Abstract
This study investigated the effects of high-fat diet on metabolic factors in the presence of acute foot-shock and psychological stresses in male Wistar rats.
The animals were divided into high-fat (45 % cow intra-abdominal fat) and normal (standard pellets) diet groups; then, each group was allocated into stressed and control groups. Stress was induced by a communication box. Blood samples were collected by retro-orbital-puncture method under isoflurane anesthesia. Plasma levels of glucose, insulin, triglyceride, cholesterol, free fatty acid and corticosterone were measured.
Water and food intake significantly decreased in high-fat diet group; however, their weight did not change compared with the normal diet group. The level of fasting plasma glucose in the high-fat diet group decreased whereas, the fasting plasma insulin level did not significantly change. Stress increased the plasma glucose level 15 minutes after oral glucose tolerance test (OGTT) in both diet subgroups. The concentration of plasma insulin increased after stress induction in fasting and 15 minutes after performing OGTT. The increase in the plasma level of corticosterone was significant in both diet subgroups of only the foot-shock stress group. Plasma level of cholesterol and triglyceride in the high-fat diet group significantly increased; however, foot-shock stress decreased only triglyceride concentration. Plasma level of the fatty acids did not change in any of the groups. Statistical analysis showed no significant interaction between high-fat diet and stress.
As a whole, the results showed that the high-fat diet used in the present study did not noticeably affect metabolic parameters even in the presence of acute stress.
Keywords: stress, high-fat diet, insulin, corticosterone, glucose
Introduction
High-fat diets accompanied by different degrees of stress, which are prevalent in the modern societies, are common predisposing factors for many metabolic disorders and chronic diseases. High levels of fat in diets and stress, as well, cause insulin resistance which is considered as a public issue in modern societies (Sandu et al., 2005[27]; Schrauwen, 2007[29]). In regard to the negative impact of the high-fat diet and stress on human health, investigators have developed several animal models for studying the effects of each of the two factors separately or in combination on glucose and lipid metabolism. Their results showed that using high-fat, derived from animal or plant sources, diets in rats led to different metabolic results (for revieew see: Buettner et al., 2007[7]). For instance, after applying high-fat diet in rodents, plasma glucose did not change or moderately increased or even highly increased at the level of type 2 diabetes (Buettner et al., 2007[7]; Ohtsubo et al., 2005[24]). The increase in plasma glucose level (Fu et al., 2009[12]; Blazquez and Quijada, 1968[5]) also accompanied with a decrease (Blazquez and Quijada, 1968[5]) or an increase (Fu et al., 2009[12]) in the plasma insulin level. On the other hand, stress usually impairs glucose metabolism. Previous studies showed that applying acute (Armario et al., 1985[2]) and/or chronic (Farias-Silva et al., 2002[10]; Thiagarajan et al., 1988[33]) stresses increase plasma glucose level which is accompanied with a decrease (Armario et al., 1985[2]) or an increase (Farias-Silva et al., 2002[10]) or even no change (Thiagarajan et al., 1988[33]) in plasma insulin level. The combination of stress and high-fat diet may cause more pronounced changes of the metabolic factors than when each of them alone is applied (Fu et al., 2009[12]). A high-fat diet also can reduce the number of insulin receptors in target tissues leading to decreased insulin function (Iwashita et al., 2002[17]).
High-fat diet can affect the organism response to stress. Most of the studies indicate that high-fat diet increases the response of HPA axis to stress (Tannenbaum et al., 1997[31]; Legendre and Harris, 2006[22]). However, some studies have demonstrated a decrease in HPA response to stress in the presence of high-fat diet (La Fleur et al., 2005[21]).
As mentioned above, although the effect of high-fat diet and stress on metabolic factors has been investigated extensively, discrepancies still exist in the results. In this regard, this study was performed to further evaluate the possible existence of interactions between the high-fat diet and stress as two important factors which can be the basis of metabolic disorders. In the present study an animal model of high-fat diet was created for rats using a diet containing 45 % cow intra-abdominal fat (which has not specifically investigated as fat component of the high-fat diet). Then, the animals were exposed to acute foot-shock and psychological stresses and changes in plasma levels of glucose, insulin, cholesterol, triglyceride, free fatty acids and also corticosterone as important metabolic indicators of stress were evaluated.
Materials and Methods
Animals
Male Wistar rats were used in this study with a mean weight of 170±5 grams at the initiation of the experiment. Each pair of the animals was kept in a cage at 22±2 °C with a constant light-dark cycle (light from 7:00 AM to 7:00 PM). Sufficient water and food was always provided for the animals except during the experiment. The animals were randomly allocated into two groups considering the type of diet and were simultaneously fed by high-fat diet (containing 45 % cow intra-abdominal fat mixed with standard pellets) (Kitraki et al., 2004[20]; Ghibaudi et al., 2002[14]) and normal diet (standard pellets, Pars production and distribution of animal feed company, Iran) for 30 days. The animals in both groups were weighted (1 g Digital balance, FWE Japan) and their water and food intake were measured.
Then the animals of each of the diet groups were randomly recruited into control and stressed groups. In the normal/high-fat diet group, one group was transported to communication box apparatus (Borje Sanat, Iran) without receiving any stress (non-stress group). The other group received electric shock through the foot from stress apparatus (foot-shock stress group), while the third group was placed in the apparatus receiving psychological stress (psychological stress group). All experimental procedures were approved by the animal care and use committee of the Shahid Beheshti University of Medical Sciences, Neuroscience Research Center.
Blood sampling
In all groups, blood sampling was performed before initiating the experiment (i.e. before starting the diet) and used as the standard basis of initiation condition. Thirty days after initiating the diets, blood sampling was performed for the stressed group at the day before stress induction, i.e. morning of the stress induction day (8-8:30 AM), immediately after stress induction (12-14 PM) and the day after stress induction (8-8:30 AM); in the group which received no stress and was just placed in the communication box, blood samples were collected in the same sequence. Retro-orbital puncture method was used for blood sampling with isoflurane (Nicholas Primal, UK) as anesthetic agent (Zardooz et al., 2010[36]). The blood samples were collected in Eppendorf tubes containing 5 µl heparin (5000 IU/ml) per one ml blood. Then the tubes were centrifuged at 3000 round per minute for 5 minutes; the separated plasma was preserved at -80 °C.
Oral glucose tolerance test (OGTT)
After 16 hours of fasting, the animals were fed by 2 g/kg glucose using 45 % glucose solution through an esophageal tube (Gasa et al., 2002[13]). Blood sampling was performed after 15 minutes (this was the peak time for plasma glucose concentration after performing OGTT which obtained from a pilot study). In all groups, before starting the experiment (i.e. before starting the diet) OGTT was performed as the standard basis for the initiation condition. Thirty days after starting the diets, OGTT was performed one day before and one day after stress induction in the stressed groups; in the control groups, it was performed one day before and one day after being placed in the communication box.
Stress inducing apparatus
A communication box consisting of 9 chambers (16×16) was used to induce stress. The device is designed so that the animals in the chambers can have visual, auditory and olfactory communication with each other. The floor of 5 chambers is from metal wire made of stainless steel which is connected to electricity to put the animals of the chambers under electrical shock. The floor of the other 4 chambers is made of plexiglas that does not conduct electricity, so the animals do not receive any electrical shock but they see the movements and hear the sounds of the other animals under electrical shock (i.e. psychological stress) (Endo et al., 2001[9]).
Stress induction
The animals of the foot-shock stress group received electrical shocks (1 mA, 1 Hz) for a 10 second duration every 60 second (Endo et al., 2001[9]). These stress exposures were carried out for 60 min between 11.00 and 13.00 h while the animals of the psychological stress were watching them under electrical shock from the other chambers of the box; however, the controls were kept in the box for one hour without receiving any stress.
Assays
Plasma glucose concentration was determined using the glucose oxidase method (Pars Azmoon, Iran). Cholesterol and triglyceride concentrations were measured by enzymatic calorimetric method (Pars Azmoon, Iran). Plasma free fatty acid was determined by rat free fatty acid calorimetric kit (Nanjing Jiancheng Bioengineering Institute, China). Rat insulin ELIZA kit (Mercodia, Sweden) and corticosterone ELIZA kit (DRG, Germany) were used to measure plasma insulin and corticosterone concentrations.
Statistical analysis
All data are expressed as the mean ± SEM. Three-way analyses of variance (ANOVA) were performed and followed by Bonferroni test by considering diet, stress and basal condition as factors. To compare diets in different days repeated measurement two-way analyses of variance (ANOVA) were used. A P-value below 0.05 was considered to be statistically significant.
Results
The impact of high-fat diet on the weight changes of the male rats
During the 30 days the animals' weight in both high-fat and normal diet groups have significantly increased compared with the weight of the first day (P<0.001) but it was not significantly different between the high-fat and the normal diet groups (Table 1(Tab. 1)). However, there was not a significant interaction between the weight changes during the time and the diet type.
Table 1. Food and water intake and weight variations in the high-fat and normal diet groups.
The impact of high-fat diet on the food and water intake in the male rats
A significant reduction in the food and water intake of the high-fat diet group was observed compared with the normal diet group (P<0.001) (Table 1(Tab. 1)).
Plasma glucose concentration in the basal condition and after OGTT before and after stress induction
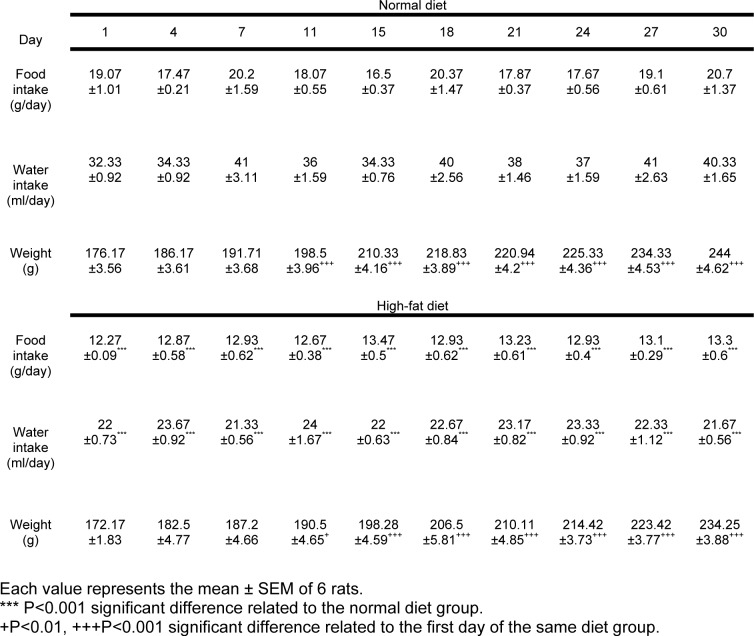
The results of three-way ANOVA showed that high-fat diet decreased fasting plasma glucose concentration in the basal condition (time zero) (P<0.05) (Figure 1a(Fig. 1)); however, a significant increase in plasma glucose concentration was observed in the high-fat diet animals after inducing foot-shock stress (P<0.01) (Figure 1a(Fig. 1)).
Figure 1. Plasma glucose concentrations in normal and high-fat diet groups before and after stress induction in fasting state (a) and 15 min after OGTT performance (b). Each column represents the mean±SEM of 6 rats.
**P<0.01, *P<0.05 significant difference versus (Before) of each group. +P<0.05 significant difference versus (After) of the control (non-stress) group of the same diet group.
Before: Before exposure to stress or being placed in the communication box
After: After exposure to stress or removing from the communication box.
Fifteen minutes after OGTT, foot-shock stress in the normal diet group and psychological stress in the high-fat diet group significantly increased glucose concentration (P<0.05) (Figure 1b(Fig. 1)). The increase in glucose concentration was significantly more in the high-fat diet animals receiving psychological stress than the control animals (non-stress) of the same diet group (P<0.05) (Figure 1b(Fig. 1)). The results of the present study did not demonstrate a significant interaction between the diet type and the response to stress in relation with glucose tolerance.
Plasma insulin concentration in the basal condition and after OGTT before and after stress induction
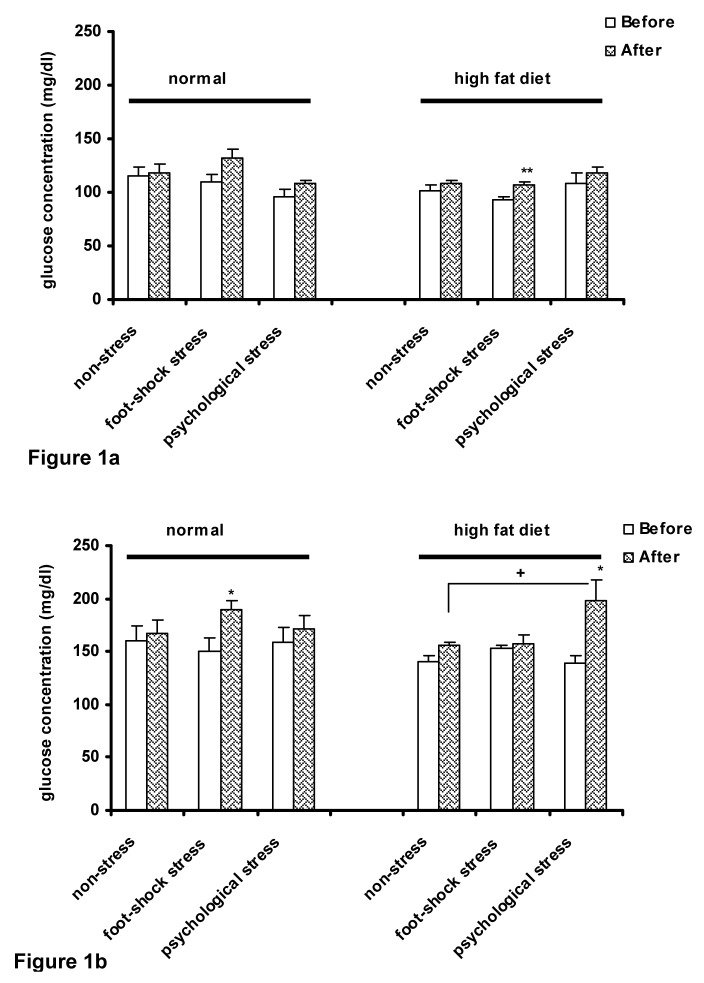
Three-way ANOVA showed that after stress induction, plasma insulin concentration in basal condition (time zero) increased which was significant in the foot-shock stress group of the animals with the normal diet (P<0.01) (Figure 2a(Fig. 2)); however, the increase in plasma concentration was significant in the animals of the high-fat diet group which were under psychological stress (P<0.05) (Figure 2a(Fig. 2)).
Figure 2. Plasma insulin concentrations in normal and high-fat diet groups before and after stress induction in fasting state (a) and after performing OGTT (b). Each column represents the mean ± SEM of 6 rats.
**P<0.01, *P<0.05 significant difference versus (Before) of each group.
Before: Before exposure to stress or being placed in the communication box
After: After exposure to stress or removing from the communication box.
The results demonstrated that 15 minutes after OGTT, plasma insulin concentration increased after stress induction; however, significant increase in plasma insulin concentration was observed only in the psychological stress group of the normal diet animals (P<0.01) (Figure 2b(Fig. 2)). There was no significant interaction between the diet type and the response to the stress.
Changes in plasma corticosterone concentration in response to the high-fat diet intake and stress induction
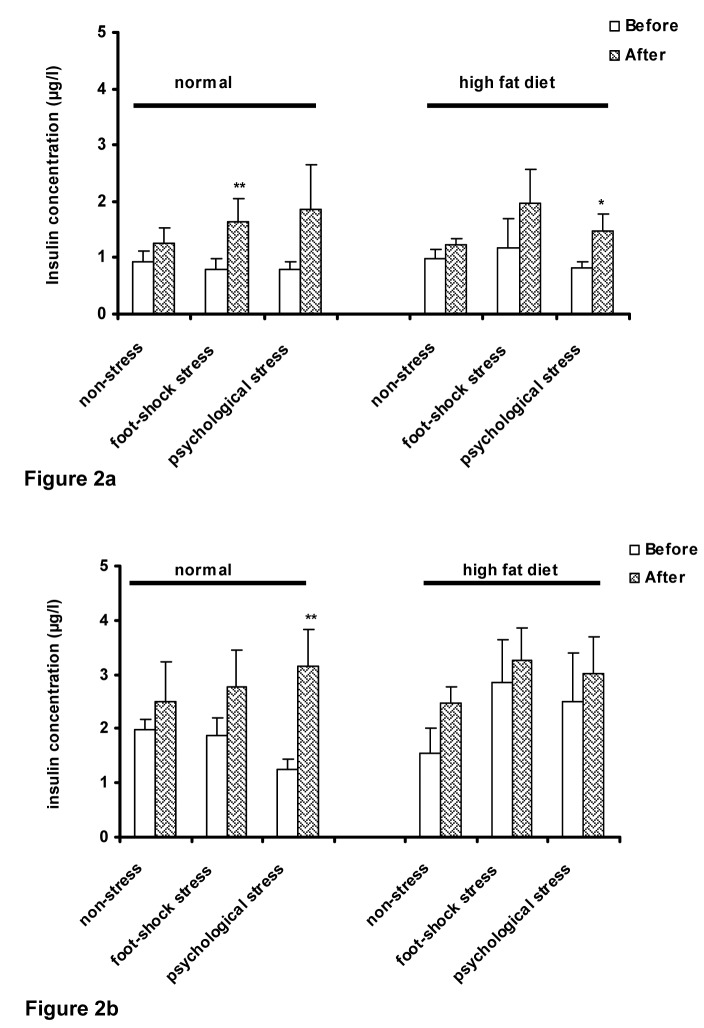
The type of the diet had no effect on changes of plasma corticosterone concentration. Foot-shock stress in the normal diet animals significantly increased plasma corticosterone concentration compared with before stress induction in the same group (P<0.001) (Figure 3(Fig. 3)), after stress induction in the psychological stress group (P<0.05) and the control group (P<0.001). Acute foot-shock stress, significantly increased plasma corticosterone concentration in the high-fat diet animals compared with before stress induction in the same group, after stress induction in the psychological stress group and the control group, as well (P<0.001) (Figure 3(Fig. 3)). Also, three-way ANOVA did not show any significant interaction between the diet type and the response to stress.
Figure 3. The effect of high-fat diet and stress on plasma corticosterone concentration. Each column represents the mean±SEM of 6 rats.
***P<0.001 significant difference relative to (Before) of each group. +++P<0.01, +P<0.05 significant difference relative to the (After) of the psychological stress and control (non-stress) groups.
Before: Before exposure to stress or being placed in the communication box
After: After exposure to stress or removing from the communication box.
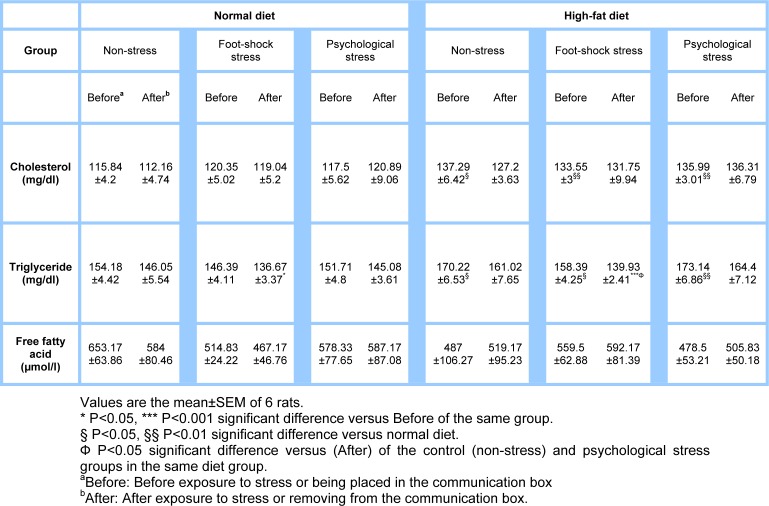
The impact of high-fat diet and stress on plasma cholesterol concentration
The high-fat diet compared with the normal diet significantly increased plasma cholesterol concentration [non-stress group (P<0.05), foot-shock and psychological stress groups (P<0.01)] (Table 2(Tab. 2)); however, stress induction did not have any significant effect on plasma cholesterol concentration in any of the groups. There was no interaction between the diet type and the response to stress, as well.
Table 2. The effect of high-fat diet and stress on the plasma cholesterol, triglyceride and free fatty acid concentrations in male rats.
The impact of the high-fat diet and stress induction on plasma triglyceride concentration
Compared with the normal diet, the high-fat diet significantly increased plasma triglyceride concentration [foot-shock stress and non-stress groups (P<0.05), psychological stress group (P<0.01)] (Table 2(Tab. 2)). Induction of foot-shock stress resulted in a significant reduction in triglyceride concentration of the high-fat diet group compared with before stress induction in the same group (P<0.001), after stress induction in the psychological stress group and the control non-stress group (P<0.05) (Table 2(Tab. 2)). The reduction was also observed in the normal diet group after foot-shock stress as compared to before stress induction (P<0.05) (Table 2(Tab. 2)). However, there was no significant interaction between the diet type and the response to stress in this part of the study, as well.
Plasma free fatty acids concentration following the use of the different diets and stress
Results showed that the high-fat diet did not make any significant changes in plasma free fatty acids concentration. Also, concentration of free fatty acids did not show any significant changes compared with before stress induction (Table 2(Tab. 2)). There was no significant interaction between the diet type and response to stress in this part.
Discussion
In the present study, high-fat diet did not significantly change plasma concentrations of glucose, insulin and corticosterone in the animals receiving acute stress compared with the controls. Consistently, in response to stress, high-fat diet did not make any significant changes in plasma concentration of free fatty acids, triglyceride and cholesterol as the factors that can reflect inappropriate lipid metabolism.
The results of this study showed no significant difference between the weights of high-fat and normal diet groups whereas, the food and water intake significantly decreased in high-fat fed rats. In agreement with our results , in a study on male rats no significant changes were observed in the animals' weight during high-fat diet (40 % fat) (Banas et al., 2009[3]); however, increase (Ghibaudi et al., 2002[14]) or decrease (Soulis et al., 2005[30]) of the weight was observed in high-fat fed rats in the previous studies. The differences between the results of the mentioned studies might be due to the type of fat and the duration of use (Grundy, 1999[15]). Healthy rats are sensitive to caloric properties of their diet and set their food intake by receiving fixed daily calories (Kamara et al., 1998[18]). Moreover, Plasma level of leptin increased following one week use of high-fat food (Soulis et al., 2005[30]). Therefore, reduction in the food intake which was observed in our experiment might be due to increased plasma leptin level beside setting the received calorie by the rats. Besides, reduced water intake in the high-fat diet group of our study, might result in weight reduction. In an experiment reduced protein intake decreased water intake by the rats (Kaunitz et al., 1956[19]). Consistently, in our study the percentage of protein intake decreased compared with the control condition which could result in reduced water intake.
The diet of the present study was not able to change glucose tolerance but significantly decreased fasting glucose concentration without any significant change in plasma insulin level. Consistent with our study, Soulis et al. (2005[30]) showed that seven days of high-fat diet (20 % corn oil) significantly decreased fasting plasma glucose in female Wistar rats while a study on male Wistar rats demonstrated an increase in fasting plasma glucose (Kitraki et al., 2004[20]); however, both of the studies indicated no changes in fasting insulin concentration and an increase in plasma leptin concentration. Since leptin alone or in combination with insulin can reduce hepatic glucose production by decreasing phosphoenolpyruvate-carboxykinase synthesis (Anderwald et al., 2002[1]) reduced plasma glucose concentration following use of high-fat diet in our study might be due to increased plasma concentration of leptin; however, plasma leptin level was not measured in the present study. Significant reduction in the consumption of fatty food in this study, augment the probability of an increase in plasma leptin concentration.
As mentioned earlier the results of the present study demonstrated that the high-fat diet could not change glucose tolerance significantly. However, Kamara et al. (1998[18]) showed that in male Sprague-Dawley rats, using high-fat diet containing 25 % corn oil for five weeks impaired glucose tolerance. They observed that in the second and the third hours after OGTT plasma glucose level increased in high-fat fed rats as compared to the controls. Besides, using fatty food containing 40 % butter for twelve weeks in male Wistar rats impaired glucose tolerance without any significant change in plasma levels of fasting glucose and insulin (Yamaguchi and Matsuoka, 1979[35]). It seems that the variety between the results of the experiments can be resulted from the type and the amount of the fat used, the duration of use, and strain of the study animals.
Stress alone can change plasma level of glucose and insulin as well as glucose tolerance. In the present study, stress increased fasting plasma glucose and insulin concentrations in both of the diet groups which are accompanied by decreased glucose tolerance. Soulis et al. (2005[30]) found that acute swimming stress in female Wistar rats decreased plasma insulin level without any changes in the basal glucose concentration while in the male rats plasma glucose concentration increased and plasma insulin concentration decreased (Kitraki et al., 2004[20]). On the other hand, induction of chronic restraint stress increased fasting plasma glucose concentration while decreasing fasting plasma insulin concentration and glucose tolerance (Zardooz et al., 2006[37]).
Consistent with our results, two previous studies showed no significant interaction between high-fat diet (20 % corn oil) and acute swimming stress in the response of plasma glucose and insulin in male and female Wistar rats (Soulis et al., 2005[30]; Kitraki et al., 2004[20]). However, high-fat diet (containing 18 % pig fat and cholesterol) in comparison with control condition (normal diet + stress) significantly increased fasting glucose concentration in response to chronic stress of electric shock accompanied with noise in male Wistar rats (Fu et al., 2009[12]). In the present study, although acute stress increased plasma insulin concentration, high-fat diet did not make any significant change in insulin response to acute stress. Compared with the control condition, 4 days of high-fat diet (40 % coconut oil and corn oil) following 3 hours of restraint stress did not significantly change plasma insulin concentration of male Sprague-dawley rats (Legendre and Harris, 2006[22]). The differences might also be related to the type of fat in the diet and the duration of use.
Although high-fat diet had no effect on basal concentration of plasma corticosterone in the present study, stress increased plasma concentration of corticosterone; however, no significant interaction was observed between high-fat diet and stress. Contrarily, using high-fat diet (20 % corn oil) in Long-Evans rats for one or three weeks significantly increased basal concentration of corticosterone. Also, in the rats which used high-fat diet for one, 9 and 12 weeks, plasma corticosterone and ACTH after restraint stress (for 20 minutes in a plastic tube) and 20, 60 and 120 minutes after the end of stress was significantly higher than those receiving stress under normal diet (Tannenbaum et al., 1997[31]). Kamara et al. (1998[18]) did not observe any changes in plasma corticosterone concentration after using 2 weeks of high-fat diet (25 % corn oil or olive oil or fish oil or soybean oil) in male Sprague-Dawley rat; however, a significant increase in plasma corticosterone concentration was observed in response to chronic restraint stress compared with the control animals (receiving stress under normal diet).
Consistent with the present study, in a study by Kitraki et al. (2004[20]) no significant change was observed in plasma corticosterone concentration in male Wistar rats following use of high-fat diet (20 % corn oil) for one week compared with the animals with normal diet. Also, compared with the controls which received stress under normal diet, high-fat diet did not significantly change plasma corticosterone response to acute swimming stress (Kitraki et al., 2004[20]). Several studies (Kitraki et al., 2004[20]; Brindley et al., 1981[6]), some mentioned above, indicate the effect of high-fat diet on HPA axis activity which increase basal level of adrenal corticosteroids. Therefore, it is claimed (Fu et al., 2009[12]; Tannenbaum et al., 1997[31]) that high-fat diet acts as a stressor; if induced chronically like using a chronic stressor it can be somehow adapted. However, like a chronic stressor it is also able to augment the response to a new stressor (Harris et al., 2004[16]). On the other hand, some researchers believe that high-fat diet (if used for a short time) just increases HPA axis response to mild stress while in severe stress, appropriate response is created without any relationship with the type of the diet used (Legendre and Harris, 2006[22]). Also, it is observed that using high-fat diet for a long time (more than 3 weeks or 10 weeks) cannot make any changes in HPA axis response to stress (Legendre and Harris, 2006[22]; Kamara et al., 1998[18]). The discrepancies which were observed in the results might reflect the various duration of high-fat diet or stress exposure, animals' age and strain as well as animals' sex. However the type of the fat also may affect the results.
In the next part of the present experiment the plasma level of free fatty acids was determined and no significant change of the plasma free fatty acid levels was observed in the high-fat fed rats, possibly because the high-fat diet was not able to increase the plasma corticosterone level. It is important to imply that corticosterone is a weak lipolytic hormone (Porterfield, 2001[25]) and also the increase of plasma glucocorticoids including corticosterone can stimulate the secretion of the triglycerides from liver (Bartlett and Gibbons, 1988[4]; Mangiapane and Brindley, 1985[23]). In addition glucocorticoids can decrease the lipoprotein lipase activity and induce hypertriglyceridemia (Taylor and Agius, 1988[32]). Thus it can be concluded that the level of the plasma free fatty acids may be increased after using high-fat diet.
Stress alone can increase plasma fatty acid concentration which is positively associated with stress duration (Ferguson and Shultz, 1975[11]). Sapira et al. (1965[28]) showed that the concentration of circulating fatty acids increased after at least nine hours of restraint stress. In the present study acute stress was not able to change plasma free fatty acids even in the presence of high-fat diet. The reason may be related on one hand to the type of fat used in our study and on the other hand to the type, duration and intensity of the stresses.
Other results of the study included the significant increase in plasma cholesterol and triglyceride concentrations affected by high-fat diet and reduction in triglyceride concentration after inducing physical stress. Consistent with the present study, stress decreased blood triglyceride concentration in rats (Robertson and Smith, 1976[26]; Tsikunov et al., 2006[34]). In the present and the mentioned studies, reduced concentration of triglyceride affected by stress might be due to impact of acute stress on the immediate decrease in endogenic triglyceride secretion (Robertson and Smith, 1976[26]). On the other hand, increased levels of triglyceride and cholesterol might be due to their high levels in the high-fat diet used. Besides, as using high-fat diet containing high calorie results in saturation of liver and muscle glycogen stores while glucose store is more than the required for ATP production, liver initiates endogenic triglyceride synthesis through glucose metabolism; as a result, blood VLDL and triglyceride levels increase (Chait et al., 1979[8]). However, no interaction was observed between high-fat diet and stress in relation with free fatty acid, cholesterol and triglyceride.
Altogether, this study also led to some what different results. Although 45 % intra-abdominal cow fat reduced some behaviors related to body metabolism like food and water intake, it was not so effective on metabolic and hormonal parameters and even their responses to acute foot-shock and psychological stresses.
Notes
This paper is part of a MSc thesis by Jamileh Ghalami.
Acknowledgements
This work was supported by a grant from Neuroscience Research center, Shahid Beheshti University of Medical Sciences. The authors would like to express their gratitude to Mr. Bahman Pasha for his help in preparing high-fat diet.
References
- 1.Anderwald C, Muller G, Koca G, Furnsinn C, Waldhausl W, Roden M. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol Endocrinol. 2002;16:1612–28. doi: 10.1210/mend.16.7.0867. [DOI] [PubMed] [Google Scholar]
- 2.Armario A, Castellanos JM, Balasch J. Chronic noise stress and insulin secretion in male rats. Physiol Behav. 1985;34:359–361. doi: 10.1016/0031-9384(85)90196-9. [DOI] [PubMed] [Google Scholar]
- 3.Banas SM, Rouch C, Kassis N, Markaki EM, Gerozissis K. A dietary fat excess alters metabolic and neuroendocrine responses before the onset of metabolic diseases. Cell Mol Neurobiol. 2009;29:157–168. doi: 10.1007/s10571-008-9307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett SM, Gibbons GF. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988;249:37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazquez E, Quijada CL. The effect of a high-fat diet on glucose, insulin sensitivity and plasma insulin in rats. J Endocrinol. 1968;42:489–494. doi: 10.1677/joe.0.0420489. [DOI] [PubMed] [Google Scholar]
- 6.Brindley DN, Cooling J, Glenny HP, Burditt SL, McKechnie IS. Effects of chronic modification of dietary fat and carbohydrate on the insulin, corticosterone and metabolic responses of rats fed acutely with glucose, fructose or ethanol. Biochem J. 1981;200:275–83. doi: 10.1042/bj2000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 8.Chait A, Brunzell JD, Johnson DG, Benson JW, Werner P, Palmer JP, et al. Reduction of plasma triglyceride concentration by acute stress in man. Metabolism. 1979;28:553–561. doi: 10.1016/0026-0495(79)90197-5. [DOI] [PubMed] [Google Scholar]
- 9.Endo Y, Yamauchi K, Fueta Y, Lrie M. Changes of body temperature and plasma corticosterone level in rats during psychological stress induced by the communication box. Med Sci Monit. 2001;7:1161–1165. [PubMed] [Google Scholar]
- 10.Farias-Silva E, Sampaio-Barros MM, Amaral MEC, Carneiro EM, Boschero AC, Grassi-Kassisse DM, et al. Subsensitivity to insulin in adipocytes from rats submitted to foot-shock stress. Can J Physiol Pharmacol. 2002;80:783–789. doi: 10.1139/y02-104. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson JH, Shultz TD. Plasma free fatty acid composition before and after cold exposure in the white rat. Int J Biochem. 1975;6:69–72. [Google Scholar]
- 12.Fu JH, Xie SR, Kong SJ, Wang Y, Wei W, Shan Y, et al. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp Clin Endocrinol Diabetes. 2009;117:354–360. doi: 10.1055/s-0028-1119406. [DOI] [PubMed] [Google Scholar]
- 13.Gasa R, Clark C, Yang R, DePaoli-Roach AA, Newgard CB. Reversal of diet-induced glucose intolerance by hepatic expression of a variant glycogen-targeting subunit of protein phosphatase-1. J Biol Chem. 2002;277:1524–1530. doi: 10.1074/jbc.M107744200. [DOI] [PubMed] [Google Scholar]
- 14.Ghibaudi L, Cook J, Farley C, Van Heek M, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague-Dawley rats. Obes Res. 2002;10:956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. The optimal ratio of fat-to-carbohydrate in the diet. Annu Rev Nutr. 1999;19:325–341. doi: 10.1146/annurev.nutr.19.1.325. [DOI] [PubMed] [Google Scholar]
- 16.Harris RB, GU H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. 2004;81:557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Iwashita S, Tanida M, Suzuki M. Decreased skeletal muscle insulin receptors in high-fat diet-related hypertensive rats. Nutr Res. 2002;22:1049–1053. [Google Scholar]
- 18.Kamara K, Eskay R, Castonguy TH. High-fat diets and stress responsivity. Physiol Behav. 1998;64:1–6. doi: 10.1016/s0031-9384(97)00534-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaunitz H, Slanetz CA, Johnson RE, Guilman J. Influence of diet composition on caloric requirement, water intake and organ weight of rats during restricted food intake. J Nutr. 1956;60:221–228. doi: 10.1093/jn/60.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Kitraki E, Soulis G, Gerozissis K. Impaired neuroendocrine response to stress following a short term fat-enriched diet. Neuroendocrinology. 2004;79:338–345. doi: 10.1159/000079665. [DOI] [PubMed] [Google Scholar]
- 21.La Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories,damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 22.Legendre A, Harris RBS. Exaggerated response to mild stress in rat fed high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1288–R1294. doi: 10.1152/ajpregu.00234.2006. [DOI] [PubMed] [Google Scholar]
- 23.Mangiapane EH, Brindley DN. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1985;233:151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Porterfield SP. Endocrine physiology. 2nd. Missouri: Mosby; 2001. pp. 143–144. [Google Scholar]
- 26.Robertson RP, Smith PH. Stress-induced inhibition of triglyceride secretion in vivo sand rats. Metabolism. 1976;25:1583–1590. doi: 10.1016/0026-0495(76)90111-6. [DOI] [PubMed] [Google Scholar]
- 27.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat–fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 28.Sapira JD, Lipman R, Shapiro AP. Effect of restraint on free fatty acid mobilization in rats. Psychosom Med. 1965;27:165–170. doi: 10.1097/00006842-196503000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Schrauwen P. High-fat diet, muscular lipotoxicity and insulin resistance. Proc Nutr Soc. 2007;66:33–41. doi: 10.1017/S0029665107005277. [DOI] [PubMed] [Google Scholar]
- 30.Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869–880. doi: 10.1007/s10571-005-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, Dawn McArthur M, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol Endocrinol Metab. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- 32.Taylor R, Agius L. The biochemistry of diabetes. Biochem J. 1988;25:625–640. doi: 10.1042/bj2500625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiagarajan AB, Gleiter CH, Nutt DJ. Electroconvulsive shock does not increase plasma insulin in rats. Convuls Ther. 1988;4:292–296. [PubMed] [Google Scholar]
- 34.Tsikunov SG, Klyueva NN, Kusov AG, Vinogradova TV, Klimenko VM, Denisenko AD. Changes in the lipid composition of blood plasma and liver in rats induced by severe psychic trauma. Bull Exp Biol Med. 2006;141:636–638. doi: 10.1007/s10517-006-0240-y. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi K, Matsuoka A. The effects of electric stress on pancreatic B cell function in rats fed a high-fat diet (I). Glucose tolerance and glucose-induced insulin release from the perfused pancreas. Nippon Naibunpi Gakkai Zasshi. 1979;55:1469–1481. doi: 10.1507/endocrine1927.55.12_1469. [DOI] [PubMed] [Google Scholar]
- 36.Zardooz H, Rostamkhani F, Zarinhalam J, Shahrivar FF. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res. 2010;59:973–978. doi: 10.33549/physiolres.931896. [DOI] [PubMed] [Google Scholar]
- 37.Zardooz H, Zahedi Asl S, Gharib Naseri MK, Hedayati M. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol Behav. 2006;89:373–378. doi: 10.1016/j.physbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]