Abstract
BACKGROUND
Thermal injury is associated with several biochemical and histopathological alteration in tissue. Analysis of these objective parameters in research and clinical field are common to determine healing rate of burn wound. Negative pressure wound therapy has been achieved wide success in treating chronic wounds. This study determines superficial burn wound healing with intermittent negative pressure wound therapy under limited access and conventional dressings
METHODS
A total 50 patients were randomised into two equal groups: limited access and conventional dressing groups. Selective biochemical parameters such as hydroxyproline, hexosamine, total protein, and antioxidants, malondialdhyde (MDA), wound surface pH, matrix metalloproteinase-2 (MMP-2), and nitric oxide (NO) were measured in the granulation tissue. Histopathologically, necrotic tissue, amount of inflammatory infiltrate, angiogenesis and extracellular matrix deposition (ECM) were studied to determine wound healing under intermittent negative pressure.
RESULTS
Patients treated with limited access have shown significant increase in the mean hydroxyproline, hexosamine, total protein, reduced glutathione (GSH), glutathione peroxidase (GPx), and decrease in MDA, MMP-2, wound surface pH, and NO. Histopathologic study showed that there was a significant difference after 10 days of treatment between limited access vs conventional dressing group, Median (Q1, Q3)=3 (2, 4.25) vs 2 (1.75, 4).
CONCLUSION
Limited access was shown to exert its beneficial effects on wound healing by increasing ground substance, antioxidants and reducing MMP-2 activity, MDA, NO and providing optimal pH, decreasing necrotic tissue, amount of inflammatory infiltrate, increasing ECM deposition and angiogenesis.
Key Words: Burn, Healing, Intermittent negative pressure, Limited access dressing, Conventional dressing
INTRODUCTION
Patients with deep and extensive wounds of major burn with multiple injuries are difficult to treat.1 Although the pathophysiological mechanisms of tissue injury remain unclear, there is increasing evidence that oxidative stress have an important role in the development of multiorgan failure after thermal injury.2 In thermal trauma, tissues are subjected to ischemia.3 It was shown that oxidative stress due to burn injury initiate an inflammatory cascade that includes acute phase protein synthesis, upregulation of inflammatory adhesion molecules and pro-inflammatory cytokine, such as interleukin-1 (IL-1) and tumour necrosis factor-a (TNF-a).4
These activated inflammatory cascade causes local and systemic neutrophil sequestration, which is source of reactive oxygen species (ROS)5 and contribute to delay healing in burn wound.6 It has been shown that burn injury, associated with lipid peroxidation is an important cause of oxidative damage to cellular membranes, and eventually cell death.7 Increased plasma malondialdehyde (MDA) levels and concentrations of lipid peroxides in the systemic circulation have been described after burn trauma.8
Enzymatic antioxidants offer defense mechanism against ROS includes Superoxide dismutase (SOD), catalase, glutathione peroxidase and a glutathione (GSH) is one of the major constituents of cellular defense mechanisms against oxidative stress.9 Tissue ischemia after burn depletes intracellular antioxidant levels10 and induces apoptosis in fibroblasts during wound healing.11 It was demonstrated that after burn injury there was an increase in the total proteolytic activity in human burn skin with increased activity of MMP-2 and 9.12 Elevated levels of MMP-2 and 9 also have been reported in human burn wound fluid.13
The relationships between wound pH and healing of wounds was previously investigated and was found that weak acidic environments significantly inhibit protease activity and may potentially promote wound healing.14 Burn trauma produces a significant inflammatory response15 and up-regulation of the inducible nitric oxide synthatase (iNOS) leads to excess NO production, which contributes to post-burn cellular damage.16 Despite recent advances in the management of burn care, thermal trauma is difficult to manage.17
Negative pressure wound therapy (NPWT) is non-invasive adjuvant therapy to treat burn wounds. It is claimed to increase blood supply to the wound and to induce the formation of granulation tissue, causes rapid healing through stimulating of re-epithelialization, proliferation of endothelial, fibroblasts cells thereby angiogenesis and collagen reorganization, decreases bacterial load, eliminates exudate and slough, reduces oedema and swelling, and maintains an optimal moist environment for proper healing.18
The clinical evidence supporting the use of continuous NPWT on burn wounds has been based largely on clinician perception, case series, small cohort studies and weakly powered randomised trials that constitute a substantial number of publications but an overall low amount of evidence. Evidences are lacking on use of intermittent negative pressure dressing that is more economical and acceptable clinically than continuous NPWT.19 In this survey, a prospective randomized study was carried out to compare wounds treated with limited access dressing (cycle of 30 minutes suction and 3 1/2 hours rest) and with conventional dressing biochemically and histologically to assess the rates of wound healing.
MATERIALS AND METHODS
The study is prospective randomized clinical trial which was carried out in the Department of Plastic surgery and Burns, Kasturba Hospital, Manipal, India. Institutional Ethics committee of Kasturba Medical College and Hospital, Manipal University approved the study protocol and study was registered to Clinical Trials Registry India, (Government of India) - CTRI number: CTRI/2015/01/005419. Informed consent was obtained from all patients or their next of kin before inclusion into the study.
Fifty patients of age 12 to 45 years (Mean age of 38.5 years) ailing from burn injury less than 40% total body surface area (TBSA) were enrolled in to the study. After examined inclusion criteria (less than 40% TBSA burn wounds) and exclusion criteria (Patients with collagen disorders, diabetic patients, leprosy patients, pregnant women, liver cirrhosis, HIV +ve ), they were randomly allocated to the limited access dressing (LAD) group (n=25), and conventional dressing group (n=25) (Figure 1) using simple randomization method, generating tables of random numbers through www.random.org. Numbers were assigned to a treatment group and sealed in opaque envelopes containing labelled paper with treatment and patient ID. Patient demographics and wound characterisation at baseline were shown in Table 1. On 0th day biopsies taken from both groups, LAD group- patients were treated using LAD with intermittent negative pressure therapy. Conventional closed dressing group was treated with daily dressing changes using squeezed 5% povidoneiodine gauze which is a routine protocol in our burn unit.
Fig. 1.
Consort flow chart.
Table 1.
Patient demographics and wound characterisation at baseline
| LAD group | Conventional group | |
|---|---|---|
| Number of patients | 25 | 25 |
| Age (Mean±SD) Age range (years) |
32.3±9.50 12-45 |
34.5±13.0 17-43 |
| Mean wound size (cm2) | 19 (range=9-36) | 18 (range=10-39) |
| Mechanism of injury (no.) Flames Scalds |
23 | 22 |
| 2 | 3 | |
| Female | 9 (36.0 %) | 10 (40.0 %) |
| Male | 16 (64.0 %) | 15 (60.0 %) |
LAD: Limited access dressing; SD: Standard deviation
Standard L-Hydroxyproline, bovine serum albumin (BSA), standard glutathione, l-chloro-2,4-dinitrobenzene, nictoinamide adenine dinucleotide phosphate (reduced form), glutathione reductase (type III, Baker’s yeast), cumene hydrogen peroxide (Sigma–Aldrich, St. Louis, MO, USA), thiobarbituric acid, tri-chloroacetic acid, 1,1,3,3-tetramethoxypropane, N-ethylmaleimide (NEM), (SD Fine Chemicals Ltd, Boisar), MMP-2 Elisa kit (Uscn Life Science Inc. USA), pH paper strips, ortho phosphoric acid, (Merck, India), graded alcohol, Van Gieson stain (Sigma Aldrich Chemical Company, Bengaluru, India).
The granulation tissues were dried at 60°C for 24 hr. It was weighed and kept in glass stoppered test tubes. 6NHCl was added in each tube so that it contained 40 mg of the dried granulation tissue per ml of acid. The tubes were kept on boiling water bath for 24 h for hydrolysis. The hydrolysate was then cooled and excess of acid was neutralised by 10N NaOH using phenolphthalein/ Methyl red as an indicator. The volume of neutral hydrolysate was diluted to a concentration of 20 mg/ml of dried granulation tissue in the final hydrolysate with distilled water.20 The hydrolysate was used for the estimation of hydroxyproline and hexosamine
Granulation tissue samples wet weight was noted and homogenized by Rotex homogenizer in ice-cold 0.2 M phosphate buffer (pH=7.4). Homogenates were centrifuged at 15,000 rpm for 30 min in cooling centrifuge and supernatant was then used for determine total protein, antioxidants (GSH, GPx), oxidative biomarker (MDA). For MMP-2 assay, tissues we rinsed in ice-old PBS (0.1 mol/L, pH=7.0-7.2) to remove excess blood thoroughly and weighed. Minced the tissues to small pieces and homogenized them in 5-10 mL of PBS with a glass homogenizer on ice. The resulting suspension was sonicated with an ultrasonic cell disrupter or subjected to two freeze-thaw cycles to further break the cell membranes. After that, the homogenates were centrifugated for 5 minutes at 50×g, to remove the supernatant, aliquot and store at ≤-80oC.
For Nitric oxide (NO) assay tissue was homogenized in isotonic solution of PBS containing 10 mM N-ethylmaleimide (NEM) and 2.5 mM EDTA. The addition of NEM/EDTA served the purpose of blocking SH-groups and inhibiting transition metal-catalysed transnitrosation reactions, preventing artificial nitrosation, as well as thiolate and ascorbate-mediated degradation of endogenous RSNOs and nitrite. Protein concentration was determined according to Lowry method using purified bovine serum albumin as standard. Tissue hydrolysate was prepared and used for estimation of hydroxyproline,20 hexosamine,21 total protein,22 reduced glutathione (GSH),23 glutathione peroxidase (GPx),24 malondialdehyde (MDA),25 matrix metalloproteinase-2 (MMP-2),26 wound surface pH,27 and nitric oxide (NO).28
Wound biopsies on 0th day and 10th were collected, then fixed in 10% buffered formalin, dehydrated through graded alcohol series (50%, 70%, 90% and 100%), cleared in xylene and embedded (Leica EG1150 H) in paraffin wax (mp=560C). Serial sections of 5-µm thickness were cut using microtome (Leica RM2255).The slides were stained with Van Gieson stain for the analyses. Each slide was given a histopathological score ranging from 1 to 12, with 1 corresponding to no healing and 12 corresponding to a completely reepithelialised wound.29 The scoring was based on the degree of cellular invasion, granulation tissue formation, vascularity, and reepithelialization. The histopathological score was assigned by investigator; code describing treatment to the patients was broken after the scoring was completed.
Statistical analysis for biochemical parameters was performed by Student’s t-test and data were expressed as mean±standard deviation (SD). Histopathological score between the groups was performed by Mann-Whitney U test using the SPSS software (15th version package, Chicago, IL, USA). The data were expressed as median and interquartile range (IQR). A p value <0.05 was considered as significant. When appropriate, statistical uncertainty was expressed by the 95% confidence levels.
RESULTS
Hydroxyproline (77.2±23.2 µg/mg dry tissue weight), hexosamine (9.4±2.8 µg/mg dry tissue weight), total protein (14.5±8.1 mg/g wet tissue weight), GSH levels (7.2±2.3 µg/mg protein), and activity of GPx (145.9±74.7 µmol/min/mg protein) were significantly higher in LAD group in comparison to the conventional group (29.7±14.8 µg/mg dry tissue weight, p=0.002 (9.1±1.96 µg/mg dry tissue weight, p=0.038), (9.21±4.2 mg/g wet tissue weight, p=0.013), (6.2±2.3 µg/mg tissue protein, p=0.039), and (115.9±68.8 µmol/min/mg tissue protein, p=0.001), respectively (Table 2).
Table 2.
Results of biochemical parameters of LAD and conventional dressing groups
| Parameters |
LAD Group (n=25)
[Mean±SD] |
Conventional dressing group (n=25)
[Mean±SD] |
p value | ||||
|---|---|---|---|---|---|---|---|
| Day 0 th | Day 10 th | Day (0 th -10 th ) | Day 0 th | Day 10 th | Day (0 th -10 th ) | ||
| Hydroxyproline (µg/mg of dry weight of tissue) | 62.1±12.6 | 139.7±25.7 | 77.2±23.2 | 67.5±10.4 | 100.5±18.0 | 32.7±14.8 | 0.002* |
| Hexosamine (µg/mg of dry weight of tissue) | 6.79±1.5 | 15.7±2.5 | 9.4±2.8 | 7.0±1.4 | 15.4±2.5 | 9.1±1.96 | 0.038* |
| Total protein (mg/g of wet weight of tissue) | 11.4±3.0 | 25.8±7.4 | 14.5±8.1 | 12.0±2.5 | 21.5±5.1 | 9.21±4.2 | 0.013* |
| GSH (µg/mg tissue protein) | 15.9±4.3 | 22.9±3.3 | 7.2±2.3 | 14.9±3.5 | 21.2±3.5 | 6.2±2.3 | 0.039* |
| GPx (µMoles NADPH oxidized/min/mg tissue protein) | 269.6±65.8 | 414.9±70.5 | 145.7±74.7 | 280±83.2 | 384.1±90.1 | 115.9±68.8 | 0.001* |
| MDA ( nmole/mg tissue protein) | 20.1±4.45 | 6.79±2.24 | 13.6±6.62 | 19.7±4.17 | 9.45±4.0 | 9.86±3.35 | 0.006* |
| MMP-2 (ng/mg tissue protein) | 0.96±0.80 | 0.66±0.42 | 0.54±0.45 | 0.77±0.52 | 0.56±0.49 | 0.41±0.34 | 0.024* |
| Wound surface pH | 8.1±0.35 | 7.5±0.33 | 0.86±0.48 | 8.1±0.35 | 7.9±0.57 | 0.25±0.16 | 0.011* |
| Nitric oxide (µmole/mg tissue) | 4.08±1.5 | 3.0±1.5 | 1.02±0.35 | 3.6±1.4 | 2.8±1.6 | 0.8±0.53 | 0.140 |
SD: Standard deviation; LAD: Limited access dressing; GSH: Reduced glutathione; GPx: Glutathione peroxidase; MDA: Malondialdhyde; NADPH: Nicotinamideadenine dinucleotide phosphate; MMP-2: Matrixmetalloproteinase-2
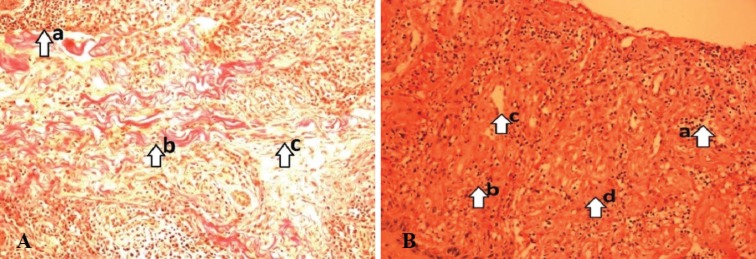
MDA was significantly higher in conventional dressing group (10.6±3.8 nmol/mg protein) when compared to the LAD group (6.5±2.24 nmol/mg protein, p=0.006). A significant decrease was noticed in the level of MMP-2 (0.54±0.45 ng/mg protein), wound surface pH (0.86±0.48), NO (1.02±0.35) when compared to the conventional group (0.41±0.34 ng/mg protein, p=0.024), (0.16±0.25, p=0.011), and (0.8±0.53, p=0.140), respectively (Table 3). On day 0th, both the LAD group [Figure 2A (VG)] and conventional group showed a necrotic tissue with increased cellular infiltration [Figure 3A (VG)]. On day 10, LAD group showed an increase in extracellular matrix (ECM) deposition, decrease in cellular infiltration and increased angiogenesis [Figure 2B (VG)] when compared to conventional dressing group [Figure 3B (VG)].
Table 3.
Results of histological study of LAD and conventional dressing groups
| Group | N |
Day 0
Median (Q 1 , Q 3 ) |
Day 10
Median (Q 1 , Q 3 ) |
Day (0-10) Median
(Q 1 , Q 3 ) |
Wilcoxon Mann Whitney U test |
|---|---|---|---|---|---|
| p value | |||||
| LAD | 25 | 5 (3 , 7) | 9 (7, 9) | 3 (2, 4.25) | 0.008 |
| Conventional dressing group | 25 | 4 (3, 6) | 6 (5, 7.25) | 2 (1.75, 4) |
A Mann Whitney U test was performed to determine the significant difference in the average (Median) histological score of LAD and conventional group. The result indicates that there was a significant difference in the average score of the two groups (p=0.008). Median (Q1, Q3): Median interquartile range; LAD: Limited access dressing
Fig. 2.
A: PreLAD (0th day) (arrow) numerous neutrophils infiltration (a), minimum number of fibroblasts (b), fewer collagen fibers (c). (Photograph with Olympus PM20 photomicroscope 20X magnification). [VG stain]. B: PostLAD (10th day) (arrow) maximum number of fibroblasts(a), fewer inflammatory cells(b), More proliferating blood capillaries. (neovascularization) (c), Collagen bundles organized well between the cells (d). (Photograph with Olympus PM20 photomicroscope 20X magnification; VG stain
Fig. 3.
A: Pre conventional (0th day) - (arrow) numerous neutrophils infiltration (a), Minimum number of fibroblasts (b), poor collagen fibers (c). (Photograph with Olympus PM20 photomicroscope 20X magnification; VG stain). B: Post conventional (10th day) (arrow) numerous neutrophils infiltration (a), poorly developed matrix minimum number of fibroblasts (b), Poor collagen bundles (c). (Photograph with Olympus PM20 photomicroscope 20X magnification; VG stain
On day 0, histological score in LAD versus conventional dressing group was 5 (Q1, Q3) (3, 7) and 4 (3, 6), respectively. On day 10, histological score in LAD versus conventional dressing group was 9 (Q1, Q3) (7, 9) versus 6 (5, 7.25). The test was performed for the difference of scores of day 10 and day 0 (LAD versus conventional dressing group) which was 3 (Q1, Q3) (2, 4.25) versus 2 (1.75, 4) (p=0.008). Consistent with these findings, the histopathologic score of wounds from LAD group was significantly higher with a decreased cellular filtration and increased fibroblasts, collagen deposition, increased the number of capillary vessels per high power field than conventional dressing group.
DISCUSSION
Thermal burn initiates systemic inflammatory reactions producing burn toxins, oxygen radicals and finally leads to peroxidation. The relationship between the amount of products of oxidative metabolism and natural scavengers of free radicals determines the outcome of tissue damage.30 This can be determined by assessment of antioxidants (GSH, GPx). The study on lipid peroxidation product, the malondialdehyde levels gives us the extent of free radical damage in the cells.31
The formation of well-vascularized granulation tissue in the wound bed is a prerequisite for burn wound healing.32 Granulation tissue composed of fibroblasts, collagen, edema, and new small blood vessels. Extracellular matrix of granulation tissue contains most dominantly collagen which assists the wound and plays an important role in homeostasis.33 Collagen not only confers strength and integrity to the tissue matrix but also plays an important role in homeostasis and epithelialization in wound healing. Collagen is composed of the amino acid, hydroxyproline, has been used as a biochemical marker for tissue collagen.34
Various studies on human wound models shown that dressing technique like moist wound dressing35 and continuous NPWT36 increased the level of hydroxyproline content in wound granulation tissue. In the present study, the mean±SD hydroxyproline content in LAD group was 77.2±23.2 µg/mg of dry weight of tissue which was significantly higher than conventional dressing group (29.7±14.8, p=0.002). Hexosamine is an important part of the extracellular matrix and one of the main glycosaminoglycan secreted during tissue repair. Increased hexosamine content reflects the stabilization of collagen molecules by enhancing electrostatic and ionic interactions with it, which in turn reflects remodeling of the new extracellular matrix produced.37 Hence, enhanced hydroxyproline and hexosamine synthesis provides strength to repaired tissue and stimulates healing. In the present study, the mean hexosamine (±SD) in LAD vs conventional dressing group was 9.4±2.8 vs 9.1±1.96 (p=0.038).
Protein is another important constituent of extracellular matrix. High protein content confirms positive effects towards cellular growth and proliferation, granulation tissue formation and epithelisation.38 In the present study, mean±SD total protein was significantly higher in LAD group in comparison to the conventional dressing group (14.5±8.1 Vs 9.21±4.2 mg/g wet tissue weight; p=0.013). GSH (tripeptide) and enzymatic antioxidants which are normally present at high concentrations in intracellular, which constitutes the major reducing capacity of the cytoplasm and protects the cellular system against the toxic effects of lipid peroxidation.39 In the present study, LAD vs conventional dressing group for mean±SD GSH was 7.2±2.3 vs 6.2±2.3 (p=0.039), and for GPx was 145.7±74.7 vs 115.9±68.8 (p=0.001). MDA is produced during the attack of free radicals to membrane lipoproteins and polyunsaturated fatty acids.40 In the present study, MDA significantly decreased in LAD group (10.6±3.8 nmole/mg protein) compared to conventional group (6.5±2.24 nmol/mg protein, p=0.006).
Burn injury leads to an increase in the total proteolytic activity in human burn skin. MMP-2 has ability to proteolytically degrade type I, IV and V collagens, elastin and vitronectin, induces apoptosis in endothelial cells and inhibits neovascularisation.41 Several studies have shown in chronic wounds, including burn wounds, elevated expression and activation of MMPs -2 and 9, which may result in chronic tissue turnover and failed wound closure. In our study, LAD vs conventional group mean±SD for MMP-2 was 0.54±0.45 vs 0.41±0.34 (p=0.024).
The pH environment of chronic wounds has been recorded within the range of 7.15–8.9.42 This variability is representative of both healing and non-healing wounds. Both acute and chronic wounds with an elevated alkaline pH have demonstrated lower rates of healing than wounds in which the pH is closer to neutral.42 Consistent with these results in the present study mean±SD of wound surface pH (0-10th day) LAD vs conventional group on the 0th day was 0.86±0.48 vs 0.16±0.25 (p=0.011] and on the 10th day was 7.5±0.33 vs 7.9±0.57.
Burn injury was associated with increased inflammatory response confirmed by increased serum nitric oxide levels. Overproduction of NO has harmful effects on vascular regulation.43 NO also interacts with the superoxide radical to yield peroxynitrite and other RNS, which are highly reactive. Peroxynitrite produces cellular death by enhancing DNA single strand breakage, cellular energy depletion and cellular necrosis.44 Consistent with above results in our study, NO mean±SD in LAD vs conventional dressing group was 1.02±0.35 vs 0.8±0.53 (p=0.140).
Various studies have shown that NPWT increase in rate of granulation tissue formation, angiogenesis, and decreased inflammation.45 Wound healing was markedly improved in LAD group (8.70±0.21) after 10 days of treatment when compared to the conventional dressing group (6.24±0.40). The Median (Q1, Q3) on 0th –10th day (LAD vs conventional dressing group was 3 (2, 4.25) vs 2 (1.75, 4; p=0.008).
LAD exerts its beneficial effects on burn wound healing by significantally increasing ground substance, antioxidants and reducing oxidative stress, MMP-2, wound surface pH, and NO. An increase in ECM deposition and angiogenesis and decrease in necrotic tissue, and amount of inflammatory infiltrate were noted when compared to the conventional dressing group. Results of our biochemical and histopathological studies supports the clinical observation indicating better clinical effect of LAD on granulation tissue formation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Tanideh N, Rokhsari P, Mehrabani D, Mohammadi Samani S, Sabet Sarvestani F, Ashraf MJ, Koohi Hosseinabadi O, Shamsian Sh, Ahmadi N. The healing effect of licorice on Pseudomonas aeruginosa infected burn wounds in experimental rat model. World J Plast Surg. 2014;3:99–106. [PMC free article] [PubMed] [Google Scholar]
- 2.Sener G, Sehirli AO, Satiroğlu H, Keyer-Uysal M, C Yeğen B. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns. 2002;28:419–25. doi: 10.1016/s0305-4179(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi AA, Amini M, Mehrabani D, Kiani Z, Seddigh A. A survey on 30 months electrical burns in Shiraz University of Medical Sciences Burn Hospital. Burns. 2008;34:111–3. doi: 10.1016/j.burns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Youn YK, LaLonde C, Demling R. The role of mediators in the response to thermal injury. World J Surg. 1992;16:30–6. doi: 10.1007/BF02067111. [DOI] [PubMed] [Google Scholar]
- 5.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Gürbüz V, Corak A, Yeğen BC, Kurtel H, Alican I. Oxidative organ damage in a rat model of thermal injury: the effect of cyclosporin A. Burns. 1997;23:37–42. doi: 10.1016/s0305-4179(96)00072-1. [DOI] [PubMed] [Google Scholar]
- 7.Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999;25:113–8. doi: 10.1016/s0305-4179(98)00124-7. [DOI] [PubMed] [Google Scholar]
- 8.Demling R, Ikegami K, Lalonde C. Increased lipid peroxidation and decreased antioxidant activity correspond with death after smoke exposure in the rat. J Burn Care Rehabil. 1995;16:104–10. doi: 10.1097/00004630-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 10.Sener G, Sehirli AO, Satiroglu H, Keyer-Uysal M, C Yegen B. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns. 2002;28:419–25. doi: 10.1016/s0305-4179(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi A1, Aoshiba K, Nagai A. Apoptosis of wound fibroblasts induced by oxidative stress. Exp Lung Res. 2002;28:275–84. doi: 10.1080/01902140252964366. [DOI] [PubMed] [Google Scholar]
- 12.StricklinGP , Nanney LB. Immunolocalization of collagenase andTIMP in healing human burn wounds. J Invest Dermatol. 1994;103:488–92. doi: 10.1111/1523-1747.ep12395601. [DOI] [PubMed] [Google Scholar]
- 13.Neely AN, Brown RL, Clendening CE, Orloff MM, Gardner J, Greenhalgh DG. Proteolytic activity in human burn wounds. Wound Repair Regen. 1997;5:302–9. doi: 10.1046/j.1524-475X.1997.50404.x. [DOI] [PubMed] [Google Scholar]
- 14.Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms and antimicrobial efficacy. Wound Repair Regen. 2014;22:174–86. doi: 10.1111/wrr.12125. [DOI] [PubMed] [Google Scholar]
- 15.Preiser JC, Reper P, Vlasselaer D, Vray B, Zhang H, Metz G, Vanderkelen A, Vincent JL. Nitric oxide production is increased in patients after burn injury. J Trauma. 1996;40:368–71. doi: 10.1097/00005373-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen SM, Wurster SH, Nanney LB. Expression of inducible nitric oxide synthase in human burn wounds. Wound Repair Regen. 1998;6:142–8. doi: 10.1046/j.1524-475x.1998.60208.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanideh N, Haddadi MH, Rokni-Hosseini MH, Hossienzadeh M, Mehrabani D, Sayehmiri K, Koohi-Hossienabadi O. The healing effect of scrophularia striata on experimental burn wounds infected to pseudomonas aeruginosa in rat. World J Plast Surg. 2015;4:16–23. [PMC free article] [PubMed] [Google Scholar]
- 18.González Alaña I, Torrero López JV, Martín Playá P, Gabilondo Zubizarreta FJ. Combined use of negative pressure wound therapy and Integra® to treat complex defects in lower extremities after burns. Ann Burns Fire Disasters. 2013;26:90–3. [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P. Exploiting potency of negative pressure in wound dressing using limited access dressing and suction-assisted dressing. Indian J Plast Surg. 2012;45:302–15. doi: 10.4103/0970-0358.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuman Re, Logan Ma. The determination of collagen and elastin in the tissues. J Biol Chem. 1950;186:549–56. [PubMed] [Google Scholar]
- 21.Johansen PG, Marshall RD. Neuberger A: Carbohydrates in protein 2 The hexose, hexosamine, acetyl and amide-nitrogen content of hen’s-egg albumin. Biochem J. 1960;77:239–47. doi: 10.1042/bj0770239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough MH, Farr L, Randell RJ. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;93:265–75. [PubMed] [Google Scholar]
- 23.Beutler E, Duron O, Kefly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 24.Paglia DE, Valentine , WN Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 26.Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J Invest Dermatol. 1994;103:660–4. doi: 10.1111/1523-1747.ep12398424. [DOI] [PubMed] [Google Scholar]
- 27.Shukla VK, Shukla D, Tiwary SK, Agrawal S, Rastogi A. Evaluation of pH measurement as a method of wound assessment. J Wound Care. 2007;16:291–4. doi: 10.12968/jowc.2007.16.7.27062. [DOI] [PubMed] [Google Scholar]
- 28.Talas DU, Nayci A, Polat G, Atis S, Comelekoglu U, Bagdatoglu OT. The effects of dexamethasone on lipid peroxidation and nitric oxide levels on the healing of tracheal anastomoses: an experimental study in rats. Pharmacol Res. 2002;46:265–71. doi: 10.1016/s1043-6618(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 29.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–46. [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrabani D, Farjam M, Geramizadeh B, Tanideh N, Amini M, Panjehshahin MR. The healing effect of curcumin on burn wounds in rat. World J Plast Surg. 2015;4:29–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Latha B, Babu M. The involvement of free radicals in burn injury: a review. Burns. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 32.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, Zaghi AH, Khalili N. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 2009;9:23. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. 2007;5:23–6. doi: 10.1016/j.ijsu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Ghaffari A, Manafi A, Moghimi HR. Clindamycin phosphate absorption from nanoliposomal formulations through third-degree burn eschar. World J Plast Surg. 2015;4:145–152. [PMC free article] [PubMed] [Google Scholar]
- 35.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–42. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Molnar JA. Applications of negative pressure wound therapy to thermal injury. Ostomy Wound Manage. 2004;50:17–19. [PubMed] [Google Scholar]
- 37.Ricard Blum S, Ruggiero F. The collagen super family: from the extracellular matrix to the cell membrane. Pathol Biol. 2005;53:430–42. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Amini M, Kherad M, Mehrabani D, Azarpira N, Panjehshahin MR, Tanideh N. Effect of plantago major on burn wound healing in rat. J Appl Anim Res. 2010;37:53–6. [Google Scholar]
- 39.Ragini V, Prasad KVSRG, Bharathi K. Antidiabetic and antioxidant activity of Shorea tumbuggaia Rox. Int J Innov Pharm Res. 2011;2:113–21. [Google Scholar]
- 40.Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008;43:154–8. doi: 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro S, Khodalev O, Bitterman H, Auslender R, Lahat N. Different activation forms of MMP-2 oppositely affect the fate of endothelial cells. Am J Physiol Cell Physiol. 2010;298:942–51. doi: 10.1152/ajpcell.00305.2009. [DOI] [PubMed] [Google Scholar]
- 42.Gethin GT, Cowman S, Conroy RM. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int Wound J. 2008;5:185–94. doi: 10.1111/j.1742-481X.2007.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Soejima K, Traber LD, Schmalstieg FC, Halhawkins , Jodoin JM, Szabo C et al , Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163:745–52. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 44.Korhonen R, Lahti A, Kankaanranta K, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–9. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 45.Sinha K, Chauhan VD, Maheshwari R, Chauhan N, Rajan M, Agrawal A. Vacuum Assisted Closure Therapy versus Standard Wound Therapy for Open Musculoskeletal Injuries. Adv Orthop. 2013;2013:245940. doi: 10.1155/2013/245940. [DOI] [PMC free article] [PubMed] [Google Scholar]