Abstract
Importance
Marijuana use is increasingly common in the US. It is unclear whether it has long term effects on memory and other domains of cognitive function.
Objective
To study the association between cumulative lifetime exposure to marijuana use and cognitive performance in mid-life.
Design, Setting and Participants
We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study, a cohort of black and white men and women 18 to 30 years of age at baseline in 1986 (Year 0) and followed over 25 years, to estimate cumulative years of exposure to marijuana (=365 days of marijuana use) using repeated measures and to assess associations with cognitive function at Year 25. Linear regression was used to adjust for demographic factors, cardiovascular risk factors, tobacco smoking and alcohol, illicit drugs, physical activity, depression, and Mirror Star Tracing Test (a measure of cognitive function) at Year 2.
Main Outcome Measures
Three domains of cognitive function were assessed at Year 25 using the Rey Auditory Verbal Learning Test (verbal memory), the Digital Symbol Substitution Test (processing speed) and the Stroop Interference Test (executive function).
Results
Among 3385 Year 25 CARDIA participants with cognitive function measurements, 2852 (84%) reported past marijuana use, but only 392 (9%) continued to use marijuana into middle age. Current use of marijuana was associated with worse verbal memory and processing speed; cumulative lifetime exposure was associated with all three domains of cognitive function. After excluding current users and adjusting for potential confounders, cumulative lifetime exposure to marijuana remained strongly associated with verbal memory. For each 5 years of past exposure, verbal memory was 0.13 standardized units lower (95% confidence interval (CI):−0.24 to −0.02, p=0.02), corresponding to 1 of 2 participants on average remembering one word less from a list of 15 words for every 5 years of use. After adjustment, we found no associations with lower executive function (−0.03, 95%CI: −0.12 to 0.07) or processing speed (−0.04, 95%CI: −0.16 to 0.08).
Conclusion and Relevance
Past exposure to marijuana is associated with worse verbal memory, but does not appear to impact other domains of cognitive function.
Marijuana use is common among adolescents and young adults. Data from the US in 2012 indicate that among 12th graders (aged 17–18 years old), 37% had used marijuana within the last year, 23% within the last 30 days and 6.5% daily.1 If marijuana has significant adverse long-term effects, marijuana use early in life may have important public health consequences. Long-term effects from marijuana use, however, can be difficult to detect.
Impaired cognitive function (CF) is an acute effect of marijuana use,2 and there is increasing evidence that those effects may persist later in life.3–5 Heavy, long term use of marijuana has been associated with cognitive impairment, particularly in learning and remembering new information.3,6 Evidence from population-based studies, however, is scarce and it remains unclear whether there are long-term effects from low intensity or occasional marijuana use earlier in life3, and whether the magnitude and persistence of impairment depends on the duration of cannabis use or the age of exposure.4,5
With 25 years of repeated measurements of marijuana exposure starting in early adulthood, the Coronary Artery Risk Development in Young Adults (CARDIA) Study provides a unique opportunity to study the long-term effects of marijuana exposure among community-based adults. In Year 25, CARDIA measured cognitive performance using standardized tests of verbal memory, processing speed and executive function. We used these measurements to study the association between cumulative years of exposure to marijuana use and cognitive performance in mid-life among CARDIA participants with marijuana exposures typical of the communities in which they live
METHODS
Study Design and Sample
We used data collected over 25 years in the Coronary Artery Risk Development in Young Adults (CARDIA) study, a population-based epidemiological study of 5115 adults aged 18 to 35 years at baseline.7 Participants were recruited in 1985 and 1986 by random selection of telephone numbers from designated census tracts in Birmingham, AL; Chicago, IL; Minneapolis, MN; and by random selection from the membership list of a health care plan in Oakland, CA. The sampling scheme was designed to achieve a balance at each of the 4 sites by race (self-identified “black, not Hispanic” and “white, not Hispanic”), sex, education (high school degree or less, more than high school), and age (18–24 years, 25–30 years). All subjects gave informed consent before entering the study and at each visit and the approval of institutional review boards was obtained at each site.
Marijuana Exposure: Current and Cumulative
Current marijuana use was assessed at each in-person CARDIA visit (at baseline and after 2,5,7,10,15,20 and 25 years of follow up) using the following survey question: “During the last 30 days, on how many days did you use marijuana?” Direct self-reported lifetime exposure was assessed using the question: “About how many times in your lifetime have you used marijuana?”. We used current and lifetime use to compute marijuana-years, with one year of exposure equivalent to 365 days of marijuana use (see example in eMethods in Supplement).8 We assumed that current use at each visit (i.e. the number of days of using marijuana during the month before each visit) reflected the average number of days of use during the months before and after each visit. We estimated the cumulative lifetime use by adding up the total number of days using marijuana over follow-up. We adjusted our estimate upwards whenever directly self-reported lifetime use was higher than our computed estimates.8
Outcome Measure
CF was assessed by trained and certified CARDIA technicians who administered a battery of three cognitive tests at the Year 25 visit.9 The Rey Auditory Verbal Learning Test (RAVLT) mainly assesses verbal memory through the ability to memorize and retrieve lists of 15 words. The RAVLT yields three separate scores; in the main analyses we used the delayed (25 min) free recall score only (and tested the other two in sensitivity, Supplement).10 The Digit Symbol Substitution Test (DSST) assesses visual motor speed, executive function, sustained attention, and working memory; we refer to this domain as processing speed.11 The Stroop Interference Test evaluates the ability to view complex visual stimuli and to respond to one stimulus dimension while suppressing the response to another dimension; we refer to this domain as executive function.12,13 The resulting interference score provides a measure of how much additional executive processing is needed to respond to an incongruent trial; thus, a higher interference score indicates worse performance on the task. The inverse of this score was used in the present analyses such that increasing scores indicate better performance. Each measure was standardized by dividing the score by the within-CARDIA standard deviation and subtracting the mean such that absolute and relative differences in these standardized measures are comparable.
Other Covariates
Cigarette smoking behavior was evaluated during each in-person CARDIA visit and at yearly contacts over the phone between CARDIA visits. These data were used to estimate cumulative lifetime exposure to cigarettes in terms of pack-years, with 1 pack-year of exposure equivalent to smoking 1 pack of cigarettes per day for a year.8 We estimated lifetime alcohol consumption in “drink-years,” defining 1 drink-year as the amount of alcohol consumed in 1 year by a person consuming 1 drink/day (eMethods in Supplement).14 Acute heavy exposure to alcohol (bingeing) was defined as reporting 5 or more drinks on one occasion, and we estimated total lifetime episodes. We estimated total number of lifetime exposures to cocaine (including other forms of cocaine such as crack, powder, free base), amphetamines (speed, uppers, methamphetamines) and heroin (eMethods in Supplement).15,16 Education was measured as the maximum educational grade attained for each participant across reports at each visit. Physical activity was measured with the CARDIA Physical Activity History questionnaire, which queries the amount of time per week spent in 13 categories of leisure, occupational, and household physical activities over the past 12 months.17 Self-reported depression was measured every five years starting at the Year 5 visit using the Center for Epidemiologic Studies Depression scale (CES-D).18 We used cardiovascular risk factor measurements including blood pressure, blood cholesterol (total-, LDL-,HDL-cholesterol and triglycerides), fasting glucose and body mass index (BMI), and calculated cumulative exposures to these and for physical activity and depression (area under the curve for continuous measurements, see eMethods in Supplement).19 The number of years using antidepressant medication was computed by adding the number of years reporting the use of one or more antidepressant medication (eMethods in Supplement). Self-reported schizophrenia was based on self-reported mental disease, reasons for hospitalizations and reasons for taking a psychoactive medication (eMethods in Supplement). At Year 2, the mirror star tracing test was conducted to elicit reactive blood pressure. In the mirror star-tracing test, participants had to trace the outline of a star from a reversed image displayed in a mirror while staying within narrow limits.20,21 Study participants were instructed to draw stars as quickly as possible with the fewest possible errors. If they moved out of the limits of the star, an error was scored. Total stars completed and total numbers of errors over three minutes were recorded. Although initially intended as a stressor to measure blood pressure reactivity and not as a cognitive test,20,22 some have suggested that the mirror star tracing test measures aspects of executive function.21,23
Statistical analyses
We used descriptive statistics to compare participants with different levels of exposure to marijuana at the Year 25 visit. We then described unadjusted associations between marijuana use (current and lifetime) and each CF measure, before and after standardization. Current and lifetime marijuana exposure were strongly associated with each other, and their potential effects on CF were difficult to tease apart due to co-linearity and potential interactions in their effects on CF. Given our primary goal of assessing potential effects of cumulative exposure, we eliminated the obscuring influence of current marijuana uses by excluding the minority of CARDIA participants who were currently using marijuana at the Year 25 visit in our primary analyses. We used linear regression to assess independent associations between years of exposure to marijuana and CF outcomes. We estimated a sequence of models: the first model was unadjusted; the second model controlled for the covariates used to achieve a balance of sampling in CARDIA: age, race/ethnicity, sex, study center and years of education. The third additionally controlled for covariates potentially associated with both marijuana and cognition: alcohol, cocaine, amphetamines and heroin, age participants started smoking cigarettes, cardiovascular risk factors, physical activity, BMI, depression, and diabetes at the Year 25 visit. Education, drink-years of alcohol, physical activity and BMI were flexibly modeled using restricted cubic splines with three knots at the quartiles of their distributions. To minimize potential bias due to informative censoring, we used inverse probability of censoring weights (IPCW) (eMethods in Supplement).24 We adjusted for the mirror star tracing score at Year 2 (near baseline) to minimize reverse causation as an explanation for any associations between marijuana use, and we also assessed correlations between mirror star tracing and Year 25 cognition and marijuana use to further investigate this potential issue (see eMethods and eResults in Supplement for details). Schizophrenia and psychotropic medication have been associated with both cognitive impairment and marijuana use and could therefore act as confounder of the association between marijuana and CF.25,26 We evaluated the sensitivity of the analyses to inclusion of self-reported schizophrenia (see eMethods) as a covariate in the multivariate adjusted models and by exclusion of participants with self-reported schizophrenia. We also tested the sensitivity of the results by inclusion of psychoactive medications in the main multivariate model. We also tested the association between cumulative years of exposure to marijuana with the components of the RAVLT (eResults and eFigure 1 in Supplement). Tests of statistical significance were 2-tailed, with an alpha level of 0.05. All analyses were conducted using STATA 13 (StataCorp LP, College Station, TX).
RESULTS
Of the 3,499 participants re-assessed at the Year 25 visit, 3,385 (97%) had data on CF and 3,326 (95%) had complete data on all three cognitive outcomes. Attrition was more common among men, blacks, heavy marijuana users, tobacco smokers and cocaine users (eResults in Supplement). Most participants (n=2852, 84%) reported having used marijuana before or during the 25 years of follow-up, but most had relatively few cumulative years of exposure (Table 1). Total years of marijuana exposure was strongly associated with other participant characteristics including race/sex, education, study site, other substance use, physical activity, BMI, HDL-cholesterol and triglycerides, total number of stars completed and errors on the mirror star tracing test and weakly associated with depressive symptoms and antidepressant medication use (Table 1).
Table 1.
Characteristics of 3385 CARDIA Participants with Cognitive Function Test Results at the Year 25 Visit, by Cumulative Marijuana Use Behavior.
| Variable | No marijuana use |
Ever marijuana usea |
p- valuec |
|||
|---|---|---|---|---|---|---|
| <0.5 marijuana- years |
0.5–2 marijuana- years |
2–5 marijuana- years |
>5 marijuana- years |
|||
| N, (row %) b | 533 (16) | 1505 (44) | 800 (24) | 236 (16) | 311 (9) | |
| Demographics | ||||||
| - Age, mean (SD), y | 49.7 (3.9) | 50.1 (3.6) | 50.7 (3.4) | 50.1 (3.6) | 49.8 (3.7) | <0.01 |
| - Race-sex, N (col. %)d | ||||||
| Black women | 193 (36) | 475 (32) | 147 (18) | 50 (21) | 71 (23) | |
| Black men | 88 (17) | 189 (13) | 180 (23) | 81 (34) | 95 (31) | <0.01 |
| White women | 130 (24) | 527 (35) | 223 (28) | 47 (20) | 45 (14) | |
| White men | 122 (23) | 314 (21) | 250 (31) | 58 (25) | 100 (32) | |
| - College education at any visit, N (%) |
306 (57) | 931 (62) | 405 (51) | 89 (38) | 105 (34) | <0.01 |
| - Years of education, median (IQR), y |
16 (14, 18) | 16 (14, 18) | 15 (12, 16) | 14 (13, 16) | 14 (12, 16) | <0.01 |
| - Study center, N (col. %) | ||||||
| Birmingham, AL | 240 (45) | 355 (24) | 132 (17) | 45 (19) | 43 (14) | |
| Chicago, IL | 138 (26) | 344 (23) | 201 (25) | 49 (21) | 48 (15) | <0.01 |
| Minneapolis, MI | 101 (19) | 347 (23) | 264 (33) | 72 (31) | 113 (36) | |
| Oakland, CA | 54 (10) | 459 (31) | 203 (25) | 70 (30) | 107 (34) | |
| Substance use exposure | ||||||
| Current marijuana use, N (col. %)e | ||||||
| - No current use | 533 (100) | 1483 (99) | 743 (93) | 153 (65) | 81 (26) | |
| - 1–10 days per month | 0 (0) | 12 (1) | 23 (3) | 16 (7) | 9 (3) | <0.01 |
| - 11 to 29 days per month | 0 (0) | 10 (1) | 34 (4) | 64 (27) | 159 (51) | |
| - 30 days per month (everyday) | 0 (0) | 0 (0) | 0 (0) | 3 (1) | 62 (19) | |
| Cigarette smoking, N (col. %) | ||||||
| Current use | ||||||
| - Never smoker | 462 (87) | 923 (61) | 219 (27) | 59 (25) | 60 (19) | |
| - Current smoker | 17 (3) | 162 (11) | 199 (25) | 78 (33) | 124 (40) | <0.01 |
| - Former smoker | 54 (10) | 420 (28) | 382 (48) | 99 (42) | 127 (41) | |
| - Age started smoking among ever cigarette smokers, median (IQR) |
22 (17, 32) | 18 (15, 21) | 16 (15, 19) | 17 (15, 20) | 17 (15, 21) | <0.01 |
| Cumulative use | ||||||
| - Pack-years over lifetime among ever cigarette smokers, median (IQR) f |
2 (0, 12) | 5 (1, 13) | 9 (2, 17) | 9 (3, 18) | 10 (3, 21) | <0.01 |
| Alcohol use, N (col. %)g | ||||||
| Current use | ||||||
| - Abstainer | 362 (68) | 682 (45) | 310 (39) | 84 (35) | 92 (30) | |
| - Light to moderate | 171 (32) | 802 (53) | 453 (57) | 141 (60) | 189 (61) | <0.01 |
| - Heavy | 0 (0) | 17 (1) | 36 (5) | 10 (4) | 29 (9) | |
| Cumulative use | ||||||
| - Drink-years, median (IQR)h | 8 (3, 17) | 14 (6, 26) | 21 (11, 37) | 24 (11, 48) | 35 (17, 68) | <0.01 |
| - Binge drinking days, cumulative use, N (col %)i |
||||||
| - Never reported bingeing | 432 (81) | 817 (54) | 241 (30) | 57 (24) | 51 (16) | |
| - ≤ 250 days | 64 (12) | 431 (29) | 260 (33) | 67 (28) | 85 (27) | <0.01 |
| - > 250 days | 37 (7) | 257 (17) | 299 (37) | 112 (47) | 175 (56) | |
| Illicit drug use j | ||||||
| Current use | ||||||
| - Cocaine, crack, speed or metamphetamine, N (%) |
2 (0) | 11 (1) | 26 (3) | 15 (6) | 33 (11) | <0.01 |
| - Heroin, N (%) | 0 (0) | 2 (0) | 6 (1) | 2 (1) | 4 (1) | <0.01 |
| Cumulative use | ||||||
| - Cocaine, crack, speed or metamphetamine, N (col. %) |
||||||
| - Never reported using | 526 (99) | 1000 (66) | 202 (25) | 46 (19) | 47 (15) | |
| - 1 to 25 days | 4 (1) | 338 (22) | 217 (27) | 58 (25) | 63 (20) | <0.01 |
| - > 25 to 250 days | 1 (<1) | 132 (9) | 253 (32) | 77 (32) | 109 (35) | |
| - > 250 days | 2 (<1) | 35 (2) | 128 (16) | 55 (23) | 92 (30) | |
| - Heroin, N (col. %) | ||||||
| - Never reported using | 528 (99) | 1469 (98) | 695 (87) | 202 (86) | 266 (86) | |
| - 1– 25 days | 5 (<1) | 29 (2) | 63 (8) | 19 (8) | 21 (7) | <0.01 |
| - ≥ 25 days | 0 (0) | 7 (<1) | 42 (5) | 15 (6) | 24 (7) | |
| Physical activity | ||||||
| - Physical activity score, median (IQR)k |
233 (83,409) |
267 (136,485) |
309 (151,529) |
263 (133, 475) |
322 (171, 520) |
<0.01 |
| Anthropomorphic variables | ||||||
| - BMI, mean (SD)l | 31.5 (7.3) | 30.1 (7.6) | 29.8 (6.8) | 30.1 (29.1) | 29.5 (6.0) | <0.01 |
| Cardiovascular risk factors | ||||||
| - Systolic blood pressure, mean (SD), in mmHg |
120 (17) | 118 (16) | 119 (16) | 122 (16) | 123 (16) | 0.5 |
| - Diastolic blood pressure, mean (SD), in mmHg |
75 (12) | 74 (11) | 75 (11) | 76 (12) | 77 (11) | 0.4 |
| - LDL-cholesterol, mean (SD), in mg/dl |
113 (34) | 112 (3) | 112 (33) | 109 (32) | 110 (32) | 0.7 |
| - HDL-cholesterol, mean (SD), in mg/dl |
56 (16) | 60 (18) | 57 (18) | 57 (20) | 56 (18) | <0.01 |
| - Triglycerides, mean (SD), in mg/dl |
109 (71) | 107 (79) | 123 (95) | 115 (68) | 129 (124) | <0.01 |
| - Diabetes, N (%) | 77 (15) | 204 (14) | 114 (14) | 26 (11) | 33 (11) | 0.3 |
| Psychological variables | ||||||
| - Depression, current CES-D ≥16/30, N (%) m |
72 (14) | 216 (15) | 118 (15) | 47 (20) | 60 (20) | 0.03 |
| - Years of antidepressant medication use, N (col. %) n |
||||||
| - Never reported using | 453 (85) | 1166 (77) | 616 (77) | 180 (76) | 251 (81) | |
| - 1 to 5 years | 57 (11) | 263 (17) | 138 (17) | 40 (17) | 51 (16) | <0.01 |
| - 5 or more years | 22 (4) | 76 (5) | 46 (6) | 16 (7) | 9 (3) | |
| - Self-reported schizophrenia o | 1 (0) | 6 (0.4) | 5 (0.6) | 2 (1) | 1 (0.3) | 0.4 |
|
Cognitive function at the Year 2 visit (Mirror star tracing)p |
||||||
| - Number of stars completed, median, (IQR) |
4 (3, 6) | 4 (3, 7) | 4 (2, 6) | 4 (2, 6) | 4 (2, 6) | <0.01 |
| - Number of errors, median, (IQR) |
21 (7, 48) | 23 (8, 48) | 24 (9, 53) | 23 (9, 49) | 27 (12, 54) | 0.03 |
Abbreviations: BMI: body mass index; CARDIA, Coronary Artery Risk Development in Young Adults study; CES-D: Center for Epidemiologic Studies Depression scale; Col. %: column percent; IQR, interquartile range; LDL: low density lipoprotein (LDL); HDL: high density lipoprotein; N: number of participants; SD, standard deviation
Cumulative lifetime exposure to marijuana joints in terms of marijuana-years, with 1 marijuana-year of exposure equivalent to 365 days used marijuana (1 year × 365 days/y) (see Methods and eMethods in Supplement).8,41
Row percent are presented for binary variables and column percents for variables with more than one category.
P values are from 1-way analyses of variance for age, BMI, systolic and diastolic blood pressure, LDL- and HDL-cholesterol and triglycerides; from a χ2 test for race-sex, college education, center, income, and alcohol use category; and from a Kruskal-Wallis nonparametric test for pack-years, years of education, cigarettes smoked per day, drink-years, binge-drinking days and physical activity. All P values are two sided.
By design, the CARDIA study sampled self-identified white men, white women, black men and black women in roughly equal numbers for participation in the study.42
Categories based on the answer to the question: “During the last 30 days, on how many days did you use marijuana?”
Cumulative lifetime exposure to cigarettes in terms of pack-years, with 1 pack-year of exposure equivalent to 7300 cigarettes (1 year × 365 days/y × 1 pack/d × 20 cigarettes/pack)(Methods).8
Categories of alcohol consumption were based on the sex-specific weekly maximum drinking limits published by the National Institute on Alcohol Abuse and Alcoholism [for men >14 (women >7) standard drinks/week or >4 (>3) drinks/day].43
Drink-years in those reporting ever drinking alcohol. A drink-year was defined as the total amount of ethanol consumed by a person who had one alcoholic drink per day for 1 year (1 drink-year = 17.24 ml of ethanol/drink × 1 drink/day × 365 days/year = 6,292.6 ml of ethanol).
Binge-drinking days defined as >4 drinks per episode (eMethods in Supplement). If bingeing were to be constant over 25 years in one individual, 250 binge drinking days would correspond to 10 episodes of bingeing per year over 25 years.
Current use defined as any use within the last 30 days. The number of days on the illicit drug listed over the study duration was computed using current exposure at each visit and replaced by lifetime exposure when the latter was higher. Cocaine included other forms of cocaine such as crack, powder, free base; amphetamines included speed, uppers and metamphetamines (Methods and eMethods in Supplement).
Physical activity measured with the CARDIA Physical Activity History questionnaire, which queries the amount of time per week spent in 13 categories of leisure, occupational, and household physical activities over the past 12 months.17
Calculated as weight in kilograms divided by height in meters squared.
Self-reported depression was measured every five years starting at the Year 5 visit by using the Center for Epidemiologic Studies Depression scale (CES-D).18 A score of ≥16 used as the cut-off value for both sexes as an indication of the clinically significant depressive symptoms.44
Antidepressant and mood stabilizing medications recorded at each clinical visits (eMethods in Supplement).
Self-reported schizophrenia based on self-reported mental disease, reasons for hospitalizations and reasons for taking an psychoactive medication (eMethods in Supplement)
Mirror star tracing performed at the Year 2 visit to test blood pressure reactivity which tests cognitive domains similar to the Stroop test (see Methods). 280 out of 3,385 participants (8%) with missing data on mirror star tracing.
In unadjusted analyses, current marijuana use at the Year 25 visit was associated with worse verbal memory (RAVLT) and processing speed (DSST) (eTable 1), while lifetime exposure was associated with worse performance on all three CF measures (Table 2). In preliminary analyses, we found evidence of a negative interaction between years of marijuana use and current use at the Year 25 visit in both unadjusted (p<0.001) and multivariate adjusted models (p=0.03) for the RAVLT, such that past marijuana use appeared to be less important as a predictor of verbal memory among participants who were currently using marijuana (eResults in Supplement). With or without exclusion of current users, lifetime exposure to marijuana was associated with reductions in all three CF measures (Table 2).
Table 2.
Association between cognitive function and exposure to marijuana among 3385 CARDIA participants at Year 25
| Marijuana exposure | N | Rey Auditory Verbal Learning– Test (RAVLT) |
N | Digit Symbol Substitution Test (DSST) |
N | Stroop Interference Test e |
|||
|---|---|---|---|---|---|---|---|---|---|
| Raw mean (SD) |
Standar dized mean |
Raw mean (SD) |
Standar dized mean |
Raw mean (SD) |
Standar dized mean |
||||
| All Participants | 3365 | 8.3 (3.7) | 0.00 | 3370 | 69.9 (16) | 0.00 | 3352 | −23 (11) | 0.00 |
| Overall exposure | |||||||||
| - Never exposed | 531 | 8.6 (3.2) | 0.09 | 531 | 70 (16) | −0.02 | 528 | − 23 (12) | −0.07 |
| - Past exposure | 2443 | 8.4 (3.2) | 0.03 | 2448 | 71 (16) | 0.04 | 2436 | − 22 (11) | 0.03 |
| - Current exposure | 391 | 7.2 (3.4) | −0.32 | 391 | 67 (15) | −0.21 | 388 | − 24 (12) | −0.08 |
| p-valuec | <0.001 | <0.001 | 0.09 | ||||||
|
Current use (last 30 days)a |
|||||||||
| - No current use | 2974 | 8.5 (3.2) | 0.04 | 2979 | 70 (16) | 0.03 | 2964 | − 23 (11) | 0.01 |
| - 1–10 days per month | 226 | 7.5 (3.5) | −0.24 | 226 | 68 (15) | −0.15 | 224 | − 23 (11) | −0.05 |
| - 11 to 29 days per month | 100 | 7.3 (3.3) | −0.31 | 101 | 68 (16) | −0.13 | 100 | − 22 (10) | 0.02 |
| - 30 days per month (daily) |
65 | 6.2 (3.4) | −0.66 | 64 | 61 (13) | −0.55 | 64 | − 27 (15) | −0.37 |
| p-valuec | <0.001 | <0.001 | 0.1 | ||||||
|
Lifetime exposure, cumulativeb |
|||||||||
| - Never used marijuana | 531 | 8.6 (3.2) | 0.09 | 531 | 70 (16) | −0.02 | 528 | − 23 (12) | −0.07 |
| - 1 day to <0.5 marijuana- years |
1496 | 8.8 (3.2) | 0.14 | 1498 | 72 (16) | 0.16 | 1493 | − 22 (11) | 0.06 |
| - 0.5 to <2 marijuana- years |
792 | 8.1 (3.2) | −0.08 | 799 | 68 (16) | −0.10 | 791 | − 23 (11) | 0.00 |
| - 2 to <5 marijuana-years | 236 | 7.5 (3.3) | −0.24 | 233 | 65 (17) | −0.28 | 234 | − 23 (10) | −0.05 |
| - >5 marijuana-years | 310 | 6.9 (3.4) | −0.43 | 309 | 65 (15) | −0.28 | 306 | − 24 (12) | −0.12 |
| p-valuec | <0.001 | <0.001 | 0.02 | ||||||
|
Lifetime exposure, cumulativeb, excluding current usersf |
|||||||||
| - Never used marijuana | 531 | 8.6 (3.2) | 0.09 | 531 | 70 (16) | −0.02 | 528 | − 23 (12) | −0.07 |
| - 1 day to <0.5 marijuana- years |
1474 | 8.8 (3.1) | 0.15 | 1476 | 73 (16) | 0.16 | 1472 | − 22 (11) | 0.06 |
| - 0.5 to <2 marijuana- years |
735 | 8.0 (3.2) | −0.08 | 742 | 68 (16) | −0.10 | 734 | − 23 (10) | 0.02 |
| - 2 to <5 marijuana-years | 153 | 7.5 (3.3) | −0.24 | 150 | 64 (17) | −0.35 | 151 | − 24 (11) | −0.10 |
| - >5 marijuana-years | 81 | 6.9 (3.2) | −0.43 | 80 | 66 (15) | −0.27 | 79 | − 25 (11) | −0.19 |
| p-valuec | <0.001 | <0.001 | 0.02 | ||||||
Abbreviations: SD: Standard deviation, N: number included
Current exposure to marijuana assessed through the question: “During the last 30 days, on how many days did you use marijuana?” (see Methods).
Cumulative exposure to marijuana expressed in ‘marijuana-years’, with 1 marijuana-year of exposure equivalent to 365 days of marijuana use (see Methods).
P values are from 1-way analyses of variance. All P values two sided.
The inverse of the Stroop score used in the present analyses to allow interpretation of worse CF with negative standardized scores for all three CF tests.
Current marijuana users at the Year 25 visit excluded (N=391)
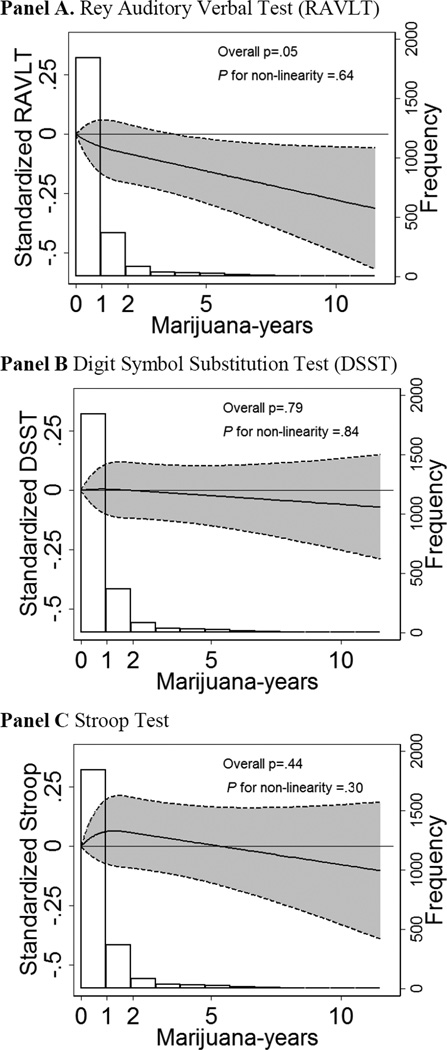
In fully adjusted analyses excluding current users, lifetime exposure to marijuana remained strongly associated with worse verbal memory (RAVLT), even after extensive adjustment for other factors associated with marijuana use and mirror star tracing scores at the Year 2 visit (Table 3). The association was dose-dependent, with no evidence of non-linearity (Figure); each additional 5 years of exposure to marijuana was associated with 0.13 lower standard deviations in the verbal memory test (RAVLT, 95% confidence interval (CI): 0.02–0.24, p=0.02; Table 3). In contrast, adjusted models demonstrated no association of cumulative marijuana exposure with processing speed and executive function (DSST and Stroop, Table 3). In multivariate adjusted analyses, total number of stars completed and errors were not associated with higher marijuana use at the Year 2 visit and over 25 years of follow-up (eMethods and eResults in Supplement). Total number of stars completed and errors were associated with CF scores at the Year 25 visit in both unadjusted and adjusted analyses (eMethods and eResults in Supplement). In exploratory analyses, the attenuation of the association between marijuana exposure and all three measures of CF was mostly seen after adjustment for race-sex strata and education. Sensitivity analyses demonstrated no evidence of significant interactions by race or sex (p>0.10 for all tests).
Table 3.
Association between cognitive function (CF) and cumulative lifetime exposure to marijuana in ‘marijuana-years’ among those without recent use.a
| Standardized difference in each CF measure (95% CI)c | ||||
|---|---|---|---|---|
| Cognitive Function Measure |
Unadjusted model |
Adjusted for age, race, sex, education, study center, and with IPCW f |
Additionally adjusted for substance use, depression and cardiovascular risk factors g |
Additionally adjusted for mirror star tracing at the Year 2 visit h |
| - Cumulative lifetime exposure in marijuana-years b | ||||
|
Rey Auditory Verbal Learning Test (RAVLT) |
||||
| - Never used marijuana | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| - 1 day to <0.5 marijuana-years | 0.06 (−0.04 to 0.16) | −0.01 (−0.11 to 0.08) | −0.02 (−0.12 to 0.08) | −0.03 (−0.13 to 0.08) |
| - 0.5 to <2 marijuana-years | −0.17 (−0.28 to −0.06) | −0.07 (−0.18 to 0.04) | −0.07 (−0.21 to 0.06) | −0.08 (−0.22 to 0.06) |
| - 2 to <5 marijuana-years | −0.33 (−0.51 to −0.15) | −0.11 (−0.28 to 0.06) | −0.09 (−0.28 to 0.09) | −0.08 (−0.27 to 0.11) |
| >5 marijuana-years | −0.52 (−0.75 to −0.29) | −0.27 (−0.49 to −0.05) | −0.31 (−0.54 to −0.07) | −0.25 (−0.50 to −0.01) |
| p-value for trend | <0.001 | 0.007 | 0.01 | 0.04 |
| For every 5 marijuana-years | −0.34 (−0.45 to −0.24) | −0.15 (−0.24 to −0.05) | −0.15 (−0.25 to −0.04) | −0.13 (−0.24 to −0.02) |
| p-value | <0.001 | 0.002 | 0.005 | 0.02 |
|
Digit symbol substitution test (DSST) |
||||
| - Never used marijuana | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| - 1 day to <0.5 marijuana-years | 0.17 (0.07 to 0.27) | 0.03 (−0.06 to 0.12) | 0.06 (−0.04 to 0.16) | 0.06 (−0.04 to 0.16) |
| - 0.5 to <2 marijuana-years | −0.08 (−0.19 to 0.03) | −0.03 (−0.13 to 0.07) | 0.07 (−0.06 to 0.19) | 0.05 (−0.08 to 0.18) |
| - 2 to <5 marijuana-years | −0.33 (−0.51 to −0.16) | −0.12 (−0.28 to 0.04) | −0.03 (−0.21 to 0.15) | −0.02 (−0.20 to 0.17) |
| >5 marijuana-years | −0.25 (−0.48 to −0.02) | −0.04 (−0.24 to 0.15) | 0.12 (−0.08 to 0.33) | 0.13 (−0.09 to 0.34) |
| p-value for trend | <0.001 | 0.26 | 0.5 | 0.5 |
| For every 5 marijuana-years | −0.31 (−0.41 to −0.20) | −0.08 (−0.17 to 0.01) | −0.01 (−0.10 to 0.08) | −0.03 (−0.12 to 0.07) |
| p-value | <0.001 | 0.08 | 0.8 | 0.6 |
| Stroop interference test d | ||||
| - Never used marijuana | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| - 1 day to <0.5 marijuana-years | 0.12 (0.02 to 0.22) | 0.06 (−0.05 to 0.17) | 0.05 (−0.06 to 0.17) | 0.05 (−0.07 to 0.17) |
| - 0.5 to <2 marijuana-years | 0.09 (−0.02 to 0.20) | 0.10 (−0.02 to 0.23) | 0.13 (−0.04 to 0.29) | 0.11 (−0.06 to 0.27) |
| - 2 to <5 marijuana-years | −0.03 (−0.21 to 0.15) | 0.10 (−0.09 to 0.29) | 0.08 (−0.13 to 0.29) | 0.10 (−0.11 to 0.31) |
| >5 marijuana-years | −0.12 (−0.36 to 0.11) | −0.08 (−0.32 to 0.17) | −0.02 (−0.30 to 0.24) | −0.09 (−0.37 to 0.20) |
| p-value for trend | 0.12 | 0.7 | 0.9 | 0.7 |
| For every 5 marijuana-years | −0.09 (−0.20 to 0.01) | −0.02 (−0.12 to 0.09) | −0.01 (−0.13 to 0.10) | −0.04 (−0.16 to 0.08) |
| p-value | 0.08 | 0.8 | 0.8 | 0.5 |
Abbreviations: CF: Cognitive function; IPCW: Inverse probability of censoring weighting; Ref.: Reference
Cumulative exposure to marijuana expressed in ‘marijuana-years’, with 1 marijuana-year of exposure equivalent to 365 days of marijuana use (see Methods). Current marijuana users within the 30 days prior of the Year 25 visit excluded (N=392).
Years of marijuana exposure was modeled first as a 5-level categorical predictor, and then as a continuous linear predictor, per 5 marijuana-years (separate models).
Linear regression models used to determine the association between CF scores and cumulative exposure to marijuana use. Negative standardized scores indicate worse CF.
The inverse of the Stroop score used in the present analyses to allow interpretation of worse CF with negative standardized scores for all three CF tests.
Adjusted for age, race/ethnicity, sex, study site and years of education. Analyses weighted by the inverse probability of censoring (IPCW) to address potential bias by informative censoring (eMethods).
Model described in f additionally adjusted for cumulative and current exposure to licit and illicit substances and other covariates (see Methods).
Model described in g additionally adjusted for total number of stars completed and errors made drawing the stars. Participants with missing data on mirror star tracing excluded (N=280).
Figure. Associations between lifetime exposure to marijuana and cognitive function (CF).
Years of marijuana modeled flexibly and current marijuana users at the Year 25 visit excluded (N=392). Results are adjusted for age, race/ethnicity, sex, study site, education, cigarette smoking, alcohol, illicit drug use, cardiovascular risk factors, depression, mirror star tracing at the Year 2 visit and differential likelihood of follow up (see Methods). All test results standardized, such that a 1 unit negative deviation indicates a standard deviation worse CF than the mean. Histograms describe the distribution of marijuana-years in CARDIA participants with any exposure to marijuana by presenting the frequency of participants in each considered interval. The inverse of the Stroop score used in the present analyses to allow interpretation of worse CF with negative standardized scores for all three CF tests. RAVLT - Rey Auditory Verbal Learning Test; DSST – Digit Symbol Substitution Test.
Our method of identifying participants with a potential diagnosis of schizophrenia through self-reported mental disease, reasons for hospitalizations and reasons for taking psychoactive medication identified 28 participants in the entire CARDIA cohort (0.6%; 28/5114). Of those, 14 attended the Year 25 visit (0.4%; 14/3371) compared to 14 not attending (0.8%; 14/1716; P=0.07 for not attending the Year 25 visit). Results were virtually unchanged when including this covariate in the main multivariate adjusted model and the IPCWs or excluding these participants from the main analyses. Similarly, inclusion of the predictor of anti-depressant medication led to similar results.
COMMENT
In this large, community-based cohort of white and black young adults followed over 25 years, we found a dose-dependent independent association between cumulative lifetime exposure to marijuana and worsening verbal memory in middle age. For each additional 5 marijuana-years of exposure (1825 days of use), verbal memory was 0.13 standard deviations lower than for never users after full adjustment, corresponding to less than 1 of 2 participants remembering one word less from a list of 15 words for every 5 years of use, on average. We found no significant associations of cumulative exposure with executive function or processing speed.
Our findings are consistent with previous studies demonstrating associations between heavy marijuana exposure and CF, but the association with lower levels of marijuana exposure has not previously been demonstrated.3–5,27,28 In one study, for example, the association with verbal memory was only apparent among heavy long-term marijuana users (N=51),4 defined as using marijuana every day or nearly every day for over 20 years (23.9 years of use) compared to more recent use (10.2 years of use, N=51) or non-users (N=33). In another, investigators used 38-years of follow-up data from 1037 participants in a birth cohort in New Zealand and found that persistent regular cannabis use (4 days/week or more) was associated with neuropsychological decline, while those who reported non-regular use (50.6% of the total) showed no decline in IQ or neuropsychological performance.5 Similarly, a longitudinal study with 10 years of follow-up found evidence of a cognitive decline with heavy marijuana use,28 but those who stopped using during follow-up did not show a decline in IQ score. In contrast, with more detailed measurement of lifetime marijuana exposure in a larger sample, we were able to detect a negative association at lower levels of cumulative use and among persons with remote past exposure to marijuana.
The extent of association between worse verbal memory and cumulative marijuana use is of uncertain clinical significance. In the context of cognitive decline after stroke, Levine et al used a 0.5 SD cut-off for defining a clinically meaningful decline in global cognition.29 The point estimate for verbal memory in our study for those with 5 marijuana-years of exposure (0.13 SD; 95% CI: −0.24 to −0.02) is of lesser magnitude than the decline found in the study by Levine et al. and the confidence intervals excludes the 0.5 SD cut-off. However, participants with up to 10 marijuana-years of exposure might have a significant decline in verbal memory given the lower bound of the 95% CI. Similarly, participants with current daily marijuana in the month before the Year 25 visit might have a clinically significant decrease in verbal memory and other measures of CF (eResults in Supplement).
The mechanism by which marijuana exposure might impact verbal memory is unclear, but might be explained by the potential effect of tetrahydrocannabinol (THC) on how information is processed in the hippocampus.30 Marijuana use has been associated with functional changes in the activation brain regions involved in associative learning,31 particularly in the para-hippocampal regions and the dorsolateral prefrontal cortex.31–33 Some have found suggestions of lower hippocampal and amygdala volumes in heavy, long-term users (>5 joints daily for more than 10 years),34 as well as alterations in the cerebellum, the frontal cortex,31 and medial temporal cortex33, though other researchers were unable to confirm these findings.30,35 Numerous methodological issues such as variation in imaging techniques and in measurement of exposures, dose-threshold effects and small sample sizes limit the possibility for drawing strong conclusions on the published findings.31,33
Our study has important limitations. We constructed a marijuana exposure measurement from self-reported information collected prospectively and periodically over 25 years, but self-report is not always reliable,36 measurements were infrequent, and age of exposure, especially during adolescence and young adulthood was not queried. However, even if imprecise, the repeated question over the 25 years was prospectively obtained and allowed us to demonstrate a potential deleterious association, one that is not easily studied without a large, well-characterized cohort with long-term follow-up such as CARDIA. Another limitation is the availability of CF measurements at only 1 time point, which limits our ability to pinpoint when a change in the outcome might have occurred and relate it in time to a change in exposure. We found no significant change in the measure of association between cumulative marijuana exposure and measures of CF after inclusion of mirror star tracing score measured early in life (Year 2 visit). Even with this adjustment, we cannot rule out reverse causation as an explanation for our results.5 While some have suggested that the mirror star tracing test measures aspects of executive function,21,23 no study has compared the CF domains measured in the mirror star tracing test and those measured in the other tests used at the Year 25 visit. Factors strongly associated with marijuana use could confound the association between marijuana and CF. The New Zealand study, for example, has been criticized for lack of adequate control over socio-economic status (SES), even though additional analyses have shown that controlling for SES did not attenuate the association between sustained daily marijuana use and worse intellectual quotient (IQ).38 In our study, the observed associations were substantially attenuated by control for core demographic variables, including education, race and gender. However, adjustment for a host of additional behavioral, psychosocial, and cardiovascular risk factors available, including self-reported schizophrenia and psychoactive medication, did not further attenuate the estimates.
We found past exposure to marijuana use to be significantly associated with worse verbal memory in middle age. Future studies with multiple assessments of cognition, brain imaging, and other functional outcomes should further explore these associations and their potential clinical and public health implications. In the meantime, with recent changes in legislation and the potential for increasing marijuana use in the US,39 continuing to warn potential users about the possible harm from exposure to marijuana seems reasonable.40
Supplementary Material
Acknowledgments
Funding/Support: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
Role of the Sponsors: The funding organizations were independent of the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation of the manuscript. Before submission for publication, the manuscript was reviewed and approved by the CARDIA P&P committee.
Footnotes
Author Contributions:
Dr Auer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Auer, Pletcher, Vittinghoff.
Acquisition of data: Jacobs, Kertesz, Pletcher, Sidney, Yaffe.
Analysis and interpretation of data: Albanese, Auer, Glymour, Jacobs, Künzi, Pletcher, Yaffe, Vittinghoff.
Drafting of the manuscript: Auer.
Critical revision of the manuscript for important intellectual content: Albanese, Glymour, Jacobs, Kertesz, Levine, Whitmer, Pletcher, Sidney, Yaffe, Vittinghoff.
Statistical analysis: Auer, Künzi, Pletcher,Vittinghoff.
Obtained funding: Auer, Pletcher, Sidney, Yaffe.
Administrative, technical, or material support: Sidney, Yaffe, Pletcher.
Study supervision: Pletcher.
Conflict of interest: None
REFERENCES
- 1.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2012. [Accessed March 21st, 2013]. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2012.pdf. [Google Scholar]
- 2.Solowij N. Cannabis and Cognitive Functioning. Cambridge University Press; 1998. [Google Scholar]
- 3.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003 Jul;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 4.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002 Mar 6;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 5.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012 Oct 2;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez R, Martin EM, Grant I. Marijuana. In: Kalechstein A, van Gorp WG, editors. Neuropsychology and Substance Use: State-of-the-Art and Future Directions. Taylor & Francis: 2007. pp. 139–170. [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 8.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012 Jan 11;307(2):173–181. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013 Feb;73(2):170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 11.Wechsler D. Administration and scoring manual for the Wechsler Adult Intelligence Scale-III. London: Psychological Corporation; 2008. [Google Scholar]
- 12.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991 Mar;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 13.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 14.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2005 Mar 1;161(5):423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- 15.Kertesz SG, Khodneva Y, Richman J, et al. Trajectories of Drug Use and Mortality Outcomes Among Adults Followed Over 18 Years. J Gen Intern Med. 2012 Jul;27(7):808–816. doi: 10.1007/s11606-011-1975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kertesz SG, Pletcher MJ, Safford M, et al. Illicit drug use in young adults and subsequent decline in general health: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Drug Alcohol Depend. 2007 May 11;88(2–3):224–233. doi: 10.1016/j.drugalcdep.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and Reliability of Short Physical Activity History: Cardia and the Minnesota Heart Health Program. Journal of Cardiopulmonary Rehabilitation and Prevention. 1989;9(11):448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977 Jun 1;1(3):385–401. 1977. [Google Scholar]
- 19.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014 Apr 15;129(15):1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasprowicz AL, Manuck SB, Malkoff SB, Krantz DS. Individual differences in behaviorally evoked cardiovascular response: temporal stability and hemodynamic patterning. Psychophysiology. 1990 Nov;27(6):605–619. doi: 10.1111/j.1469-8986.1990.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhu N, Jacobs DR, Meyer KA, et al. Cognitive function in a middle aged cohort is related to higher quality dietary pattern 5 and 25 years earlier: the CARDIA study. The journal of nutrition, health & aging. 2015 Jan;19(1):33–38. doi: 10.1007/s12603-014-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldstein SR, Bachen EA, Manuck SB. Active coping and cardiovascular reactivity: a multiplicity of influences. Psychosom Med. 1997 Nov-Dec;59(6):620–625. doi: 10.1097/00006842-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Gardner RM. The reverse affect test: a new interference task. Perceptual and motor skills. 1985 Apr;60(2):384–386. doi: 10.2466/pms.1985.60.2.384. [DOI] [PubMed] [Google Scholar]
- 24.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012 Jun;42(6):1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 26.McLoughlin BC, Pushpa-Rajah JA, Gillies D, et al. Cannabis and schizophrenia. Cochrane Database Syst Rev. 2014;10:CD004837. doi: 10.1002/14651858.CD004837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pope Hg JY-TD. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275(7):521–527. [PubMed] [Google Scholar]
- 28.Fried P, Watkinson B, James D, Gray R. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. Cmaj. 2002 Apr 2;166(7):887–891. [PMC free article] [PubMed] [Google Scholar]
- 29.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev. 2008 Jun;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 31.Batalla A, Bhattacharyya S, Yucel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager G, Van Hell HH, De Win MM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007 Mar;17(4):289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des. 2014;20(13):2138–2167. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- 34.Yucel M, Solowij N, Respondek C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008 Jun;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 35.Tzilos GK, Cintron CB, Wood JB, et al. Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict. 2005 Jan-Feb;14(1):64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- 36.van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Validation of self-reported cannabis dose and potency: an ecological study. Addiction. 2013 Oct;108(10):1801–1808. doi: 10.1111/add.12226. [DOI] [PubMed] [Google Scholar]
- 37.Rogeberg O. Correlations between cannabis use and IQ change in the Dunedin cohort are consistent with confounding from socioeconomic status. Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4251–4254. doi: 10.1073/pnas.1215678110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffitt TE, Meier MH, Caspi A, Poulton R. Reply to Rogeberg and Daly: No evidence that socioeconomic status or personality differences confound the association between cannabis use and IQ decline. Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):E980–E982. doi: 10.1073/pnas.1300618110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacula RL, Sevigny EL. Natural experiments in a complex and dynamic environment: the need for a measured assessment of the evidence. Journal of policy analysis and management : [the journal of the Association for Public Policy Analysis and Management] 2014 Winter;33(1):232–235. doi: 10.1002/pam.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014 Jun 5;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancox RJ, Poulton R, Ely M, et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010 Jan;35(1):42–47. doi: 10.1183/09031936.00065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bild DE, Jacobs DR, Jr, Sidney S, Haskell WL, Anderssen N, Oberman A. Physical activity in young black and white women. The CARDIA Study. Ann Epidemiol. 1993 Nov;3(6):636–644. doi: 10.1016/1047-2797(93)90087-k. [DOI] [PubMed] [Google Scholar]
- 43.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician's Guide. [Accessed Sept 25th, 2013];2005 http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm.
- 44.Radloff LS, Locke BZ. The community mental health assessment survey and the CES-D Scale. In: Weissman MM, Myers JK, Ross CE, editors. Community surveys of psychiatric disorders. New Brunswick, NJ: Rutgers University Press; 1986. pp. 177–189. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.