Abstract
Granulocyte colony stimulating factor (G-CSF) may enhance recovery from stroke through neuroprotective mechanisms if administered early, or neurorepair if given later. Several small trials suggest administration is safe but effects on efficacy are unclear. We searched for randomised controlled trials (RCT) assessing G-CSF in patients with hyperacute, acute, subacute or chronic stroke, and asked Investigators to share individual patient data on baseline characteristics, stroke severity and type, end-of-trial modified Rankin Scale (mRS), Barthel Index, haematological parameters, serious adverse events and death. Multiple variable analyses were adjusted for age, sex, baseline severity and time-to-treatment. Individual patient data were obtained for 6 of 10 RCTs comprising 196 stroke patients (116 G-CSF, 80 placebo), mean age 67.1 (SD 12.9), 92% ischaemic, median NIHSS 10 (IQR 5–15), randomised 11 days (interquartile range IQR 4–238) post ictus; data from three commercial trials were not shared. G-CSF did not improve mRS (ordinal regression), odds ratio OR 1.12 (95% confidence interval 0.64 to 1.96, p = 0.62). There were more patients with a serious adverse event in the G-CSF group (29.6% versus 7.5%, p = 0.07) with no significant difference in all-cause mortality (G-CSF 11.2%, placebo 7.6%, p = 0.4). Overall, G-CSF did not improve stroke outcome in this individual patient data meta-analysis.
The impact of stroke on individuals, carers and society is huge and is the third leading cause of death worldwide1. Recent progress in acute treatments is encouraging (e.g. mechanical thrombectomy) but they can often only apply to a small proportion of the stroke population. Beyond the acute phase there are very few effective treatments and novel approaches are required.
An ischaemic stroke leads to mobilisation of CD34+ haematopoietic stem cells (HSC), which occurs in bursts over the first 10 days post stroke2,3; those with higher levels of CD34+ cell mobilisation have a better neurological outcome2. Intentional recruitment of CD34+ HSCs from bone marrow to peripheral blood with granulocyte-colony stimulating factor (G-CSF) is a clinical process termed peripheral blood stem cell (PBSC) mobilisation. Although the mechanism is poorly understood, G-CSF alone or with chemotherapy is used routinely in clinical practice to reduce the duration of neutropenia in patients with haematological disease, or for mobilising and harvesting HSCs for subsequent autologous or allogenic infusion. Its use in stroke is under investigation in both animals and humans.
In experimental ischaemic stroke, a number of groups have demonstrated G-CSF to be neuroprotective at various doses4, in the presence of thrombolysis5, induce functional recovery6 and promote angiogenesis and neurogenesis7,8. G-CSF given early causes a reduction in stroke lesion volume9. Consequentially, the use of G-CSF in stroke has progressed into phase II/III clinical trials analysing the effects of G-CSF in hyperacute, acute, subacute and chronic stroke. We have therefore performed an individual patient data meta-analysis on the effects of G-CSF on stroke with the following aims:
(1) To assess the safety of G-CSF administered after ischaemic and haemorrhagic stroke in an individual patient data meta-analysis.
(2) To assess the efficacy of G-CSF treatment after ischaemic and haemorrhagic stroke.
(3) To assess the effect of time of administration on safety and efficacy.
Results
The initial search highlighted 310 publications; once duplicates, non-stroke studies, experimental studies and review articles were excluded, a total of 10 randomised controlled trials were identified (Table 1)10,11,12,13,14,15,16,17,18,19. We received individual patient data from 5 trials10,14,15,17,19,20 and there was sufficient detail in the primary publication of another trial to be included in the analysis11. Risk of bias in the included studies has been previously reported in our Cochrane review21, which did not include results from one trial included in this analysis19.
Table 1. Trial design of identified randomised controlled trials of G-CSF and stroke.
| Study | Design | Participants | Interventions | Time of administration | Comments on the study |
|---|---|---|---|---|---|
| Hyperacute administration | |||||
| AXIS 2010* | Double blind RCT, dose escalation | N = 44, ischaemic MCA stroke | G-CSF (Filgrastim), i.v. 30-180 μg/kg or placebo over 3 days | <12 hours | G-CSF appears safe |
| AXIS-2 2013* | Double blind RCT | N = 328 ischaemic MCA stroke | G-CSF (Filgrastim), i.v. 135 mcg/kg or placebo | <9 hours | G-CSF appears safe. No beneficial effect of G-CSF observed |
| Acute & subacute administration | |||||
| Prasad 2011 | Open label RCT | N = 10, ischaemic stroke | G-CSF (Filgrastim) s.c. 10 μg/kg or placebo for 5 days (no placebo) | <7 days | G-CSF appears safe |
| Shyu 2006 | Single blind RCT | N = 10, ischaemic stroke | G-CSF (Filgrastim) s.c. 15 μg/kg or placebo for 5 days | <7 days | G-CSF appears safe. Improvement in NIHSS, ESS and BI at 12 months |
| STEMS-1 2006 | Double blind RCT, dose escalation | N = 36, ischaemic stroke | G-CSF (Filgrastim) s.c. 1-10 μg/kg or placebo for 1 or 5 doses | 7 to 30 days | G-CSF mobilises PBSCs post stroke, appears safe |
| STEMS-2 2010 | Double blind RCT | N = 60, ischaemic or haemorrhagic stroke | G-CSF (Filgrastim) s.c. 10 μg/kg or placebo (2:1) for 5 days | 3 to 30 days | G-CSF appears safe. PBSCs tracked in vivo |
| STEMTHER 2010 | Open label RCT | N = 20, ischaemic stroke | G-CSF (Leukostim) s.c. 10 μg/kg for 5 days (no placebo) | <48 hours | G-CSF appears safe |
| Zhang 2006* | Double blind RCT | N = 45, ischaemic stroke | G-CSF 2 μg/kg s.c. for 5 days | <7 days | Improved NIHSS by day 20 in the G-CSF group. Abstract only. |
| Chronic administration | |||||
| Floel 2011* | Double blind RCT | N = 41, ischaemic stroke | G-CSF (Filgrastim) s.c. 10 μg/kg or placebo for 10 days | >4months | Feasible and safe administration |
| STEMS-3 | Double blind 2 × 2 factorial RCT | N = 60, ischaemic or haemorrhagic stroke | G-CSF (Filgrastim) s.c. 10 μg/kg or placebo s.c. for 5 days, & PT vs. no PT | 3 months to 2 years | G-CSF appears safe in chronic stroke and improves quality of life |
*Not included in the independent patient data analysis; RCT, randomised controlled trial; MCA, middle cerebral artery; i.v, intravenous; s.c., subcutaneous; NIHSS, National Institutes of Health stroke scale; ESS, European Stroke Scale; BI, Barthel index; PBSC, peripheral blood stem cells; PT physiotherapy.
One study could only be identified as an abstract (so was not included after attempted contact with the author)12, and there was no response from the Chief Investigators of three commercial trials (company Axaron/Sygnis) following repeated attempts13,16,18. A recent meta-analysis of Chinese origin describing results in favour of G-CSF includes 4 Chinese publications that did not appear in our systematic searches22. Attempts to obtain further detail on these publications from the authors were unsuccessful.
Data comprising 196 stroke patients (116 G-CSF, 80 placebo) revealed a mean age 67.1 (standard deviation, SD 12.9), 92% ischaemic stroke, mean NIHSS 10.3 (SD 5.8), and randomisation at 11 days (interquartile range, IQR, 4–238) post ictus (Table 2). Although the data were more limited, the groups appeared to be reasonably well matched for stroke risk factors including hypertension, diabetes and dyslipidaemia (Table 2).
Table 2. Baseline characteristics.
| Placebo | G-CSF | All | |
|---|---|---|---|
| n = 80 | n = 116 | n = 196 | |
| Age | 66 (13.1) | 67.8 (12.8) | 67.1 (12.9) |
| Male | 45 (56.3) | 65 (56) | 110 (56) |
| Days from stroke | 11.5 [5–286] | 10 [4–120] | 11 [4–238] |
| Type | |||
| Ischaemic | 73 (91.3) | 107 (92.2) | 180 (92) |
| Haemorrhagic | 7 (8.8) | 9 (7.8) | 16 (8) |
| Baseline NIHSS | 9.6 (5.5) | 10.7 (6.1) | 10.3 (5.8) |
| n = 67 | n = 99 | n = 166 | |
| Hypertension | 44 (65.7) | 64 (64.6) | 108 (65) |
| Diabetes | 13 (19.4) | 21 (21.2) | 34 (20.5) |
| Dyslipidaemia | 30 (44.8) | 48 (48.5) | 78 (47) |
| n = 62 | n = 94 | n = 156 | |
| Atrial Fibrillation | 13 (21) | 17 (18.1) | 30 (19.2) |
| Previous stroke | 12 (19.4) | 21 (22.3) | 33 (21.2) |
| Previous TIA | 9 (14.5) | 14 (14.9) | 23 (14.7) |
| IHD | 14 (22.6) | 24 (25.5) | 38 (24.4) |
| PVD | 2 (2.2) | 2 (3.1) | 4 (2.6) |
| OCSP Classification | |||
| LACS | 15 (18.8) | 19 (16.4) | 34 (17.3) |
| PACS | 18 (22.5) | 32 (27.6) | 50 (25.5) |
| TACS | 26 (32.5) | 39 (33.6) | 65 (33.2) |
| POCS | 3 (3.8) | 4 (3.4) | 7 (3.6) |
N(%); median [interquartile range]; IHD, ischaemic heart disease; PVD, peripheral vascular.
disease; TIA, transient ischaemic attack; OCSP, Oxford Clinical Stroke Project; LACS, lacuna.
syndrome; PACS, partial anterior circulation stroke; TACS, total anterior circulation stroke.
POCS, posterior circulation stroke.
In univariate and covariate-adjusted analyses (ordinal logistic regression), there was no significant difference between treatment with G-CSF and placebo for end-of-trial mRS: odds ratio (OR) 1.12, 95% confidence interval (CI) 0.64 to 1.96 (p = 0.69), Table 3. There was also no significant difference between groups in NIHSS or Barthel Index (analysed by ANCOVA). A total of 16 (8%) haemorrhagic strokes meant there were too few cases to perform analysis of the effects of G-CSF by stroke pathology. Treatment with G-CSF did not significantly affect outcome (mRS) in different stroke subtypes according to the Oxford Clinical Stroke Project (OCSP) classification23, when compared to placebo (data not shown). There was a non-significant trend towards improved Health Utility Status scores in the treatment group (adjusted mean difference 0.088, p = 0.11, Table 3).
Table 3. The effect of G-CSF compared to placebo on secondary outcome measures.
| Placebo | G-CSF | Unadjusted Odds Ratio or Between-Group Difference (95% CI) | P value | Adjusted Odds Ratio or Between-Group Difference (95% CI)* | P value | |
|---|---|---|---|---|---|---|
| n = 79 | n = 116 | |||||
| N° with SAE† | 14 (7.5) | 34 (29.6) | 1.93 (0.95 to 3.89) | 0.07 | 1.82 (0.89–3.75) | 0.10 |
| Death end of trial† | 3 (3.8) | 8 (7.0) | 1.88 (0.48 to 7.3) | 0.36 | 1.49 (0.36–6.16) | 0.59 |
| Vascular occlusive events† | 6 (7.6) | 12 (11.2) | 1.42 (0.51 to 3.96) | 0.52 | 1.21 (0.43–3.45) | 0.72 |
| n = 73 | n = 109 | |||||
| End of trial mRS‡ | 3.03 (1.3) | 3.26 (1.3) | 1.3 (0.8 to 2.2) | 0.24 | 1.12 (0.64 to 1.96) | 0.62 |
| n = 72 | n = 114 | |||||
| End of trial NIHSS§ | 7.7 (8.5) | 9.2 (10.7) | 1.6 (−1.5 to 4.5) | 0.30 | 0.7 (−1.9 to 3.2) | 0.62 |
| n = 75 | n = 114 | |||||
| End of trial BI§ | 67.1 (29.6) | 63.3 (34.4) | −3.8 (−13.4 to 5.7) | 0.43 | −0.5 (−7.6 to 6.7) | 0.90 |
| n = 56 | n = 83 | |||||
| Health Utility Index§ | 0.392 (0.364) | 0.463 (0.338) | 0.071 (−0.048 to 0.19) | 0.24 | 0.088 (−0.019 to 0.195) | 0.11 |
| n = 80 | n = 116 | |||||
| Peak WCC | 7.46 (3.46) | 31.34 (17.54) | 23.88 (19.96–27.8) | <0.0001 | 23.83 (19.87–27.8) | <0.0001 |
Data shown are number (%) for categorical events, mean (standard deviation) for mRS, NIHSS and BI.
*Adjusted for age, sex, baseline NIHSS and time-to-treatment. Analysed by
†Logistic regression
‡ordinal logistic regression and
§ANCOVA. mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; SAE, serious adverse event; OR odds ratio; CI, confidence interval; WCC, white cell count.
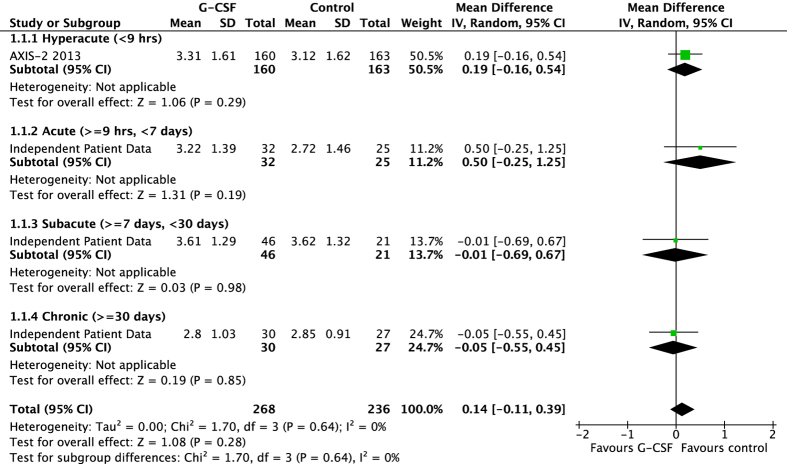
Ordinal analysis indicated a mild but significant influence on outcome in those whom received the treatment/placebo at later time points, OR 1.002, 95% CI 1.0004 to 1.0037 p = 0.015. There was no significant interaction, however, between treatment allocation and time of administration (p = 0.40) within the ordinal model. The effect of time of administration is also represented in Fig. 1 whereby the end-of-trial mRS scores are categorised by time-to-treatment. The data represents mean differences in mRS scores between treatment and placebo groups and is subdivided into hyperacute, acute, subacute and chronic administration times. The figure demonstrates chronic administration trending towards favouring G-CSF, and earlier hyperacute and acute administration trending to favour placebo. Of note, in this summary analysis, we have included data from AXIS-218 despite being unable to obtain individual patient data from the commercial sponsor; we extracted the mean mRS score from each treatment group in this study and calculated the standard deviation from confidence intervals published, thereby providing the data in the hyperacute category.
Figure 1. The effect of time of administration on end-of-trial modified Rankin scale according to treatment group; subgroups are divided into hyperacute (<9 hours), acute (9 hours to <7 days) subacute (7 to 30 days) and chronic (>30 days) phases of stroke.
There were more serious adverse events in the G-CSF group, not reaching significance (p = 0.07, unadjusted analysis); rate of death did not differ between the groups (G-CSF 7% vs. control 4%, p = 0.34, Table 3). There were no reports of new or recurrent haemorrhagic strokes in either group (although one suffered with haemorrhagic transformation of infarction in the control group) and there was no significant difference in the number of vaso-occlusive events, incorporating arterial ischaemia and veno-occlusive disease, by end-of-trial. The distribution of the timing of the vascular events, relative to the first dose of G-CSF, did not differ between groups (log rank test p = 0.51). There was no significant relationship between peak white cell count and vascular events.
Discussion
G-CSF offers a potential multimodal therapy for both ischaemic and haemorrhagic strokes and this individual patient data meta-analysis has highlighted a number of areas requiring further exploration. Overall, G-CSF had a neutral impact on functional outcome, the modified Rankin score. The time of G-CSF administration significantly influenced functional outcome but no interaction between G-CSF and time was observed, which might be expected if this significant finding was secondary to a G-CSF treatment effect. Patients treated with G-CSF were 1.8 times more likely to suffer from a serious adverse event but this was not statistically significant (p = 0.11 in adjusted analyses).
To determine the optimal time of G-CSF administration, it is best considered in two distinct paradigms: G-CSF enhancing neuroprotection or neurorepair. Two randomised controlled trials have explored the former, AXIS 1 and 218,24, administering G-CSF in the hyperacute phase. AXIS-1 reported a small safety study in 44 patients within 12 hours of stroke onset, whilst the follow up trial, AXIS-2, enrolled 328 patients with ischaemic stroke in the MCA territory within 9 hours. There was no difference in efficacy between treatment and placebo groups: G-CSF mean mRS 3.31 (95% CI 3.06–3.56) vs placebo mRS 3.12 (95% CI 2.87–3.37). There were no significant differences in safety or mortality between groups though the absolute number of deaths was higher in the treatment group (22% vs 18%, p = 0.4). These neutral results suggest that assessment of G-CSF in the hyperacute phase of stroke is unlikely to continue. A potential reason for treatment failure could simply be due to giving the drug too late; the preclinical data suggests efficacy if given with 4 hours post onset9, whilst mean time to treatment in AXIS-2 was 7 hours. The inability to translate promising pre-clinical treatments to the bedside is also a likely consequence of the experimental models not adequately simulating human stroke. Whilst G-CSF has been shown to be effective in aged rodent models and in models with co-morbidities25,26,27, the problems of age and co-pathologies are often concurrently present in clinical studies, which may be a significant factor in the failure of phase II/III trials. Furthermore, the aging brain is more susceptible to ischaemic damage, demonstrating earlier inflammatory responses to ischaemia28 and impaired neurogenesis in the peri-infarct area29, which can potentially inhibit neurological recovery.
Chronic administration tests the concept of neurorepair, recovery derived through mobilisation of peripheral blood stem cells (or CD34+ cells). Most pre-clinical data, however, have been developed in models that administer the drug in the hyperacute phase9, and the mechanism of recovery is thought largely to be secondary to attenuation of apoptosis in the ischaemic penumbra7. However, independent of this, neurogenesis in areas remote to the infarct is seen following treatment with G-CSF30. G-CSF also promotes neurogenesis and angiogenesis in peri-ischaemic areas8.
Preclinical studies of G-CSF administration in the subacute phase have been performed with additional haematopoietic cytokines. G-CSF in combination with stem cell factor (SCF) given daily 11–20 days post-stroke produced significant improvements in functional and cognitive outcomes when compared to both control and administration in the acute phase (days 1–10)31. Similarly, G-CSF and SCF combined induced significant and sustained functional recovery in stroke rats with administration as late as 3.5 months post ictus32. More recently, however, experimental studies assessed G-CSF in post-stroke aged rats in combination with bone marrow-derived mononuclear cells33 and pre-differentiated mesenchymal cells34; 28 days of combined treatment did not enhance recovery in comparison to G-CSF alone in either experiment, further questioning the capability of the aged brain to respond to regenerative therapies.
An area of concern in human clinical studies is the potential risk of G-CSF to induce thrombotic events secondary to an inevitable leucocytosis, and therefore risk of exacerbating recurrent ischaemic stroke or inducing vascular events. Leucocytosis increases with repeated daily dosing and subsequently returns to normal within 5 days of the final dose10,17. Thrombotic complications would therefore be more likely to occur over the first 5–20 days after randomisation; our data depicts no differences in vascular event rates during this initial time period and no difference overall between groups until end-of-trial follow-up. A second area of safety concern is with increased risk of intracerebral haemorrhage as seen in other trials of colony stimulating factors (erythropoietin)35, though there is conflicting preclinical literature as to whether G-CSF attenuates or potentiates risk of haemorrhage when used in conjunction with thrombolysis36,37. There were no haemorrhagic events in the treatment group of this patient safety set, and none reported in AXIS-2 where two thirds of the cohort was thrombolysed18.
Our study has a number of limitations to consider. First, one company that ran three studies failed to respond to repeated requests to share data with the collaboration16,18,24. In particular, absence of the largest dataset (AXIS-2) could have confounded our analyses. We have attempted to overcome this by including their summary data in the subgroup analyses (Fig. 1). One of these study assessing G-CSF in the chronic phase (n = 41) is also absent16. There are published concerns about this study: motor function assessments were initially conducted 3 to 7 days after treatment had started (i.e. no pre-treatment values) and participants had almost completely recovered from their initial stroke deficits. Second, our overall sample size is relatively small meaning any findings could be due to chance. Third, the heterogeneity in trial design, such as route and dose of G-CSF and study quality21 could have lead to either over- or under- estimates of treatment efficacy and safety.
In summary, G-CSF did not improve stroke outcome in this individual patient data meta-analysis. There are insufficient data on G-CSF administration in the subacute and chronic phases of stroke and further clinical trials should be considered. It seems sensible to adopt an administration time when the acute and potentially toxic inflammatory reaction has started to settle, and treat when the microenvironment favours a remodelling and neuroreparative phase. We suggest a period up to 4 weeks post stroke when most patients are still receiving active stimulus with a rehabilitation programme, perhaps further enhancing neuroregeneration19. A trend to an increase in serious adverse events in the G-CSF group highlights the importance of continued safety surveillance in future studies.
Methods
Identification of relevant trials
Randomised controlled trials of G-CSF and stroke were sought using electronic searches (Cochrane Library, Medline, Embase, PubMed) up to May 2016, and in a Cochrane Collaboration review of colony stimulating factors and stroke21. Key search terms included granulocyte-colony stimulating factor, G-CSF, ischaemic and haemorrhagic stroke, and randomised controlled trial (exploding the search terms). PRISMA guidelines for reporting have been followed.
Target trials
Randomised controlled trials of G-CSF given in the hyperacute (<9 hours), acute (9 hours to <7 days), subacute (7 to 30 days), and chronic (>30 days) phases of stroke, and involving participants with ischaemic stroke and/or spontaneous intracerebral haemorrhage.
Data acquisition
Investigators and authors were approached to share individual patient data from their respective trials including: patient demographics-age, sex; risk factors-hypertension, atrial fibrillation, diabetes, ischaemic heart disease, previous stroke; stroke details-date and time, subtype, severity (National Institutes of Health Stroke Scale, NIHSS); trial design-blinding; treatment-start date, length of treatment, treatment received, length of follow up; and outcomes and their date-functional (modified Rankin Scale [mRS], Barthel Index [BI]), impairment (NIHSS), quality of life (Euro-Qol-5D, as health utility status [HUS]), haematology (leucocyte count), vascular events (ischaemic stroke, haemorrhagic stroke, myocardial infarction, systemic embolus, venous thrombo-embolism), and serious adverse events.
Outcome measures
The number of participants with a serious adverse event, arterial and venous vascular events, and death were key components of safety. Functional outcome was assessed by the mRS at final follow-up. Other outcomes included NIHSS, BI, number of people with an infection and health utility index.
Data analysis
Following receipt of individual patient data from corresponding chief investigators, the information was tabulated, checked for errors and compared to the primary publication of respective trials. Individual patient data were merged to form a common data set. One trial collected information on stroke severity using the Scandinavian Stroke Scale10; these data were converted to the equivalent NIHSS score using a published formula38. Univariate and multiple variable analyses were performed, with the latter adjusted for age, sex, severity (NIHSS) and time to treatment. The effects of time-to-treatment were analysed in an ordinal logistic regression model, with mRS set as the outcome variable (ordinal shift analysis), and age, sex, NIHSS, time-to-treatment and treatment-time interaction (trt*time) as the predictor variables. Safety and efficacy were assessed in pre-specified sub groups: stroke subtype and time to administration. Statistical significance was taken at p < 0.05. No data was imputed for missing values; patients who had died were assigned scores of −1 for BI, 6 for mRS, and 43 for NIHSS.
Additional Information
How to cite this article: England, T. J. et al. Granulocyte-Colony Stimulating Factor (G-CSF) for stroke: an individual patient data meta-analysis. Sci. Rep. 6, 36567; doi: 10.1038/srep36567 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
P.B. was the Chief Investigator for the Stem Cell Trial of Recovery EnhanceMent After Stroke (STEMS-1, funded by the Stroke Association) and STEMS-2 (funded by the Medical Research Council). He is the Stroke Association Professor of Stroke Medicine. NS was the Chief investigator for STEMS-3 funded by National Institute for Health Research-Research for Patient Benefit. There were no external sources of funding for this work.
Footnotes
Author Contributions T.E. and P.B. designed the study; T.E. wrote the main manuscript and prepared the figure and tables; N.S., P.B., A.A., A.B., A.K. and K.P. reviewed and commented on the manuscript.
References
- Murray C. J. & Lopez A. D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349, 1269–1276 (1997). [DOI] [PubMed] [Google Scholar]
- Dunac A. et al. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol 254, 327–332 (2007). [DOI] [PubMed] [Google Scholar]
- Hennemann B. et al. Mobilization of CD34+ hematopoietic cells, colony-forming cells and long-term culture-initiating cells into the peripheral blood of patients with an acute cerebral ischemic insult. Cytotherapy 10, 303–311 (2008). [DOI] [PubMed] [Google Scholar]
- Schabitz W. R. et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34, 745–751 (2003). [DOI] [PubMed] [Google Scholar]
- Kollmar R., Henninger N., Urbanek C. & Schwab S. G-CSF and rt-PA for the treatment of experimental embolic stroke. Cerebrovascular Diseases 23, 23 (2007). [Google Scholar]
- Gibson C. L., Bath P. M. & Murphy S. P. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. Journal of Cerebral Blood Flow & Metabolism 25, 431–439 (2005). [DOI] [PubMed] [Google Scholar]
- Schneider A. et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. Journal of Clinical Investigation 115, 2083–2098 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehara Y. et al. Potentiation of neurogenesis and angiogenesis by G-CSF after focal cerebral ischemia in rats. Brain Research 1151, 142–149 (2007). [DOI] [PubMed] [Google Scholar]
- England T. J., Gibson C. L. & Bath P. M. Granulocyte-colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: A systematic review. Brain Res Rev 62(1), 71–82 (2009). [DOI] [PubMed] [Google Scholar]
- Sprigg N. et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092). Stroke 37, 2979–2983 (2006). [DOI] [PubMed] [Google Scholar]
- Shyu W. C., Lin S. Z., Lee C. C., Liu D. D. & Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ Canadian Medical Association Journal 174, 927–933 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. J. et al. A Short-Term Assessment of Recombinant Granulocyte-Stimulating factor (RHG-CSF) in Treatment of Acute Cerebral Infarction. Cerebrovascular Diseases 21, 143 (2006). [Google Scholar]
- AXIS study collaborative, g. et al. Ax 200 (G-CSF) for the treatment of ischemic stroke. Stroke 39, 561 (2008). [Google Scholar]
- Alasheev A. M., Belkin A. A., Liderman L. N., Ivanov R. A. & Isakova T. M. Granulocyte-colony stimulating factor for acute ischemic stroke: a randomized controlled trial (STEMTHER). Translational Stroke Research 2, 358–365 (2011). [DOI] [PubMed] [Google Scholar]
- Prasad K. et al. Mobilization of Stem Cells Using G-CSF for Acute Ischemic Stroke: A Randomized Controlled, Pilot Study. Stroke Res Treat 2011, 283473, doi: 10.4061/2011/283473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A. et al. Granulocyte-Colony Stimulating Factor (G-CSF) in Stroke Patients with Concomitant Vascular Disease-A Randomized Controlled Trial. PLoS One 6, e19767, doi: 10.1371/journal.pone.0019767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. J. et al. Stem-cell trial of recovery enhancement after stroke 2 (STEMS2). Randomised placebo-controlled trial of granulocyte-colony stimulating factor in mobilising bone marrow stem cells in sub-acute stroke. Stroke 43, 405–411 (2012). [DOI] [PubMed] [Google Scholar]
- Ringelstein E. B. et al. Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for Ischemic Stroke trial. Stroke 44, 2681–2687, doi: 10.1161/strokeaha.113.001531 (2013). [DOI] [PubMed] [Google Scholar]
- Sprigg N. et al. Granulocyte Colony Stimulating Factor and Physiotherapy after Stroke: Results of a Feasibility Randomised Controlled Trial: Stem Cell Trial of Recovery EnhanceMent after Stroke-3 (STEMS-3 ISRCTN16714730). PLoS One 11, e0161359, doi: 10.1371/journal.pone.0161359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. et al. Stem cell Trial of recovery Enhancement after Stroke 3 (STEMS3). International Journal of Stroke 8, 3 (2013).23280261 [Google Scholar]
- Bath P. M., Sprigg N. & England T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev 6, CD005207, doi: 10.1002/14651858.CD005207.pub4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z. Z. et al. The Efficacy and Safety of Granulocyte Colony-Stimulating Factor for Patients with Stroke. J Stroke Cerebrovasc Dis, doi: 10.1016/j.jstrokecerebrovasdis.2014.11.033 (2015). [DOI] [PubMed] [Google Scholar]
- Bamford J., Sandercock P., Dennis M., Burn J. & Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337, 1521–1526 (1991). [DOI] [PubMed] [Google Scholar]
- Schabitz W. R. et al. AXIS: A Trial of Intravenous Granulocyte Colony-Stimulating Factor in Acute Ischemic Stroke. Stroke 41, 2545–2551 (2010). [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A. et al. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke 41, 1027–1031 (2010). [DOI] [PubMed] [Google Scholar]
- Lan X., Qu H., Yao W. & Zhang C. Granulocyte-colony stimulating factor inhibits neuronal apoptosis in a rat model of diabetic cerebral ischemia. Tohoku Journal of Experimental Medicine 216, 117–126 (2008). [DOI] [PubMed] [Google Scholar]
- Zhao L. R., Singhal S., Duan W. M., Mehta J. & Kessler J. A. Brain repair by hematopoietic growth factors in a rat model of stroke. Stroke 38, 2584–2591 (2007). [DOI] [PubMed] [Google Scholar]
- Buga A. M., Di Napoli M. & Popa-Wagner A. Preclinical models of stroke in aged animals with or without comorbidities: role of neuroinflammation. Biogerontology 14, 651–662, doi: 10.1007/s10522-013-9465-0 (2013). [DOI] [PubMed] [Google Scholar]
- Buga A. M. et al. The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. J Cell Mol Med 12, 2731–2753, doi: 10.1111/j.1582-4934.2008.00252.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehara Y. et al. G-CSF enhances stem cell proliferation in rat hippocampus after transient middle cerebral artery occlusion. Neuroscience Letters 418, 248–252 (2007). [DOI] [PubMed] [Google Scholar]
- Kawada H. et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation 113, 701–710 (2006). [DOI] [PubMed] [Google Scholar]
- Zhao L. R. et al. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke 38, 2804–2811 (2007). [DOI] [PubMed] [Google Scholar]
- Buga A. M. et al. Granulocyte colony-stimulating factor and bone marrow mononuclear cells for stroke treatment in the aged brain. Curr Neurovasc Res 12, 155–162 (2015). [DOI] [PubMed] [Google Scholar]
- Balseanu A. T. et al. Multimodal Approaches for Regenerative Stroke Therapies: Combination of Granulocyte Colony-Stimulating Factor with Bone Marrow Mesenchymal Stem Cells is Not Superior to G-CSF Alone. Frontiers in aging neuroscience 6, 130, doi: 10.3389/fnagi.2014.00130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H. et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40, e647–e656, doi: 10.1161/STROKEAHA.109.564872 (2009). [DOI] [PubMed] [Google Scholar]
- dela Pena I. C. et al. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J Cereb Blood Flow Metab 35, 338–346, doi: 10.1038/jcbfm.2014.208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier S. et al. Impact of the neutrophil response to granulocyte colony-stimulating factor on the risk of hemorrhage when used in combination with tissue plasminogen activator during the acute phase of experimental stroke. Journal of neuroinflammation 11, 96, doi: 10.1186/1742-2094-11-96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. J., Ali M., Lyden P. D. & Bath P. M. Interconversion of the National Institutes of Health Stroke Scale and Scandinavian Stroke Scale in acute stroke. J Stroke Cerebrovasc Dis 18, 466–468 (2009). [DOI] [PubMed] [Google Scholar]