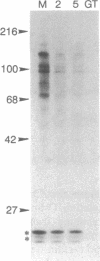
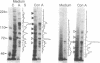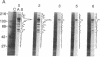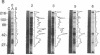Abstract
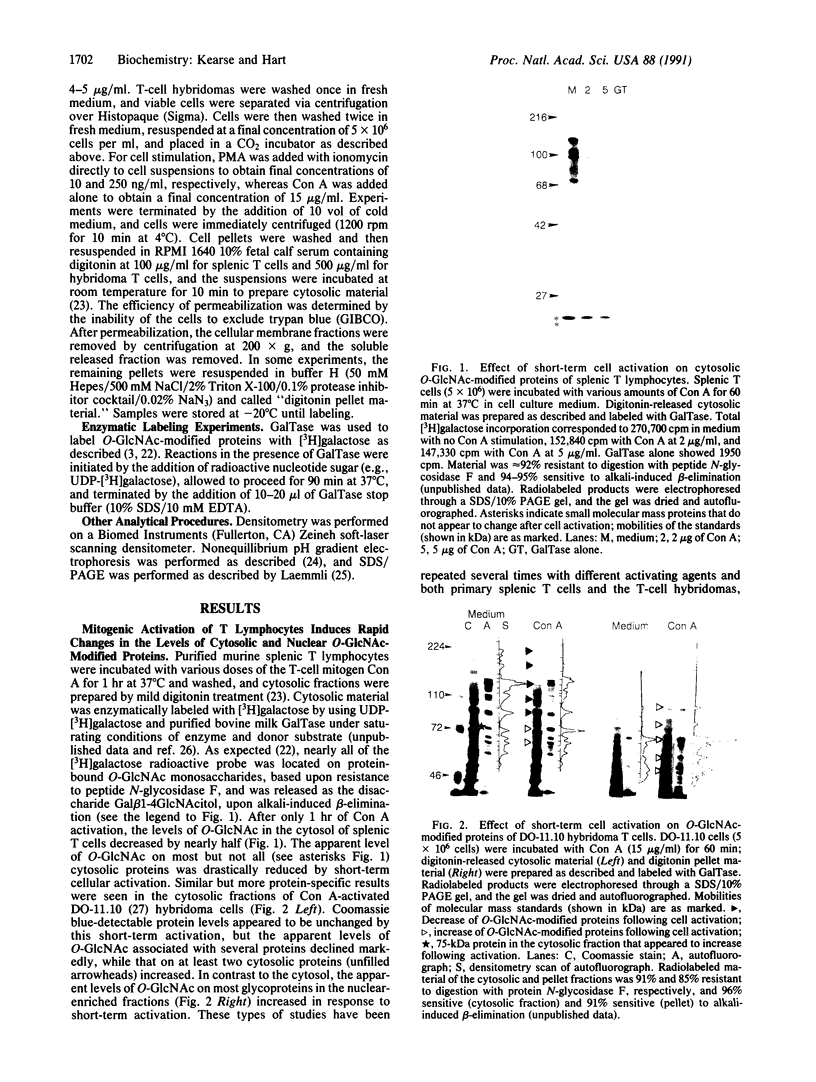
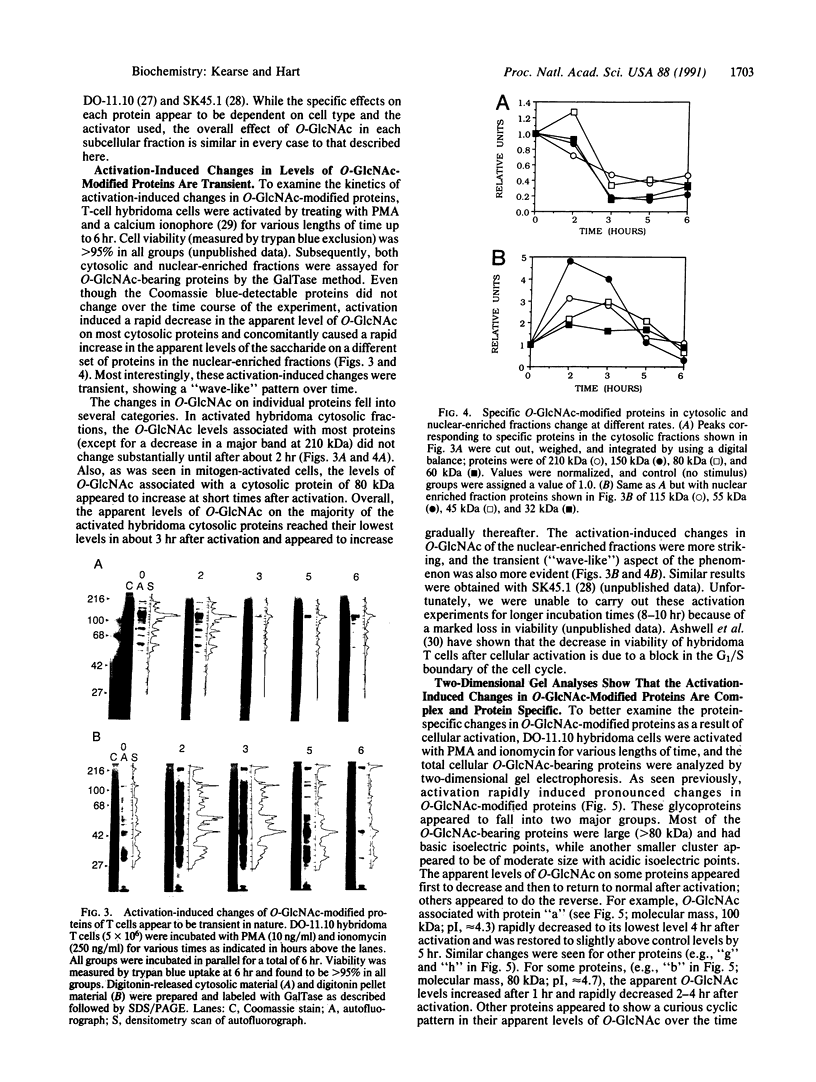
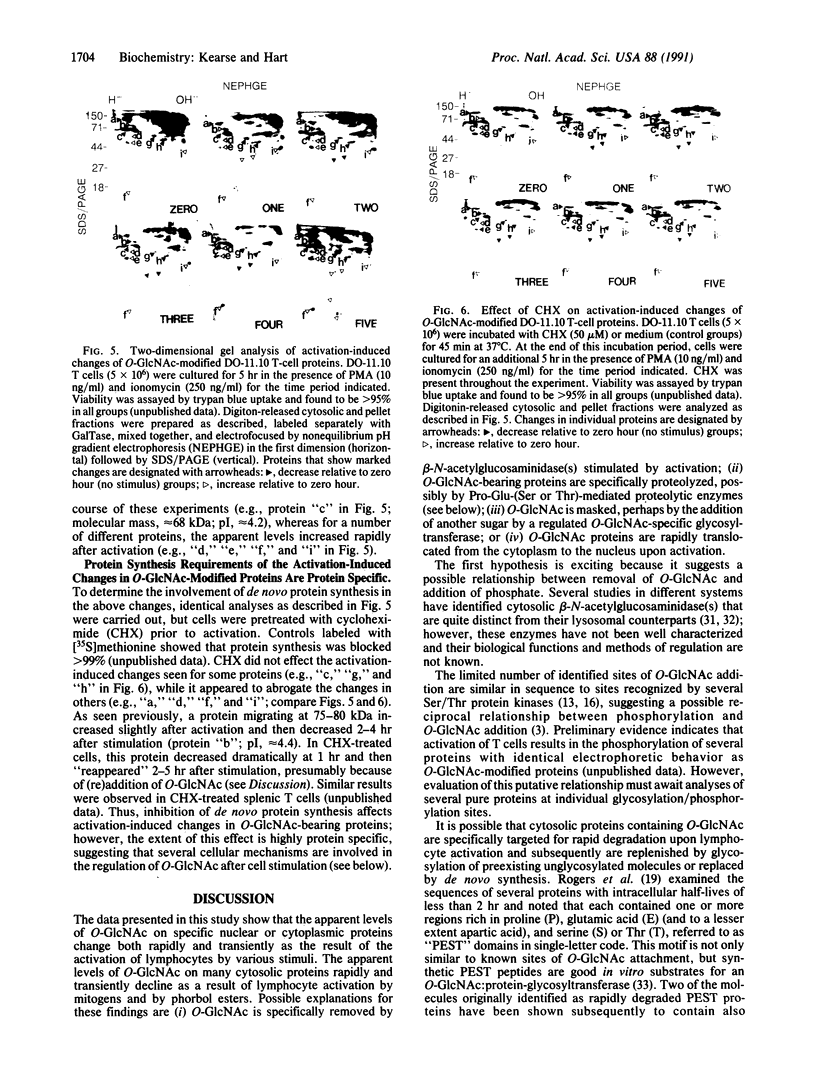
A unique form of nucleoplasmic and cytoplasmic protein glycosylation, O-linked GlcNAc, (O-GlcNAc) is present on proteins ranging from those of yeast to man, including many chromatin proteins, transcription factors, nuclear pore proteins, and certain types of cytoskeletal proteins. In this report we have studied the effects of cellular activation on O-GlcNAc-modified proteins, using T lymphocytes as a model system. Results indicate that the apparent levels of O-GlcNAc on many nuclear proteins increases rapidly after lymphocyte activation, returning to control levels after a few hours. In contrast, the apparent levels of O-GlcNAc on a distinct population of cytosolic proteins decreases rapidly after cellular activation and also returns to control levels after a few hours. These data are consistent with the hypothesis that O-GlcNAc is a regulatory modification and suggest that O-GlcNAc modification may play an important role in the early stages of T-lymphocyte activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Cunningham R. E., Noguchi P. D., Hernandez D. Cell growth cycle block of T cell hybridomas upon activation with antigen. J Exp Med. 1987 Jan 1;165(1):173–194. doi: 10.1084/jem.165.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall F. L., Mitchell J. P., Vulliet P. R. Phosphorylation of synapsin I at a novel site by proline-directed protein kinase. J Biol Chem. 1990 Apr 25;265(12):6944–6948. [PubMed] [Google Scholar]
- Haltiwanger R. S., Holt G. D., Hart G. W. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990 Feb 15;265(5):2563–2568. [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–874. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. Nucleoplasmic and cytoplasmic glycoproteins. Ciba Found Symp. 1989;145:102-12, discussion 112-8. doi: 10.1002/9780470513828.ch7. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Holt G. D., Haltiwanger R. S. Nuclear and cytoplasmic glycosylation: novel saccharide linkages in unexpected places. Trends Biochem Sci. 1988 Oct;13(10):380–384. doi: 10.1016/0968-0004(88)90179-x. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Haltiwanger R. S., Torres C. R., Hart G. W. Erythrocytes contain cytoplasmic glycoproteins. O-linked GlcNAc on Band 4.1. J Biol Chem. 1987 Nov 5;262(31):14847–14850. [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Suzuki K. Neutral beta-N-acetylhexosaminidases of rat brain. Purification and enzymatic and immunological characterization. J Biol Chem. 1983 Jun 10;258(11):6991–6999. [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1781–1785. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Hart G. W. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989 Apr 21;57(2):243–251. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Lysosomal enzyme targeting. Biochem Soc Trans. 1990 Jun;18(3):367–374. doi: 10.1042/bst0180367. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Overdijk B., Van der Kroef W. M., Van Steijn G. J., Lisman J. J. Isolation and further characterization of bovine brain hexosaminidase C. Biochim Biophys Acta. 1981 Jun 15;659(2):255–266. doi: 10.1016/0005-2744(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Shaper J. H. Bovine beta 1----4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990 Feb 25;265(6):3324–3331. [PubMed] [Google Scholar]
- Schalch D. S., Heinrich U. E., Draznin B., Johnson C. J., Miller L. L. Role of the liver in regulating somatomedin activity: hormonal effects on the synthesis and release of insulin-like growth factor and its carrier protein by the isolated perfused rat liver. Endocrinology. 1979 Apr;104(4):1143–1151. doi: 10.1210/endo-104-4-1143. [DOI] [PubMed] [Google Scholar]
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984 Mar 10;259(5):3308–3317. [PubMed] [Google Scholar]
- Vulliet P. R., Hall F. L., Mitchell J. P., Hardie D. G. Identification of a novel proline-directed serine/threonine protein kinase in rat pheochromocytoma. J Biol Chem. 1989 Sep 25;264(27):16292–16298. [PubMed] [Google Scholar]
- White J., Haskins K. M., Marrack P., Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J Immunol. 1983 Mar;130(3):1033–1037. [PubMed] [Google Scholar]
- Whiteheart S. W., Passaniti A., Reichner J. S., Holt G. D., Haltiwanger R. S., Hart G. W. Glycosyltransferase probes. Methods Enzymol. 1989;179:82–95. doi: 10.1016/0076-6879(89)79116-3. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Shenbagamurthi P., Chen L., Cotter R. J., Hart G. W. Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha. J Biol Chem. 1989 Aug 25;264(24):14334–14341. [PubMed] [Google Scholar]