Abstract
Sustainable production of bioplastics by heterotrophic microbes has been restricted by the limited resources of organic substrates and the energy required for biomass harvest. Here, the easy-to-harvest cyanobacterium (Chlorogloea fritschii TISTR 8527), from which the biomass instantaneously settled to the bottom of liquid culture, was utilized to produce poly-3-hydroxybutyrate (PHB) using a two-stage cultivation strategy. The cells were first pre-grown under normal photoautotrophy to increase their biomass and then recultivated under a heterotrophic condition with a single organic substrate to produce the product. Through optimization of this two-stage cultivation, the mass conversion efficiency of acetate substrate to PHB was obtained at 51 ± 7% (w/w), the comparable level to the theoretical biochemical conversion efficiency of acetate to PHB. This two-stage cultivation that efficiently converted the substrate to the product, concurrent with a reduced culture biomass, may be applicable for the production of other biopolymers by cyanobacteria.
Microbial production of biodegradable plastics by heterotrophy is an effective method due to its superior product versatility and productivity compared to those obtained by photoautotrophy. However, such heterotrophic systems, which have mainly been established in bacteria, rely on a substantial consumption of composite organic compounds. Thus, a heterotrophic approach that requires a lower amount of a simple organic substrate is more desirable. Heterotrophic approaches have been described before in the two-stage cultivation of cyanobacteria, where the cells were pre-grown under photoautotrophy to increase their biomass, and subsequently cultured under heterotrophy with a single type of organic substrate. These two-stage cultivation systems have been reported in a number of cyanobacteria for the production of the biodegradable plastic poly-3-hydroxybutyrate (PHB)1,2,3, glycogen (GL)3,4,5 or lipids (LP)3,6. However, the conversion efficiency (CE) of the organic substrate to the products has not been examined in these cyanobacteria, despite the relatively low concentration (0.05–3% w/v) of the organic substrate used in the production process1,2,3,4,5,6.
The theoretical value of the maximum cellular conversion efficiency (CE) of glucose or acetate to PHB is 48% (w/w)7. Although a CE of glucose to PHB of 36–52% (w/w) has been described in bacteria, the cells also required other types of composite organic substrates, such as yeast extract and/or peptone, for the PHB production8,9,10. Thus, the definite determination of the CE from a specific organic substrate to a product was restricted by the presence of the composite organic compounds in these heterotrophic cultivations, particularly in bacterial and yeast systems. In cyanobacteria, a more definitive CE determination of a specific organic substrate to a product is possible by utilizing the two-stage culture system, where the cells are first grown under photoautotrophy, followed by heterotrophic cultivation with only a specific organic substrate.
Biomass harvest has been a major obstacle for producing bioplastic and non-excreting molecules by cyanobacteria11,12. Centrifugation, filtration and chemical flocculation have all been used for cell harvesting12,13,14, but these techniques suffer from a required extended time, cost and energy14 as well as a reduction in the economic and environmental viability of the method. Therefore, a strategy utilizing auto-sedimenting biomass with no requirement for extra energy and a reduced cost and time for cell harvesting will be a promising advantage.
To efficiently produce PHB by cyanobacteria, three key features are needed to be developed: (i) an effective cell harvesting method, (ii) a strain yielding a high biomass level and (iii) a cultivation strategy that efficiently converts the organic substrate to PHB. To establish such a system in cyanobacteria, the easy-to-harvest cyanobacterium (Chlorogloea fritschii) that has a living biomass capable of spontaneous sedimentation was selected. The cells were evaluated for their biomass yield and accumulation levels of PHB under cultivation with different nutrients, organic substrates and light/dark conditions. Subsequently, two-stage cultivation of the cells under photoautotrophy followed by heterotrophy with a single organic substrate was performed, and the CE of the organic substrate to PHB was determined.
Results
Auto-sedimentation of Chlorogloea fritschii TISTR 8527
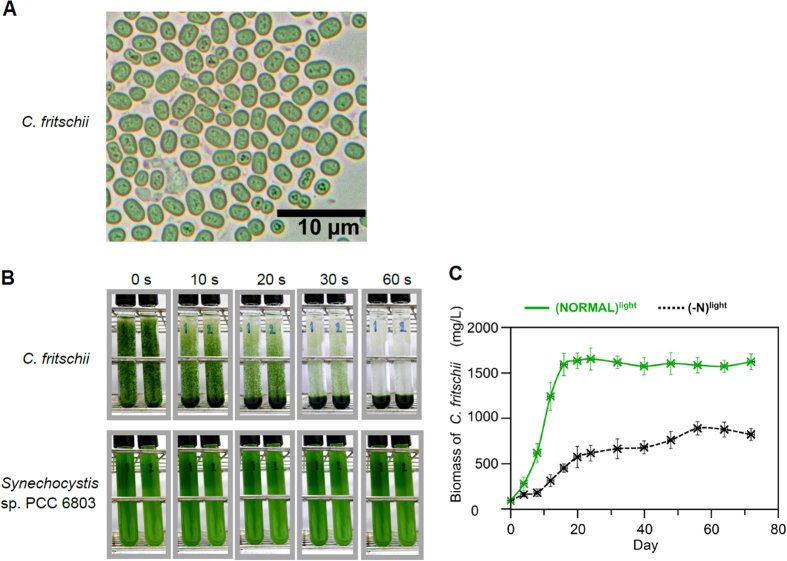
We previously screened 137 cyanobacterial strains for their PHB accumulation ability15, and found that Chlorogloea fritschii TISTR 8527 had a PHB accumulation and cell-cluster formation capability. This cyanobacterium exists in clump cell clusters (Fig. 1A) that spontaneously sedimented to the bottom of liquid medium within 1 min (Fig. 1B). In contrast, the unicellular cyanobacterium Synechocystis sp. PCC6803 does not have such a sedimentation ability (Fig. 1B). The self-sedimentation of C. fritschii enables the removal of the cell-free medium at the above portion of the sedimented cells leading to an easy harvest of the biomass. A biomass recovery of 91 ± 5% (w/w dry weight (DW)) was obtained, where 100% (w/w DW) was set as that recovered by centrifugation.
Figure 1. Auto-sedimentation and cell growth of C. fritschii.
(A) Morphology of C. fritschii cell clusters under the light microscope (1000x magnification). (B) Auto-sedimentation of the 16-d old photoautotrophic cultures. Fifteen ml of cultures (duplicate tubes: 1 and 2) were transferred to glass tubes (10-cm height) and left under natural gravity. (C) Photoautotrophic growth of C. fritschii under the normal nutrient condition (NORMAL) or nitrogen limitation (-N). Values are the average ±1 SD of four independent cultures.
Nitrate is required as a nitrogen source for rapid growth under photoautotrophy
Chlorogloea fritschii is a N2-fixing cyanobacterium. Thus, cell growth was assessed under the non-N2-fixing condition using the standard medium supplied with nitrate, and under the N2-fixing condition using the same medium without nitrate. The maximal biomass growth rate of 156 mg/L/d (during d8–d12) was obtained from the standard medium, while a 4.4-fold lower growth rate of 35 mg/L/d (during d8–d12) was derived from culture in the medium without nitrate (Fig. 1C). The time required to obtain the maximum biomass production (1595 mg/L) was 16 d in the standard medium, compared to 56 d (890 mg/L) in the medium without nitrate (Fig. 1C).
Acetate is an efficient organic substrate for PHB accumulation
Five organic compounds, each chemically equivalent or similar to a metabolite in cyanobacterial carbon metabolism, were individually supplied to C. fritschii culture and evaluated for the PHB accumulation level (Table 1). Cells were cultured under the normal nutrient condition (NORMAL) or the nitrogen-limited condition (-N) under light. The -N culture yielded higher levels of PHB accumulation than the NORMAL cultures for both concentrations of all five organic substrates (Table 1). In the -N medium, acetate at 0.2 and 0.4% (w/v) yielded the highest PHB accumulation of 5.3 and 11.8% (w/w DW), respectively, after 20 d. Under the -N condition, acetate was deemed to be the most efficient organic substrate for PHB accumulation (Table 1) and was selected for subsequent experiments. Nevertheless, it is noted that under normal condition glucose and fructose at 0.4% (w/v) induced a 4-fold higher PHB accumulation than that of acetate at the same concentration.
Table 1. PHB contents under photoheterotrophy supplied with a different organic substrate.
| Organic substrate | PHB content (% w/w DW) | |||
|---|---|---|---|---|
| NORMAL | -N | |||
| Supplied concentration (% w/v) | ||||
| 0.2 | 0.4 | 0.2 | 0.4 | |
| Acetate | 0.7 ± 0.2* | 0.8 ± 0.4* | 5.3 ± 1.2* | 11.8 ± 2.1* |
| Pyruvate | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.7 ± 0.7 | 0.5 ± 0.4 |
| Citrate | 0.4 ± 0.2 | 0.3 ± 0.1 | 4.2 ± 0.3* | 5.7 ± 0.9* |
| Glucose | 0.7 ± 0.3* | 3.4 ± 1.0* | 3.1 ± 5.3* | 6.7 ± 0.4* |
| Fructose | 1.2 ± 0.5* | 3.2 ± 0.9* | 2.8 ± 1.2* | 8.1 ± 1.4* |
5% (v/v) of C. fritschii was cultured in normal nutrient (NORMAL) or nitrogen-limiting (-N) medium containing the indicated organic substrate under light for 20 d.
Data are the average ± 1 SD from three independent cultures.
*Significantly higher PHB content (two-tailed t-test, P < 0.05.) than the PHB content obtained from the same nutrient condition, but without organic substrate (NORMAL = 0.20 ± 0.12% w/w DW, -N = 1.42 ± 0.45% w/w DW).
PHB accumulation is maximal under heterotrophy with nutrient deprivation in the dark
To increase PHB accumulation, the C. fritschii culture condition was optimized by adjusting the (i) amount of acetate supply, (ii) growth in the light or the dark and (iii) the nutrient supply as: normal nutrient condition (NORMAL), nitrogen limitation (-N), phosphorus limitation (-P), and nitrogen and phosphorus limitation (-N-P).
Under photoautotrophy, the NORMAL condition yielded low PHB levels <4% (w/w DW), while culture in the -N, -P and -N-P media yielded moderate PHB levels at 14–17% (w/w DW) at the late period of the cultivation (≥48 d, Fig. 2).
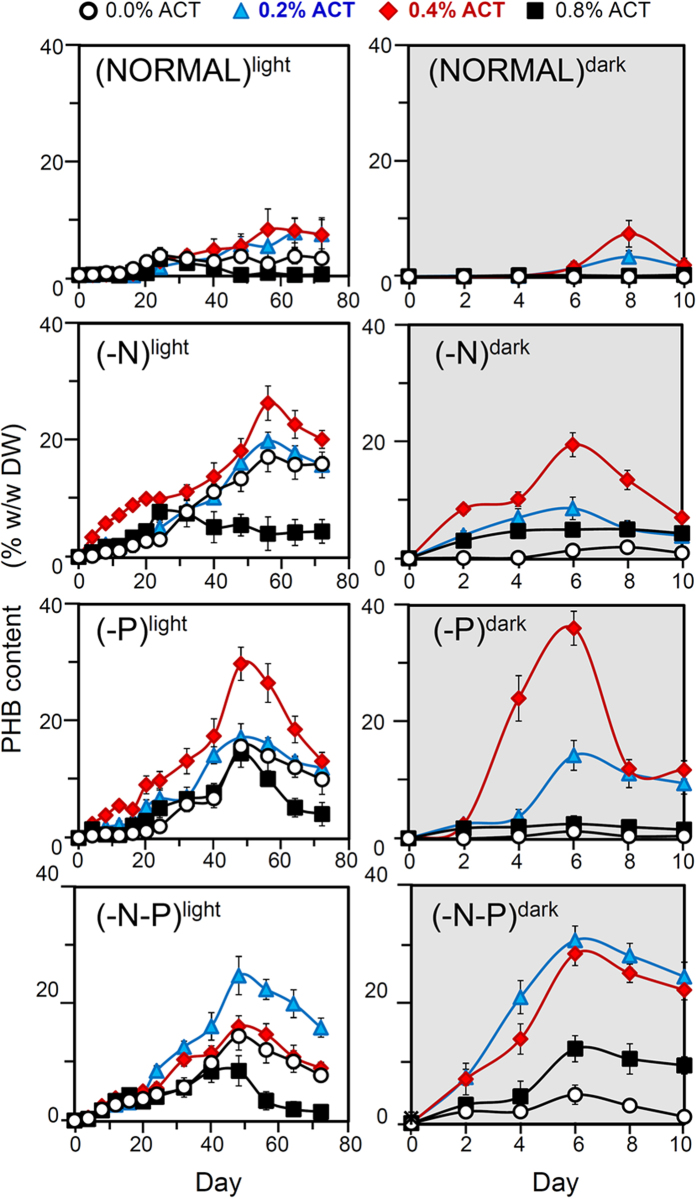
Figure 2. Cellular PHB contents under photoheterotrophy or heterotrophy in the dark with different initial levels of acetate (ACT) substrate.
Cells were cultured under normal photoautotrophy for 16 d to increase their biomass and then transferred to the normal nutrient (NORMAL), or that deficient in nitrogen (-N), phosphorus (-P) or nitrogen and phosphorus (-N-P). Data are shown as the mean ± 1 SD, derived from three to six independent experiments.
Under mixotrophy (i.e. photoheterotrophy: acetate supply in the light), cultivation in the NORMAL medium yielded low PHB levels of <10% (w/w DW) at all acetate concentrations (Fig. 2). Of particular importance is that cultivation in the -N and -P media required acetate at 0.4% (w/v) to reach peak PHB levels at 26–30% (w/w DW). In contrast, the -N-P medium required only 0.2% (w/v) acetate to achieve the peak PHB level at 24% (w/w DW). All the peak PHB levels under this mixotrophy occurred in the late cultivation period (≥48 d, Fig. 2).
Under heterotrophy (acetate supply in the dark), cultivation in the NORMAL medium yielded a low PHB level of <10% (w/w DW) at all acetate concentrations (Fig. 2), while in the -N, -P and -N-P media a 0.2–0.4% (w/v) acetate concentration gave increased PHB levels to 8–30% (w/w DW) except at 0.4% (w/v) acetate in the -P medium where a higher PHB level of 36% (w/w DW) was obtained. Increasing the acetate concentration to 0.8% (w/v) reduced the PHB accumulation level compared to that at 0.2 and 0.4% (w/v) acetate. Interestingly, the -N and -P media required acetate at 0.4% (w/v) to reach peak PHB levels of 19–36% (w/w DW), while cultivation in the -N-P medium needed a 2-fold lower acetate concentration at 0.2% (w/v) to reach the peak PHB level of 30% (w/w DW). This result reflected the more efficient conversion of acetate to PHB under the -N-P condition.
Overall results in Fig. 2 indicate that although the PHB levels were not much different in cells under photoheterotrophy and under heterotrophy in the dark, the significant shortening of the cultivation time to achieve maximum PHB level was obtained under heterotrophy in the dark.
Two-stage culture (photoautotrophy followed by heterotrophy in the dark) increased the PHB production
Next, C. fritschii was grown using the two-stage cultivation strategy. For the first stage, the cells were pre-grown under normal photoautotrophy for 16 d to achieve the maximum biomass level, whereby a PHB level of <1% w/w DW was detected in these 16-d pre-grown cells. Then, for the second stage, the cells were subjected to growth under the previously determined optimal heterotrophic condition (acetate as the substrate in the dark) to increase the PHB production. At this heterotrophic stage, the effects of different nutrient conditions (-N, -P or -N-P) and various acetate concentrations (0.2–0.8% w/v) on PHB levels were evaluated. In general, a decrease of acetate supplied to heterotrophic culture in the dark led to a decrease of PHB production in all nutrient deficient conditions, except at 0.4% (w/v) acetate under –N-P where PHB level appeared to saturate at 0.2% (w/v) acetate (Table 2). Of particular importance, heterotrophic culture in the -N and the -P medium required 0.4% (w/v) acetate to achieve the peak PHB production level of 267 and 531 mg/L, respectively, while in the -N-P medium a half-fold lower acetate concentration of 0.2% (w/v) gave the peak PHB production of 431 mg/L (Table 2).
Table 2. PHB production under a two-stage (photoheterotrophy, then heterotrophy) culture.
| Nutrient condition | Acetate supply % (w/v) | PHB content (% w/w DW) | PHB production (mg/L) | % w/w [PHB product/ACT supply]a |
|---|---|---|---|---|
| NORMAL | 0.00 | 0.1 ± 0.0 | 1 ± 1 | na |
| 0.05 | 0.1 ± 0.0 | 2 ± 1 | 0.0 ± 0.0 | |
| 0.10 | 0.6 ± 0.4 | 9 ± 5 | 0.7 ± 0.5 | |
| 0.20 | 1.5 ± 0.9 | 22 ± 13 | 0.1 ± 0.6 | |
| 0.40 | 1.8 ± 0.8 | 28 ± 15 | 0.7 ± 0.3 | |
| -N | 0.00 | 1.4 ± 0.8* | 19 ± 10* | na |
| 0.05 | 0.4 ± 0.2* | 6 ± 5 | 0.8 ± 0.9 | |
| 0.10 | 2.2 ± 0.9* | 30 ± 11* | 2.8 ± 1.0* | |
| 0.20 | 8.6 ± 1.9* | 121 ± 23* | 6.0 ± 1.1* | |
| 0.40 | 19.5 ± 2.0* | 267 ± 16* | 6.6 ± 0.4* | |
| -P | 0.00 | 1.3 ± 0.1* | 18 ± 4* | na |
| 0.05 | 1.8 ± 1.0* | 25 ± 17* | 4.6 ± 3.3* | |
| 0.10 | 7.5 ± 1.5* | 97 ± 11* | 9.5 ± 1.1* | |
| 0.20 | 14.3 ± 2.5* | 203 ± 30* | 10.0 ± 1.5* | |
| 0.40 | 36.0 ± 2.8* | 531 ± 75* | 13.2 ± 1.8* | |
| -N-P | 0.00 | 4.9 ± 1.6* | 65 ± 24* | na |
| 0.05 | 8.3 ± 1.9* | 108 ± 30* | 21.2 ± 6.0* | |
| 0.10 | 21.4 ± 2.4* | 295 ± 37* | 29.3 ± 3.7* | |
| 0.20 | 30.7 ± 2.8* | 431 ± 45* | 21.5 ± 2.2* | |
| 0.40 | 28.5 ± 1.9* | 395 ± 9* | 9.8 ± 0.2* |
Cells were pre-grown under normal photoautotrophy for 16 d and transferred to the specified heterotrophic conditions using the 6-d time culture period yielding the highest PHB contents (Fig. 3). Data are the average ± 1 SD from three to five independent cultures. Asterisks indicate significantly higher levels (P < 0.01, two-tailed t-test) than those obtained from NORMAL nutrient condition and the same acetate supply. na, not applicable. a = [(PHB production, mg/L)]/(ACT supply in the culture medium, mg/L)] × 100, where PHB production is the difference in production at a second-stage heterotrophic culture and at the end of the first-stage photoautotrophic culture.
With respect to the PHB yield of the two stage cultures, measured in terms of the % (w/w) [PHB product/acetate supply to the medium], the PHB yield was <14% (w/w) when cultured in the NORMAL, -N and -P media in the heterotrophic stage, compared to 9–29% (w/w) when cultured in the -N-P medium depending upon the acetate concentration (Table 2). The PHB yield was highest at 29% (w/w) when cultured in the -N-P medium with 0.1% (w/v) acetate (Table 2).
Conversion efficiency (CE) of acetate to PHB
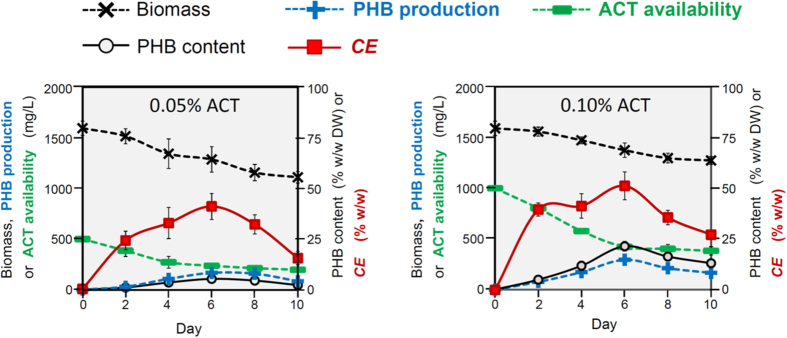
The mass conversion efficiency of acetate to PHB (CEAcetate→PHB) was determined in two-stage cultures in terms of the % (w/w) [PHB product/cellular acetate consumption]. The maximal CEAcetate→PHB of 51% was obtained from the two-stage culture in which the cells during heterotrophic stage were grown in the -N-P medium with 0.1% (w/v) acetate in the dark (Fig. 3). Reducing and increasing the acetate concentration to 0.05% and 0.2% (w/v) under the same condition decreased the CEAcetate→PHB to 41% (Fig. 3) and 33% (data not shown), respectively.
Figure 3. Conversion efficiency (CE) of acetate to PHB in the two-stage cultures of C. fritschii.
Sixteen-d photoautotrophy-grown cells were transferred to the heterotrophic -N-P medium in the dark up to 10 d with an initial acetate (ACT) concentration of 0.05% or 0.1% (w/v). The residual available ACT level in the culture medium and CE of acetate to PHB was determined at the indicated time points. Data are the average ± 1 SD from six independent cultures.
Acetyl-CoA level and PHB synthase activity
The acetyl-CoA level and PHB synthase activity of the cells cultured in the different heterotrophic media (NORMAL, -N, -P or -N-P) with added acetate at 0.1 and 0.2% (w/v) of the two-stage cultures were determined. Cells cultured in the -N medium had a two-fold increased PHB synthase activity compared to those in the NORMAL medium, while in the -P or -N-P medium it was six- to eight-fold higher (Table 3). Consistent with this, was that in the -N medium the cells had a slightly (1.2- to 1.3-fold) raised acetyl-CoA level (presumably, the primary substrate of PHB synthesis in C. fritschii), while in the -P and -N-P media the cells had a much higher level of acetyl-CoA by 2.0- to 2.8-fold, relative to those in the NORMAL medium (Table 3). The increase of added acetate from 0.1 to 0.2% (w/v) had little effect on both the enzyme activity and the acetyl-CoA levels. Overall, the results indicate that heterotrophically grown C. fritschii cells in -P or -N-P medium, rather than -N medium, had considerable increase of both the precursor and the enzyme activity required for PHB production.
Table 3. Cellular acetyl-CoA and PHB synthase activity levels after heterotrophic culture in the dark.
| Nutrient condition | Acetyl-CoA level (μg/g DW) | PHB synthase activity (nmol/min/mg protein) | ||
|---|---|---|---|---|
| NORMAL | 25.2 ± 5.1 | 20.3 ± 6.1 | 15.2 ± 4.2 | 13.2 ± 4.1 |
| -N | 30.7 ± 11.1 | 27.5 ± 9.7 | 35.1 ± 8.5* | 31.3 ± 5.5* |
| -P | 56.5 ± 10.7* | 58.4 ± 11.7* | 105.3 ± 15.3* | 97.4 ± 10.1* |
| -N-P | 52.1 ± 13.5* | 56.6 ± 12.5* | 98.5 ± 19.4* | 111.5 ± 12.3* |
Cells were cultured as described in Table 2. Data are average ± 1 SD from five independent experiments. Asterisks indicate significantly higher levels (P < 0.01, two-tailed t-test) than that obtained from NORMAL nutrient condition with the same acetate supply.
Material properties of PHB from C. fritschii
The PHB extracted from C. fritschii cultured under the two-stage culture was examined by 13C- and 1H-NMR spectra, and was found to match the respective spectra of the commercial PHB (Supplementary Fig. S1). The thermal and physical properties of C. fritschii PHB were comparable to those of the commercial PHB (Table 4), except for a slightly lower enthalpy of fusion (ΔHm) and % crystallinity (Xc) observed in C. fritschii PHB. Interestingly, C. fritschii PHB had a 27% decreased polydispersity (Mw/Mn) and a 22–43% decreased molecular weight (Mw and Mn) relative to the commercial PHB (Table 4).
Table 4. Material properties of PHB from C. fritschii.
| Sources of PHB | Thermal properties | Mechanical properties2 | Molecular weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | Tg (°C) | Tcc (°C) | ∆Hm (J/g) | Xc (%) | Elongation at break (%) | Tensile strength (MPa) | Young’s modulus (MPa) | Mw (kDa) | Mn (kDa) | Mw/Mn | |
| Commercial PHB1 | 175.4 (159) | 3.5 | 48 | 99 | 68 | 5.8 ± 1.1 | 24 ± 3 | 820 ± 300 | 970 | 330 | 2.9 |
| Chlorogloea fritschii | 171.6 (171) | 3.2 | 54 | 65 | 45 | 5.5 ± 1.8 | 23 ± 6 | 712 ± 256 | 545 | 256 | 2.1 |
Sixteen-d old photoautotrophically-grown cells were transferred to -N-P medium in the dark with 0.1% (w/v) acetate for 6 d. The dried biomass was extracted for PHB. Tm, melting temperature (first melting peak shown in parentheses); Tg, glass-transition temperature; Tcc, cold-crystallization temperature; ∆Hm, enthalpy of fusion; Xc, crystallinity; Mw, weight-average molecular weight; Mn, number-average molecular weight; Mw/Mn, polydispersity.
1Data from Sigma-Aldrich (St. Louis, MO, USA).
2The mechanical properties are shown as the mean ± 1 SD of three independent experiments.
Discussion
Microbial production of PHB and none-excreted biomolecules requires the separation of the microbial biomass from the liquid culture medium. Centrifugation and chemical flocculation have previously been used to recover the biomass16,17, but these methods are extensively energy, time and cost consuming. In the filamentous cyanobacterium Arthrospira platensis, filtration has been used for cell harvesting, but the method requires considerable amount of time and cost for filtration equipment18. Recently, Anabaena sp. producing H2 gas has been shown to float on top of the culture medium enabling its ease of biomass recovery19. However, this requires the restrictive anaerobic culturing system for H2 production19. Here, we demonstrated that the cell-cluster forming C. fritschii exhibited spontaneous sedimentation (Fig. 1B). This enables the removal of the cell-free medium at the above portion of the sedimented cells (Fig. 1B), facilitating an easy harvesting of the biomass at a 91 ± 5% (w/w) recovery. The self-sedimentation feature of C. fritschii was found in all phases of photoautotrophic growth, and the larger size of cell clusters were found at the late log phase and the stationary phase (not shown). How C. fritschii exhibited high mass density and sedimented to the bottom of the culture is still unknown. This cell clustering feature has been observed in other cyanobacteria20,21, and so it is worth examining the auto-sedimentation ability in these strains.
Culturing in the -N or –P medium under the heterotrophic condition was found to increase the PHB accumulation in C. fritschii (Fig. 2), consistent with previous reports in other cyanobacteria under photoautotrophy1,2,15 and/or heterotrophy1,2. Recently, the Synechocystis sp. PCC6803 photoheterotrophically grown with glucose was shown to have the highest PHB accumulation level in -N-P medium, about a 1.4- to 1.6-fold greater than that in the -N or -P medium3. Likewise, this study showed a 2- to 18-fold greater PHB accumulation when C. fritschii was cultured in the -N-P medium, than in the -N or -P medium under the heterotrophy with acetate supply at ≤0.2% (w/v) in the dark (Table 2). This greater PHB storage when cultured in the -N-P medium than that in the -N medium is attributed to the higher levels of acetyl-CoA and the superior PHB synthase activity found in cells cultured in the -N-P medium (Table 3). However, it is noted that broadly similar acetyl-CoA and PHB synthase activity levels were found in the -P cultures and in the -N-P cultures (Table 3), whereas the latter had a higher PHB accumulation level at almost all acetate concentrations (Table 2). This might be due to the increased levels of other enzymes and metabolites in the C. fritschii PHB synthetic pathway that have not been identified at present. In the cyanobacterium Synechocystis sp. PCC6803, where the PHB synthesis pathway has been completely identified, culturing in -N or -P medium was found to enhance the photoautotrophic PHB accumulation by increasing the activity levels of the three enzymes in the PHB synthetic pathway22,23,24.
In this study, the maximal PHB production by C. fritschii at 531 mg/L after 22 d of cultivation was obtained under the two-stage cultivation system when the heterotrophic stage was performed in the -P medium with 0.4% (w/v) acetate in the dark (Table 2). A higher cyanobacterial PHB production level of 1,597 mg/L after 19 d was previously reported in Aulosira fertilissima cultured heterotrophically in the dark with multiple types of organic substrates25. However, the CE of these substrates to PHB by A. fertilissima was not determined.
Based on the biochemical pathway, the maximal theoretical mass CEGlucose→PHB and CEAcetate→PHB is 48% (w/w)7. Bacterial CEAcetate→PHB and CEGlucose→PHB levels of 26% and 36–42% (w/w), respectively, have been described previously, but these bacteria also required other composite organic substrates, such as yeast extract and/or peptone, in the media for PHB production8,9,26. The CEGlucose→PHB above the theoretical value at 52% (w/w) was reported in the bacterium Halomonas TD01, but again this bacterium required yeast extract as a composite organic substrate for the PHB production10. The definitive determination of the CE from a specific single organic substrate to PHB requires a culture system that contains only the organic substrate of interest, as was performed in this work. Here, C. fritschii was pre-cultured under photoautotrophy that yielded high biomass level but low PHB accumulation (<1% w/w DW), and followed by heterotrophic culture with acetate as the only organic substrate to promote PHB production. In this two-stage culture a maximal CEAcetate→PHB of 51 ± 7% (w/w) was obtained (Fig. 3). This obtained CEAcetate→PHB is comparable to the theoretical biochemical efficiency of 48% (w/w)7. That this conversion is comparable to the theoretical efficiency may be attributed to the fact that C. fritschii was pre-grown under photoautotrophy, where cells would store a certain amount of energy and metabolites in their biomass and convert particular molecules to PHB during the subsequent heterotrophic culture. In support of this contention, a substantial biomass reduction (20 and 14% under 0.05 and 0.10% w/v acetate, respectively) was found 6 d after transferring photoautotrophically grown C. fritschii to the heterotrophic cultures (Fig. 3). In the second-stage cultivation, how acetate was metabolized to PHB and whether certain metabolites were converted to PHB remain to be elucidated. The metabolic flux analysis using radioactively labeled acetate and the related metabolites would provide an insight into this cellular mechanism.
In conclusion, this work demonstrated the efficient bioconversion of acetate to PHB, by the easy-to-harvest cyanobacterium C. fritschii. The results demonstrated the mass CE of acetate to PHB is at the comparable level to the theoretical efficiency. Further metabolic engineering that targets carbon storage pathways, such as that recently described by Osanai et al.22,23, and shortening culture time by optimizing light intensity, temperature and carbon supply, as demonstrated by Yu et al.27, would help to increase the CE. The established algal co-culture technique28, might be applied for concurrent cyanobacterial cultivation between a PHB-producing strain and a photoautotrophically-grown acetate-excreting strain29 to produce the biopolymer directly from CO2 and solar energy without the need for an additional organic supply.
Methods
Strain and culture conditions
The axenic culture of Chlorogloea fritschii TISTR 8527 was obtained from the Thailand Institute of Scientific and Technological Research. The strain was previously isolated from a freshwater pond in Bangkok and purified by the alkaline and ampicillin treatment method30. An approximately 5% (v/v) of a 16-d old culture was inoculated into 250-mL flasks containing 150 mL BG-11 medium31 without sodium citrate and supplemented with 20 mM HEPES-NaOH (pH 7.5) and with ferric ammonium citrate replaced by ferric chloride. Thus, this BG11 medium does not contain any organic compounds. Cells were grown at 32 °C under a continuous white light of 100 μmol/m2/s using an atmospheric CO2 supply upon shaking at 160 rpm. The limitation of combined nitrogen source and/or phosphorus in the BG11 medium was proceeded by making the medium devoid of such nutrients, as previously described3. Sodium acetate, sodium pyruvate, sodium citrate, glucose or fructose (Sigma-Aldrich, St. Louis, MO, USA) was added to the BG11 medium when required. The dark condition was acquired by wrapping each culture flask in an aluminum sheet. Wet cells were immediately frozen by liquid nitrogen and dried at 65 °C.
Quantification of PHB
The PHB content, as % (w/w DW), was determined using high-performance liquid chromatography (HPLC) as described32. Dry cells were boiled in H2SO4 to hydrolyze the PHB into crotonic acid. The crotonic acid content was then analyzed by HPLC using adipic acid as the HPLC marker. Commercial PHB (Sigma-Aldrich, St. Louis, MO, USA) was analyzed in parallel, where an 84.6 ± 4.0% (w/w) conversion of PHB to crotonic acid was obtained.
Quantification of acetate in culture medium
The acetate level in the medium was quantified using the HPLC system for organic acid analysis according to the protocol of Shimadzu (Japan). The HPLC was equipped with an InertSustain Carbon-18 column and 10 mM NH4H2PO4 (adjusted to pH 2.6 by H3PO4) as the mobile phase. The UV detection at 210 nm was used to detect acetate, while BG11 medium containing 0.1–2.0 g/L sodium acetate was used as quantification standards.
Determination of the CE of acetate to PHB
The CE proceeded by the cells was calculated according to Eq. (1),
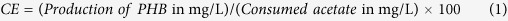 |
where Production of PHB is the difference in production at the second-stage heterotrophic culture and at the end of the first-stage photoautotrophic culture, Consumed acetate is the difference in acetate concentration in the medium at the initial time of the cultivation and at the specific time point after starting the cultivation.
Assay of PHB synthase activity and acetyl-CoA level
The PHB synthase activity was determined essentially as previously described33. Each 1-ml reaction contained 0.2 mg of crude protein, 1.5 mM 3-hydroxybutyryl-CoA substrate and 0.5 mM 5,5-dithiobis(2-nitrobenzoic acid) in Tris-glycerol buffer (25 mM Tris-HCl pH 7.5, containing 5% (v/v) glycerol). The reaction was initiated by adding 3-hydroxybutyryl-CoA and incubated at 30 °C for 10 min. The resulting thiobenzoate anion was measured spectrophotometrically at 412 nm. Protein quantification was performed using the Lowry method.
The acetyl-CoA level was determined using the acetyl-CoA assay kit MAK039 (Sigma-Aldrich, St. Louis, MO, USA). Cells were harvested and frozen in liquid nitrogen. Acetyl-CoA was specifically catabolized by the enzyme assay kit according to the manufacturer’s protocol and the resulting product was quantified by its fluorescent intensity (535-nm excitation and 587-nm emission).
Extraction of PHB and analysis of its properties
For PHB extraction, dry cells were washed in methanol to remove pigments and then the PHB was extracted in chloroform followed by precipitation using diethyl ether34. For nuclear magnetic resonance (NMR), 2 mg/mL PHB in deuterochloroform (CDCl3) was analyzed by 1H- and 13C-NMR at 25 °C using a Bruker Advance 400 MHz spectrometer (Germany). For thermal properties, 15 mg of PHB was analyzed by differential scanning calorimetry (Netzsch DSC-204-F1 machine, Germany) in the range from −50 °C to 200 °C. For mechanical analysis, the PHB film was cut into rectangles and analyzed at 25 °C using Hounsfield H10KM material testing machine (UK). For molecular weight determination, 2 mg/mL PHB in chloroform was analyzed by gel permeation chromatography (Shimadzu, Japan) using the K802.5, K803 and K804 columns (Shodex, NY, USA) according to the manufacturer’s method. Polystyrene in the range of 4.0 × 102–1.8 × 106 g/mol (Sigma-Aldrich, St. Louis, MO, USA) was analyzed as the mass standards.
Additional Information
How to cite this article: Monshupanee, T. et al. Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 6, 37121; doi: 10.1038/srep37121 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by Thailand Research Fund (RSA5980016) and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (CU-58-036-AM). The authors thank Dr. Aparat Mahakhant and the Thailand Institute of Scientific and Technological Research for the strain; Mr. Somchai Tarawat and Mr. Pakpoom Boonchuen for microscopy; Dr. Suwabun Chirachanchai for GPC; and Dr. Robert Butcher for critical proofreading. A.I. and T.M. thank Thailand Research Fund (IRG578008) for institution research grant.
Footnotes
Author Contributions T.M. designed the research with the input and suggestion from A.I; T.M. and P.N. conducted the experiments and analyzed the data; T.M. wrote the paper. All authors reviewed the manuscript.
References
- Drosg B., Fritz I., Gattermayr F. & Silvestrini L. Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem. Biochem. Eng. Q. 29, 145–156 (2015). [Google Scholar]
- Koller M. & Maršálek L. Cyanobacterial polyhydroxyalkanoate production: Status Quo and Quo Vadis? Curr. Biotechnol. 4, 464–480 (2015). [Google Scholar]
- Monshupanee T. & Incharoensakdi A. Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 116, 830–838 (2014). [DOI] [PubMed] [Google Scholar]
- Gaudana S. B., Alagesan S., Chetty M. & Wangikar P. P. Diurnal rhythm of a unicellular diazotrophic cyanobacterium under mixotrophic conditions and elevated carbon dioxide. Photosynth. Res. 118, 51–57 (2013). [DOI] [PubMed] [Google Scholar]
- Philippis R. D., Sili C. & Vincenzini M. Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. J. Gen. Microbiol. 138, 1623–1628 (1992). [Google Scholar]
- Modiri S. et al. Lipid production and mixotrophic growth features of cyanobacterial strains isolated from various aquatic sites. Microbiology 161, 662–673 (2015). [DOI] [PubMed] [Google Scholar]
- Yamane T. Yield of poly-D(-)-3-hydroxybutyrate from various carbon sources: a theoretical study. Biotechnol. Bioeng. 41, 165–170 (1993). [DOI] [PubMed] [Google Scholar]
- Fu X. Z. et al. Development of Halomonas TD01 as a host for open production of chemicals. Metab. Eng. 23, 78–91 (2014). [DOI] [PubMed] [Google Scholar]
- Lin Z. et al. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb. Cell Fact. 14, 185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D., Xue Y. S., Aibaidula G. & Chen G. Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol. 102, 8130–8136 (2011). [DOI] [PubMed] [Google Scholar]
- Molina Grima E., Belarbi E. H., Acien Fernandez F. G., Robles Medina A. & Chisti Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 20, 491–515 (2003). [DOI] [PubMed] [Google Scholar]
- Uduman N., Qi Y., Danquah M. K., Forde G. M. & Hoadley A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sust. Energy 2, 12701–12715 (2010). [Google Scholar]
- Golueke C. G. & Oswald W. J. Harvesting and processing sewage-grown planktonic algae. J. Water Pollut. Con. F. 37, 471–498 (1965). [Google Scholar]
- Wijffels R. H. & Barbosa M. J. An outlook on microalgal biofuels. Science 329, 796–799 (2010). [DOI] [PubMed] [Google Scholar]
- Kaewbai-Ngam A., Incharoensakdi A. & Monshupanee T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 212, 342–347 (2016). [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Lee K. & Oh Y. K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: a review. Bioresour. Technol. 184, 63–72 (2015). [DOI] [PubMed] [Google Scholar]
- Wan C. et al. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 184, 251–257 (2015). [DOI] [PubMed] [Google Scholar]
- Rossi N., Jaouen P., Legentilhomme P. & Petit I. Harvesting of cyanobacterium Arthrospira platensis using organic filtration membranes. Food Bioprod. Process 82, 244–250 (2004). [Google Scholar]
- Chen M. et al. Auto-flotation of heterocyst enables the efficient production of renewable energy in cyanobacteria. Sci. Rep. 4, 3998, doi: Artn 3998 Doi 10.1038/Srep03998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. H., Foulds I. & Carr N. G. Environmental conditions and morphological variation in the blue-green alga Chlorogloea fritschii. J. Gen. Microbiol. 92, 147–155 (1976). [Google Scholar]
- Silva S. M. F. & Pienaar R. N. Some benthic marine cyanophyceae of Mauritius. Botanica Marina 43, 11–27 (2000). [Google Scholar]
- Osanai T. et al. Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. PCC 6803. DNA Res. 20, 525–535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai T. et al. Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 164, 1831–1841 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener K. M. et al. Global proteomics reveal an atypical strategy for carbon/nitrogen assimilation by a cyanobacterium under diverse environmental perturbations. Mol. Cell. Proteomics 9, 2678–2689 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S. & Mallick N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 24, 803–814 (2012). [Google Scholar]
- Wu S. C., Liou S. Z. & Lee C. M. Correlation between bio-hydrogen production and polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 113, 44–50 (2012). [DOI] [PubMed] [Google Scholar]
- Yu J. et al. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 5, 8132, doi: 10.1038/srep08132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves A. L., Pires J. C. & Simoes M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: nutrients removal, biomass and lipid production. Bioresour. Technol. 200, 279–286 (2016). [DOI] [PubMed] [Google Scholar]
- Wang B., Pugh S., Nielsen D. R., Zhang W. & Meldrum D. R. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 16, 68–77 (2013). [DOI] [PubMed] [Google Scholar]
- Sena L. et al. A strategy to obtain axenic cultures of Arthrospira spp. cyanobacteria. World J. Microbiol. Biotechnol. 27, 1045–1053 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R., Deruelles J., Waterbury J. B., Herdman M. & Stanier R. Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111, 1–61 (1979). [Google Scholar]
- Schlebusch M. & Forchhammer K. Requirement of the nitrogen starvation-induced protein Sll0783 for polyhydroxybutyrate accumulation in Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 76, 6101–6107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin H. E. & Steinbuchel A. Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl. Microbiol. Biotechnol. 40, 699–709 (1994). [Google Scholar]
- Yellore V. & Desai A. Production of poly-3-hydroxybutyrate from lactose and whey by Methylobacterium sp. ZP24. Lett. Appl. Microbiol. 26, 391–394 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.