The patients received 500 mg metformin tablets thrice a day (Glenmark Pharmaceuticals Ltd.) for 2 months. The treatment was started in the menstrual phase or the follicular phase (as seen in USG). The patients were asked to maintain a menstrual calendar and to note any side effects throughout the study period. Patients were followed up every month for follicle monitoring by USG from 10th day of the last menstrual period at 2 day interval for 5-6 times. Any pregnancy during the treatment period was recorded. At the end of 2 months, all the routine investigations were done and USG was recorded. Venous blood sample was taken for estimation of progesterone, testosterone, insulin and leptin. The hormonal assays were carried out by ELISA technique. ELISA kits for leptin and insulin were supplied by DRG laboratories Ltd. ELISA kits for progesterone and testosterone were supplied by Dia Metra Laboratory Limited.
Paired t test was employed for statistical analysis of the parameters within the group at the baseline and after 2 months of treatment. Chi square test was employed to find statistical significance of ovulation. Pearson's correlation test was employed for evaluating correlation between all the parameters. Statistical analysis was done by SPSS version 10.0 statistical software. The value of p < 0.05 was taken as significant.
Results
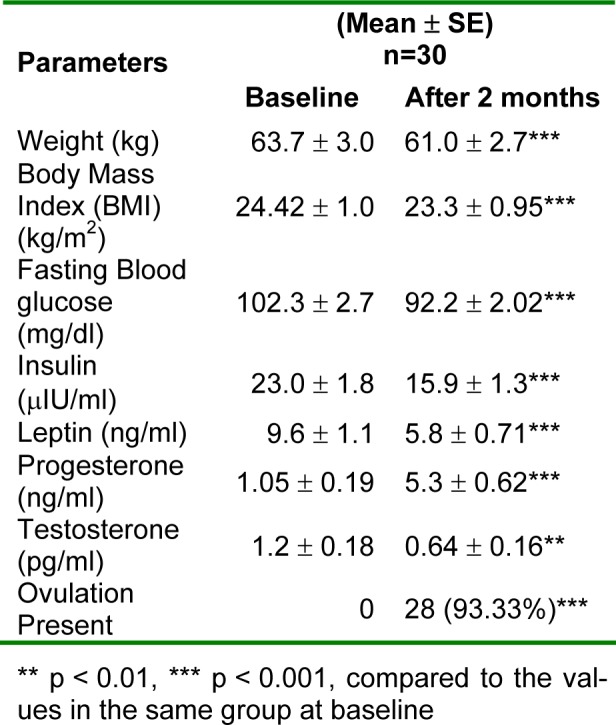
The PCOS women displayed significant benefit after 2 months treatment with metformin (Table 1(Tab. 1)). The weight and BMI reduced significantly after metformin treatment (p < 0.001). The fasting glucose levels showed a significant reduction (p < 0.001). The hormonal assays showed significant reduction in the levels of insulin, leptin and testosterone (p < 0.001), while a significant increase in progesterone levels (p < 0.01). Ovulation was achieved in 93.33 % women. The mean leptin levels after 2 months of treatment in metformin group reduced significantly (9.6 ± 1.1 to 5.8 ± 0.71 ng/ml) (p < 0.001). However, the leptin levels were found to be in the normal range (6.6-11.0 ng/ml according to the American Medical Association) (Iverson et al., 2007[22]).
Table 1. Comparison of clinical, biochemical and endocrinological data of all the study patients.
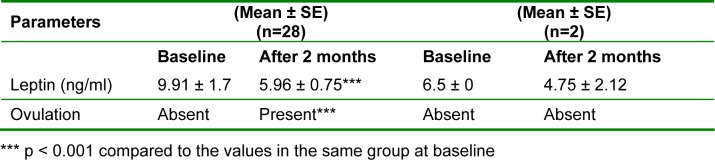
On analyzing leptin and ovulation rate, it was found that in 28 patients, who had ovulation, the mean leptin levels decreased significantly from 9.91 ± 1.7 ng/ml to 5.96 ± 0.75 ng/ml (p < 0.001). In 2 patients, who had remained anovular even after the treatment, the mean leptin levels reduced from 6.5 ± 0 ng/ml to 4.75 ± 2.12 ng/ml, which was not significant. Ovulation was achieved only with significant reduction in leptin levels (Table 2(Tab. 2)).
Table 2. Comparison of serum leptin levels and ovulation.
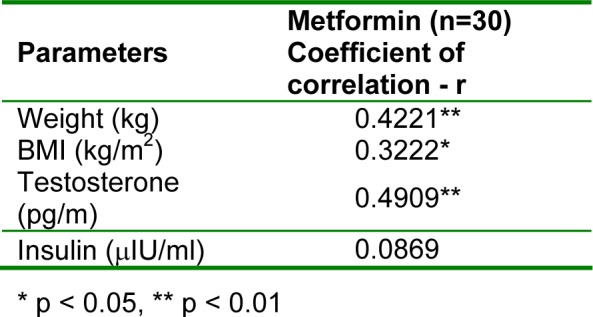
On applying Pearson's correlation test to all the parameters, it was seen that leptin showed significant positive correlation with weight (p < 0.05) and testosterone (p < 0.01), but no significant correlation with insulin (Table 3(Tab. 3)).
Table 3. Correlation of serum leptin levels with other parameters.
Discussion
Adiposity and metabolism have long been shown to regulate reproductive function, and leptin is believed to be a key hormone subserving this physiologic relationship (Ahima et al., 1996[3]). The present study reported that treatment with metformin brought a significant reduction in weight, BMI, fasting blood glucose, testosterone, insulin and leptin levels and an increase in progesterone levels, which resulted in an increase in ovulation rate (Table ). Although leptin concentration is closely related to body fat mass, yet the reduction in leptin levels cannot be fully explained by the reduction in weight and BMI, because metformin is found to reduce leptin concentration even in normal weight healthy patients (Glueck et al., 2001[20]; Fruehwald-Schultes et al., 2002[19]). Several researches have been done to analyse the molecular mechanism behind the effect of metformin on leptin levels. An in-vitro study reports that metformin inhibits leptin secretion by inhibiting MAPK signaling pathway in adipocytes (Klein et al., 2004[28]). Considering the hypothesis that PCOS is characterized by leptin resistance, a study showed that metformin restores leptin sensitivity in obese rats with leptin resistance and metformin treatment increased CSF leptin concentrations in both standard chow and high-fat-fed obese rats compared with the untreated rats (Kim et al., 2006[27]). It is suggested that the increase in CSF leptin level may be the cause of reduced resistance because the defect in leptin transport through the blood-brain barrier is a possible mechanism of leptin resistance (Caro et al., 1996[9]; Couce et al., 2001[12]; Nam et al., 2001[34]). Kim et al. (2006[27]) also reported that metformin increased hypothalamic POMC (an anorexigenic peptide) expression by leptin treatment in high-fat-fed obese rats, whereas this was not observed in untreated high-fat-fed obese rats. As the effect of leptin is associated with the activation of POMC (Kim et al., 2005[26]), failure to activate POMC expression by leptin is an evidence of leptin resistance.
In our study, we have found a strong association between leptin and ovulation rate (Table 2(Tab. 2)). This finding is supported by a study which reports that leptin induces follicular development in ob/ob mice and formation of corpora lutea and ovulation in hypogonadal mice (Barkan et al., 2005[5]). Another study showed that rats subjected to severe food restriction had reduction in ovulation and serum progesterone. Leptin administration to these rats enhanced the ovulatory process and prevented the negative effects produced by malnutrition (Roman et al., 2005[38]). However, a study reports negative effect of leptin on ovulation in rat ovary model. In this study, leptin administration resulted in fewer ovulations (Duggal et al., 2000[14]).
In the present study, leptin levels were within normal limits in PCOS patients at baseline. One reason can be that the patients in this study were not in the obese category. Rouru et al. (1997[39]) also reported similar serum leptin levels in PCOS and control subjects. They reported that serum leptin levels are related to BMI and weight, but are not significantly different in PCOS and control subjects. It has been reported in both in vitro (Kennedy et al., 1997[24]) and in vivo (Kolacynski et al., 1996[29]) studies that hyperinsulinemia stimulates leptin production from adipocytes. However, the normal leptin levels found in this and other studies lead to a possibility that insulin resistance at the levels of adipocytes counteracts the stimulatory impact of hyperinsulinemia and it may account for the maintenance of normal levels of serum leptin in PCOS (Laughlin et al., 1997[31]; Mantzoros et al., 1997[33]).
Serum leptin levels showed significant positive correlation with weight, BMI and testosterone, but did not show any correlation with insulin levels (Table 3(Tab. 3)). Similarly, Erturk et al could not find any correlation between leptin and insulin levels (Erturk et al., 2004[16]). The role of androgens in the regulation of leptin secretion is also controversial (Iuorno et al., 2007[21]). Correlation between leptin and testosterone can be substantiated by the direct effect of leptin on steroidogenesis. Karlsson et al. (1997[23]) have reported that immunoreactive leptin acts on leptin receptors in ovaries (granulosa and theca cells) and suppresses LH induced estradiol production. It may promote a steroid microenvironment in the follicle similar to that present in PCOS (Erickson et al., 1979[15]). It can be said that a significant decrease in leptin levels is associated with a decrease in androgen concentration, hence an increase in ovulation rate. Using the rat as a model, Castrogiovanni and colleagues (2003[11]) demonstrated that the administration of testosterone results in an increase in both leptin turnover and clearance. In contrast, Brzechffa et al. (1996[6]) reported that women who are relatively more insulin resistant and who exhibit high serum androgen levels have higher serum leptin concentrations than do normal women. However, Krotkiewski and colleagues (2003[30]) demonstrated that neither the acute reduction of serum androgen with a gonadotropin-releasing hormone agonist nor the administration of antiandrogens in women with PCOS alters serum leptin concentrations. Moreover, Remsberg and colleagues (2002[37]) have shown that lean women with PCOS who manifest higher androgen but lower insulin levels have lower serum leptin concentrations when compared with BMI-matched normal women. These observations suggest that insulin rather than androgen is the primary factor regulating leptin secretion, a conclusion that is consistent with our current findings.
Conclusion
Metformin reduces leptin resistance and increases ovulation in PCOS women. Leptin correlates with testosterone and BMI, but does not correlate with insulin levels. Significant reduction in leptin levels also induces ovulation.
References
- 1.Ahima RS. Revisiting leptin’s role in obesity and weight loss. J Clin Invest. 2008;118:2380–3. doi: 10.1172/JCI36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 4.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–524. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 5.Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M. Leptin induces ovulation in GnRH-deficient mice. FASEB J. 2005;19:133–135. doi: 10.1096/fj.04-2271fje. [DOI] [PubMed] [Google Scholar]
- 6.Brzechffa PR, Jakimiuk AJ, Agarwal SK, Weitsman SR, Buyalos RP, Magoffin DA. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:4166–4169. doi: 10.1210/jcem.81.11.8923878. [DOI] [PubMed] [Google Scholar]
- 7.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–548. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 8.Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001;12:65–72. doi: 10.1016/s1043-2760(00)00352-0. [DOI] [PubMed] [Google Scholar]
- 9.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity, a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 10.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 11.Castrogiovanni D, Perello M, Gaillard RC, Spinedi E. Modulatory role of testosterone in plasma leptin turnover in rats. Endocrine. 2003;22:203–210. doi: 10.1385/ENDO:22:3:203. [DOI] [PubMed] [Google Scholar]
- 12.Couce ME, Green D, Brunetto A, Achim C, Lloyd RV, Burguera B. Limited brain access for leptin in obesity. Pituitary. 2001;4:101–110. doi: 10.1023/a:1012951214106. [DOI] [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 14.Duggal PS, Vander Hoek KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, Norman RJ. The invivo and invitro effects of exogenous leptin on ovulation in the rat. Endocrinol. 2000;141:1971–1976. doi: 10.1210/endo.141.6.7509. [DOI] [PubMed] [Google Scholar]
- 15.Erickson GF, Hsueh AJW, Quigley ME, Rebar RW, Yen SS. Functional studies of aromatase activity in human granulose cells from normal and polycystic ovaries. J Clin Endocrinol Metab. 1979;49:514–519. doi: 10.1210/jcem-49-4-514. [DOI] [PubMed] [Google Scholar]
- 16.Erturk E, Kuru N, Savci V, Tuncel E, Ersoy C, Imamoglu S. Serum leptin levels correlate with obesity parameters, but not with hyperinsulinism in women with polycystic ovary syndrome. Fertil Steril. 2004;82:1364–1368. doi: 10.1016/j.fertnstert.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch RE. Body fat, puberty and fertility. Biol Rev. 1984;59:161–188. doi: 10.1111/j.1469-185x.1984.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 19.Fruehwald-Schultes B, Oltmanns KM, Toschek B, Sopke S, Kern W, Born KJ, Fehm HL, Peters A. Short-term treatment with metformin decreases serum leptin concentration without affecting body weight and body fat content in normal-weight healthy men. Metabolism. 2002;51:531–536. doi: 10.1053/meta.2002.31332. [DOI] [PubMed] [Google Scholar]
- 20.Glueck CJ, Fontaine RN, Wang P, Subbiah MTR, Weber K, Illig E, Streicher P, Sieve-Smith L, Tracy T, Kang JE, McCullough P. Metformin reduces weight, centropedal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism. 2001;50:856–861. doi: 10.1053/meta.2001.24192. [DOI] [PubMed] [Google Scholar]
- 21.Iuorno MJ, Islam LZ, Veldhuis PP, Boyd DG, Farhy LS, Johnson ML, Nestler JE, Evans WS. Leptin secretory burst mass correlates with body mass index and insulin in normal women but not in women with polycystic ovary syndrome. Metabolism. 2007;56:1561–1565. doi: 10.1016/j.metabol.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Iverson CL, Christiansen S, Flanagin AF, Fontanarosa PB, Glass RM, Gregoline B, et al. AMA Manual of Style: A Guide for Authors and Editors. 10th. New York, NY: Oxford University Press; 2007. p. 798. [Google Scholar]
- 23.Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B. Expression of functional leptin receptors in human ovary. J Clin Endocrinol Metab. 1997;82:4144–4148. doi: 10.1210/jcem.82.12.4446. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender based differences in relationship to adiposity, insulin sensitivity and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty. J Physiol. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YW, Choi DW, Park YH, Huh JY, Won KC, Choi KH, Park SY, Kim JY, Lee SK. Leptin-like effects of MTII are augmented in MSG-obese rats. Regul Pept. 2005;127:63–70. doi: 10.1016/j.regpep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metormin restores leptin sensitivity in high fat fed obese rats with leptin resistance. Diabetes. 2006;55:716–724. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- 28.Klein J, Westphal S, Kraus D, Meier B, Perwitz N, Ott V, Fasshauer M, Klein HH. Metformin inhibits leptin secretion via a mitogen activated protein kinase signaling pathway in brown adipocytes. J Endocrinol. 2004;183:299–307. doi: 10.1677/joe.1.05646. [DOI] [PubMed] [Google Scholar]
- 29.Kolacynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans in vivo and in vitro. Diabetes. 1996;45:699–701. doi: 10.2337/diab.45.5.699. [DOI] [PubMed] [Google Scholar]
- 30.Krotkiewski M, Landin K, Dahlgren E, Janson PO, Holm G. Effect of two modes of antiandrogen treatment on insulin sensitivity and serum leptin in women with PCOS. Gynecol Obstet Invest. 2003;55:88–95. doi: 10.1159/000070180. [DOI] [PubMed] [Google Scholar]
- 31.Laughlin GA, Morales AJ, Yen SSC. Serum leptin levels in women with polycystic ovary syndrome: The role of insulin resistance/hyperinsulinemia. J Clin Endocrinol Metab. 1997;82:1692–1696. doi: 10.1210/jcem.82.6.4028. [DOI] [PubMed] [Google Scholar]
- 32.Lenhard JM, Kliewer SA, Paulik MA, Plunket KD, Lehmann JM, Weiel JE. Effects of troglitazone and metformin on glucose and lipid metabolism: alterations of two distinct molecular pathways. Biochem Pharmacol. 1997;54:801–808. doi: 10.1016/s0006-2952(97)00229-3. [DOI] [PubMed] [Google Scholar]
- 33.Mantzoros CS, Dunaif A, Flier JS. Leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:1687–1691. doi: 10.1210/jcem.82.6.4017. [DOI] [PubMed] [Google Scholar]
- 34.Nam SY, Kratzsch J, Kim KW, Kim KR, Lim SK, Marcus C. Cerebrospinal fluid and plasma concentrations of leptin, NPY, and alpha-MSH in obese women and their relationship to negative energy balance. J Clin Endocrinol Metab. 2001;86:4849–4853. doi: 10.1210/jcem.86.10.7939. [DOI] [PubMed] [Google Scholar]
- 35.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 36.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 37.Remsberg KE, Talbott EO, Zborowski JV, Evans RW, McHugh-Pemu K. Evidence for competing effects of body mass, hyperinsulinemia, insulin resistance, and androgens on leptin levels among lean, overweight, and obese women with polycystic ovary syndrome. Fertil Steril. 2002;78:479–486. doi: 10.1016/s0015-0282(02)03303-4. [DOI] [PubMed] [Google Scholar]
- 38.Roman EA, Ricci AG, Faletti AG. Leptin enhances ovulation and attenuates the effects produced by food restriction. Mol Cellul Endocrinol. 2005;242:33–41. doi: 10.1016/j.mce.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Rouru J, Anttila L, Koskinen P, Penttila T-A, Irjala K, Huupponen R, Koulu M. Serum leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:1697–1700. doi: 10.1210/jcem.82.6.3996. [DOI] [PubMed] [Google Scholar]
- 40.Spicer JL, Francisco CC. The adipose obese gene product, leptin: Evidence of a direct inhibitory role on ovarian function. Endocrinology. 1997;138:3374–3379. doi: 10.1210/endo.138.8.5311. [DOI] [PubMed] [Google Scholar]
- 41.Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol. 1996;270:E1. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]