Abstract
Considering that lead caused a lot of health problems in the world, the present study was carried out to investigate the protective effect of captopril as antioxidants to reduce liver and spleen toxicity induced by lead. Animals were divided into 3 groups, the 1st group served as control group, the 2nd group received 20 mg/kg of lead acetate and the 3rd group received 50 mg/kg of captopril one hour prior to lead administration for 5 days. Results showed that lead intake caused severe alterations in the liver and spleen manifested by hepatocytes degeneration, leukocytic infiltration, fibrosis in liver and moderate to severe liver pathological score. Spleen showed ill-defined architecture, presence of large macrophages and lymphoid necrosis. Administration of captopril reduced hepatotoxicity, liver fibrosis and decrease in pathological scoring system. Moreover, reduced toxicity in spleen is represented by reduction in necrotic areas, more or less healthy lymphoid follicles and decreasing in pathological scoring system.
Keywords: Captopril, Mice, Liver, Spleen
1. Introduction
Lead is a naturally occurring bluish-gray metal found in small amounts in the Earth’s crust and can be found in all parts of our environment (Gupta, 2007). Lead is found in our food, water, air and soil. Lead emitted by smelters and boilers that burn used motor oil is frequently deposited in the soil, where it is taken up by crops (Chiras, 2009). Lead is known as an enzymatic toxicant, is neurotoxic, hemato and cardiovascular toxic, nephrotoxic, immunotoxic, carcinogenic, teratogenic and mutagenic (Kiran et al., 2009, Moreira and Moreira, 2004). Lead damages cellular materials, alters cellular genetics and produces oxidative damage. It causes hyperproduction of free radicals and decreased availability of anti oxidant reserves to respond to the resultant damage. It also interrupts enzyme activation and competitively inhibits trace mineral absorption. Lead binds to sulfhydryl proteins (interrupting structural protein synthesis), alters calcium homeostasis and lowers the levels of available sulfhydryl antioxidant reserves in the body (Lynpatrick, 2006). The toxicity of lead is closely related to age, sex, route of exposure level of intake, solubility, metal oxidation state, retention percentage, duration of exposure, frequency of intake, absorption rate, mechanisms and efficiency of excretion. Lead has been associated with various forms of cancer, nephrotoxicity, central nervous system effects and cardiovascular diseases in humans (Pitot and Dragan, 1996).
Captopril (D-3-mercapto-2-methyl-propanoyl-L-proline) is an angiotension-converting enzyme (ACE) inhibitor. Besides, its role as a treatment for hypertension (Sultana et al., 2007), it is commonly used as a cardioprotective drug (Khattab et al., 2005). Like other ACE inhibitors, captopril inhibits the conversion of angiotensin I, a relatively inactive molecule, to angiotensin II which is the major mediator of vasoconstriction and volume expansion induced by the renin–angiotensin system. Captopril, an inhibitor of angiotensin converting enzyme (ACE), has also been postulated as a free radical scavenger because of its terminal sulfhydryl group (Bagchi et al., 1989, Andreoli, 1993). Some in vitro studies indicate that captopril functions as an antioxidant both by scavenging ROS and by increasing the activities of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase (Westlin and Mullane, 1988, Kojsova et al., 2006). Captopril has been shown to decrease serum lipid peroxide concentrations in diabetic patients (Ha and Kim, 1992).
Therefore, the aim of the present work is to evaluate the possibility of reducing toxic effects induced by lead in the liver and spleen by captopril and investigate its antifibrotic effects.
2. Material and methods
2.1. Design of experiment
Thirty male Swiss albino mice (25 ± 3 g) Mice were housed in polypropylene cages inside a well-ventilated room at 22 ± 1 C and 12-h periods of light and dark, with free access to clean water and commercial mice food The experiments were approved by state authorities and followed Saudi Arabian rules for animal protection.
Mice were randomly divided into three groups, ten mice per each group. First group served as control group received saline, second group received oral administration of 20 mg/kg of lead acetate by gavage, and third received oral administration of 50 mg/kg of captopril one hour prior to administration of 20 mg/kg of lead acetate for five days, All animals were sacrificed one day-post to the end of experiment.
2.2. Liver and spleen index
At the end of the experimental period, each mouse was weighed, liver and spleen were then removed and weighed. Finally, the liver and spleen indices were calculated by dividing the weight of liver or spleen by the body weight and then multiplying by 100 and the results were statistically analyzed by SPSS 16.
2.3. Histopathological analysis
2.3.1. Histological preparation
Livers and spleens were collected and cut into small pieces, fixed in 10% neutral buffered formalin. Following fixation, specimens were dehydrated, embedded in wax, and then sectioned to 5 μm thickness. Sections were stained with hematoxylin and eosin. Also, other sections were stained with Masson trichrome stain according to Drury and Wallington (1980).
2.3.2. Pathological scoring system for changes in liver and spleen architecture
Liver sections stained with HE were examined for pathological score of the following criteria: ballooning, inflammation, apoptotic cells and fibrosis. Scoring values were registered according to Table 2. Spleen sections were examined for pathological score of the following criteria: lymphoid necrosis and red pulp expansion and scoring values registered according to Table 2 (Klopfleisch, 2013).
Table 2.
Scoring criteria for the evaluation of histopathological changes in liver architecture and in spleen architecture.
| Changes in liver architecture | |
| Ballooning degeneration | |
| Score 0 | No ballooning degeneration |
| Score 1+ | Minimal enlargement in few hepatocytes |
| Score 2+ | Mild enlargement in many hepatocytes |
| Score 3+ | Moderate enlargement in most hepatocytes |
| Score 4+ | Severe enlargement in most hepatocytes |
| Inflammation | |
| Score 0 | No inflammatory foci |
| Score 1+ | 1 inflammatory foci per 200 hpf |
| Score 2+ | 2–4 inflammatory foci per 200 hpf |
| Score 3+ | >4 inflammatory foci per 200 hpf |
| Apoptotic cells | |
| Score 0 | No apoptotic cells |
| Score 1+ | Few apoptotic cells |
| Fibrosis | |
| Score 0 | No fibrosis |
| Score 1+ | Portal/sinusoidal minimal fibrosis |
| Score 2+ | Portal/sinusoidal mild fibrosis |
| Score 3+ | Bridging fibrosis |
| Score 4+ | Cirrhosis |
| Changes in spleen architecture | |
| Lymphoid necrosis | |
| Score 0 | No necrotic foci |
| Score 1+ | <5% necrotic foci per 200 hpf (mini) |
| Score 2+ | 5–25 necrotic foci per 200 hpf (mild) |
| Score 3+ | 25–50 necrotic foci per 200 hpf (moderate) |
| Score 4+ | >50% necrotic foci per 200 hpf (severe) |
| Red pulp expansion | |
| Score 0 | No expansion |
| Score 1+ | Minimal expansion |
| Score 2+ | Mild expansion |
| Score 3+ | Moderate expansion |
| Score 4+ | Severe expansion |
2.3.3. Spleen lymphoid follicle analysis
Lymphoid follicles areas were measured by microscope measurement system (Motic-2000) in 20 different fields per section, the number of macrophages in peri-outer zone of follicles was counted 200xfield and areas of macrophages were measured by microscope system.
2.4. Statistical analysis
A one-way ANOVA was carried out, and the statistical comparisons among the groups were performed with Duncan’s test using a statistical package program (SPSS version 16.0). All P values are two-tailed and P < 0.05 was considered as significant for all statistical analyses in this study.
3. Results
3.1. Liver and spleen index
Liver index of mice group receiving lead acetate showed an insignificant increase compared to the control group, whereas the mice group receiving lead acetate and captopril showed insignificant differences compared to the control group (Table 1) and insignificant decrease compared to the group receiving lead acetate only. Spleen index of mice group received lead acetate and the other one received lead acetate and captopril showed insignificant increase compared to control group (Table 1).
Table 1.
Liver and spleen index in control, lead and lead treated with captopril groups.
| Index | Control | Lead | Lead and captopril |
|---|---|---|---|
| Liver index | 6.4 ± 0.2 | 8.04 ± 0.8 | 5.9 ± 0.6 |
| Spleen index | 0.65 ± 0.09 | 0.8 ± 0.1 | 0.72 ± 0.08 |
Data = Mean ± SEM (standard error of means).
3.2. Histopathological analysis
3.2.1. Histological examination
3.2.1.1. Liver
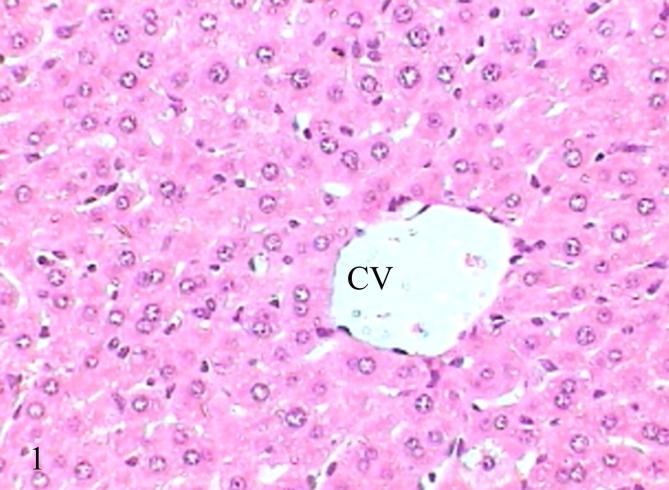
Non-treated mice liver served as control investigated for the purpose of comparison showed the normal structure of liver represented by hepatic lobules with semi circular central vein in the center of the lobule, hepatic cells were arranged in strands, blood sinusoids in between the hepatic cells and portal canals at the periphery of hepatic lobules. The portal canal comprises branches of the portal vein, hepatic artery and bile duct, often also with a lymphatic vessel lying in a small amount of connective tissue (Fig. 1).
Figure 1.
Photomicrograph of control mice liver showed normal liver consists of central vein and hepatocytes (H&E-100).
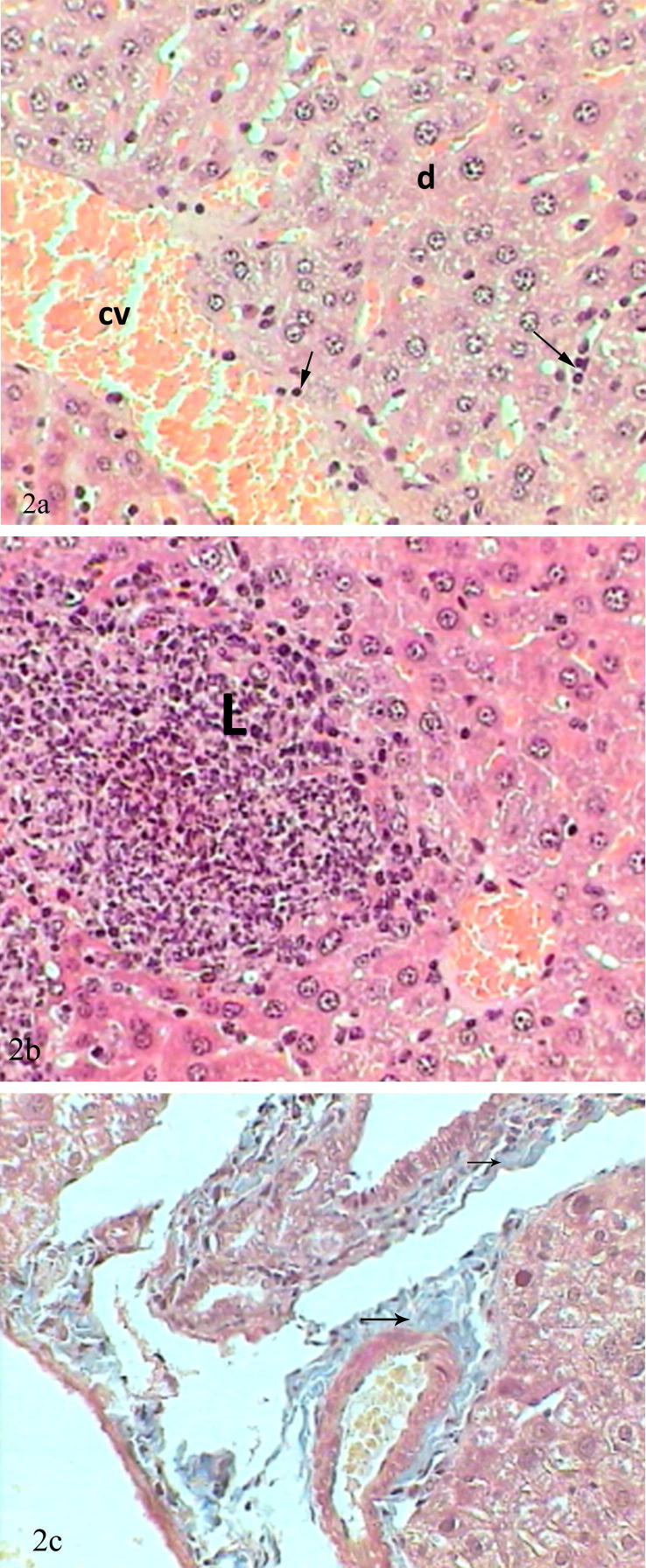
Livers of mice received oral administration of 20 mg/kg/bw of lead acetate showed marked changes in the hepatic tissue represented by congested central veins, dilatation in blood sinusoids with abundant kupffer cells, hepatic cells suffered from degeneration, its nuclei looked swollen and some of them have fragmented chromatins, whereas others showed absence of chromatin (Fig. 2a). Other sections of treated liver mice with lead acetate showed large aggregations of leukocytic infiltration (Fig. 2b), moreover, appearance of precipitation of collagenous fibers around bile duct stained blue by Masson’trichrome (Fig. 2c).
Figure 2.
(a) Photomicrograph of mice liver treated with lead acetate showed congested and dilated central vein (cv), scattered inflammatory cells (arrows) and complete degeneration of cell nuclei (d) (H&E-Mag ×400). (b) Photomicrograph of mice liver treated with lead acetate showed aggregations of lymphocytic infiltration (L) (H&E-Mag ×400). (c) Photomicrograph of mice liver treated with lead acetate showed blue stained layers of collagenous fibers (F) surrounded dilated central vein (cv) (Mtr-Mag ×400).
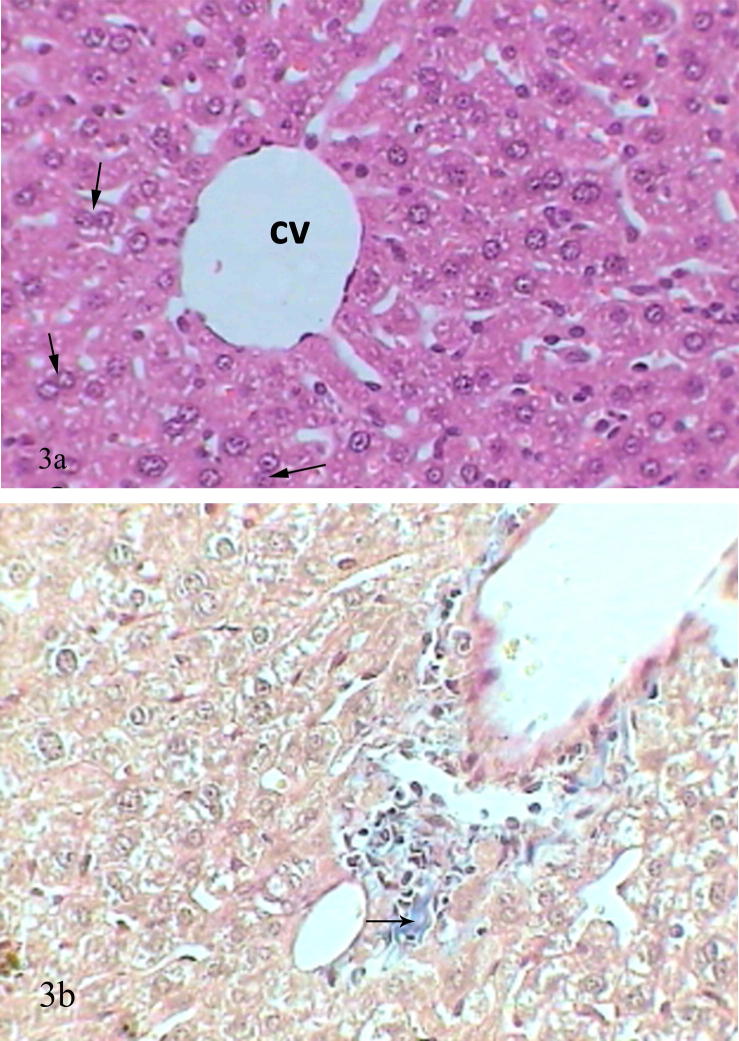
Livers of mice treated with captopril before oral administration of lead acetate revealed healthier sections compared with previous groups represented by more or less healthy hepatic cells with darkly stained nuclei, binucleated cells appeared as an evidence of regeneration, kupfer cells and scattered leukocytes were abundant in the sinusoids. Some sections showed dilated vein surrounded by leukocytic infiltration and some necrotic foci (Fig. 3a). Tissues stained by Trichrome showed small bundles of collagenous fibers were present in the liver tissue, it was obvious that captopril had antifibrotic effect (Fig. 3b).
Figure 3.
(a) Photomicrograph of mice liver treated with captopril before lead acetate treatment showed healthy central vein (cv), binucleated cells (arrows) (H&E-Mag ×400). (b) Photomicrograph of mice liver treated with captopril before lead acetate treatment showed small amounts of collagenous fibers (arrows) stained with blue colour (Mtr-Mag ×400).
3.2.1.2. Histopathological scoring system of liver
Non-treated control mice livers scored (0) normal pathological system, whereas, liver sections of mice received lead acetate scored moderate ballooning in hepatocytes (3), severe inflammation (3), presence of apoptotic cells (1) and mild fibrosis (2). Moreover, liver sections of mice treated with captopril prior to lead acetate scored minimum ballooning in hepatocytes (1), minimum inflammation (1), no apoptotic cells (0) and minimum fibrosis (1) (Table 3). Scoring system of liver revealed that group treated with captopril prior to lead acetate showed decrease in pathological score system compared to group receiving lead.
Table 3.
Pathological score for changes in liver architecture.
| Items | Control | Lead | Lead and captopril |
|---|---|---|---|
| Ballooning | 0 | 3 ± 0.1 | 1 ± 0.2 |
| Inflammation | 0 | 3 ± 0.1 | 1 ± 0.1 |
| Apoptic cells | 0 | 1 ± 0 | 0 ± 0.1 |
| Fibrosis | 0 | 2 ± 0.2 | 1 ± 0.2 |
The data are expressed as mean ± SEM (standard error of mean).
3.2.1.3. Spleen
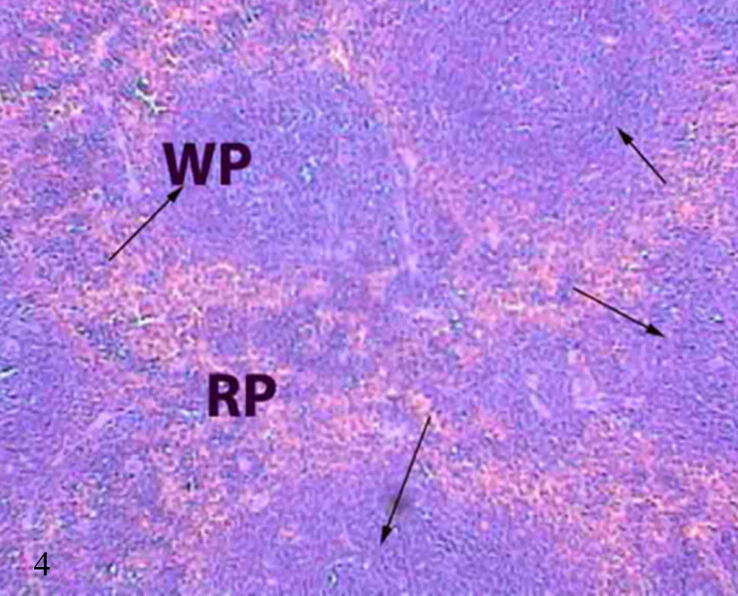
Non treated spleen sections showed normal spleen structure composed of white pulp and red pulp, besides to the fibrous capsule which covered the spleen. White pulp consisting mainly of B-lymphocytes was arranged into two zones, marginal zone (outer rim of loose lymphocytes) that contains macrophages, and mantle zone (inner rim of lymphocytes). The red pulp is the area of spleen in between white pulp and consists of open sinuses and cellular cords (Fig. 4).
Figure 4.
Photomicrograph of control mice spleen showed normal spleen consists of lymphoid follicles (arrows) called white pulp, reddish areas consist of spleen parenchyma called red pulp(H&E-Mag X100).
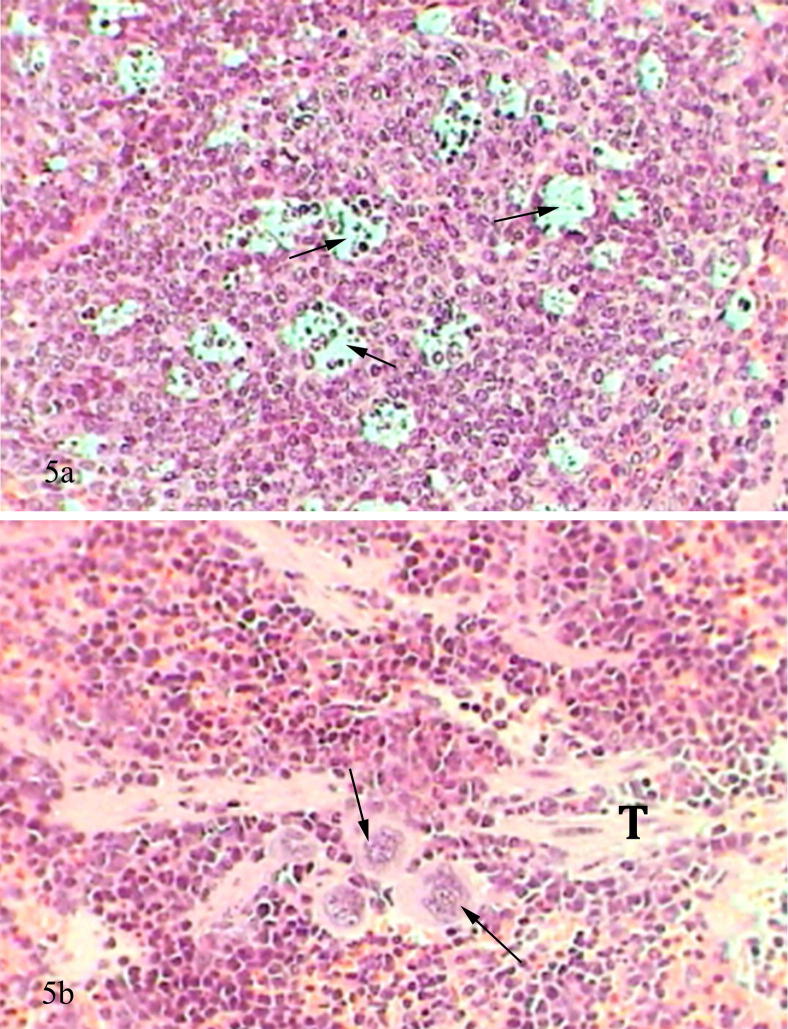
Spleen of mice receiving lead acetate showed marked changes represented by distorted spleen architecture, that it was ill-defined due to diffusion of white pulp into the red pulp in addition to the appearance of necrotic foci, large macrophages were seen in the tissue with great numbers (Fig. 5a and b).
Figure 5.
(a) Photomicrograph of mice spleen treated with lead acetate showed lymphoid follicle with numerous necrotic foci (arrows) filled by darkly stained neutrophils and basophils (H&E-Mag ×400). (b) Photomicrograph of mice spleen treated with lead acetate showed congested and ill defined spleen architecture, trabeculae (T) and large macrophages (arrows) (H&E-Mag ×400).
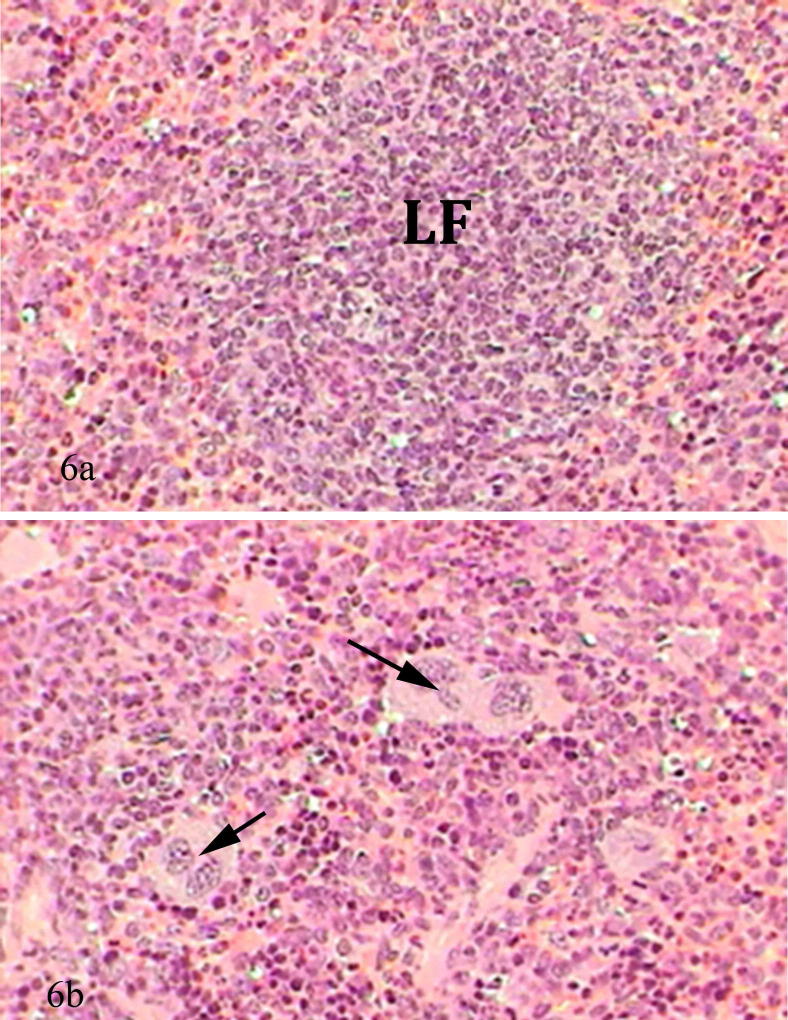
Spleen of mice treated with captopril prior to receiving lead acetate revealed a decrease in pathological alterations, to some extent well-defined spleen architecture appeared but with diffusion of red pulp into white pulp, few necrotic areas with scattered basophils and neutrophils, also macrophages were abundant (Fig. 6a and b).
Figure 6.
(a) Photomicrograph of mice spleen treated with captopril before lead acetate showed lymphoid follicle (LF) without necrosis (H&E-Mag ×400). (b) Photomicrograph of mice spleen treated with captopril before lead acetate showed expansion of red pulp and macrophages (arrows) (H&E-Mag ×400).
3.2.1.4. Histopathological scoring system of spleen
Non-treated control mice spleen scored (0) normal score of spleen, whereas, spleen sections of mice received lead acetate scored (4) severe lymphoid necrosis and (3) moderate red pulp expansion. Moreover, spleen sections of mice treated with captopril prior to lead acetate scored (1) minimum lymphoid necrosis and (2) mild red pulp expansion (Table 4). So, captopril prior to lead decreased the pathological score of spleen compared to group received lead only.
Table 4.
Pathological score for changes in spleen architecture.
| Items | Control | Lead | Lead and captopril |
|---|---|---|---|
| Lymphoid necrosis | 0 | 4 ± 0.1 | 1 ± 0.09 |
| Red pulp expansion | 0 | 3 ± 0 | 2 ± 0.1 |
The data are expressed as mean ± SEM (standard error of mean).
3.2.1.5. Lymphoid follicle analysis
Non-treated control spleen sections showed lymphoid follicle area of (81 μm3). Spleen of mice receiving lead acetate showed significant diminish P < 0.05 in lymphoid follicle area (27 μm3) compared to control, whereas, spleen of mice treated with captopril prior to lead acetate registered (80 μm3) insignificant differences compared to control and a significant increase P < 0.05 compared to group receiving lead acetate. A number of macrophages showed insignificant differences among three experimental groups (2, 3 and 3) in control, lead acetate and lead acetate treated with captopril groups respectively. Macrophage area in the non-treated control group registered (2.9 μm3), a significant increase P < 0.05 in macrophage area was registered (8.5 μm3) in group receiving lead acetate compared to control group, whereas, macrophage area in the group treated with captopril prior to lead acetate registered (4 μm3) insignificant increases compared to the control group and a significant decrease P < 0.05 compared to the lead acetate group (Table 5).
Table 5.
Spleen lymphoid follicle analysis showed area of lymphoid follicles, number of macrophages and area of macrophages in spleens of control, lead and lead treated with captopril groups.
| Items | Control | Lead | Lead and captopril |
|---|---|---|---|
| Area of lymphoid follicles μm3 | 81 ± 1.3 | 27 ± 0.3⁎a | 80 ± 1.3⁎b |
| Number of macrophages Cells/200×field | 2 ± 0.6 | 3 ± 0.2 | 3 ± 0.5 |
| Area of macrophages μm3 | 2.9 ± 0.4 | 8.5 ± 1.02⁎a | 4 ± 0.48b |
The data are expressed as mean ± SEM (standard error of mean).
P < 0.05 significant difference compared to control group.
P < 0.05 significant difference compared to lead acetate group.
4. Discussion
Lead had been a toxic problem for human beings from the earliest time. The ingested and absorbed lead stored primarily in soft tissues and bone, but the highest concentration of lead occurs within the bone, teeth, liver, lung, kidney, brain and spleen (Plumlee, 2004, Mudipalli, 2007). The present study resulted in insignificant differences in liver and spleen indices between control and experimental lead groups, that run in agreement with (Allouche et al., 2011). Histological investigations revealed that lead acetate exposure resulted in marked changes in the liver these findings agreed with (Jankeer and El-Nouri, 2009, Muselin et al., 2010, Suradkar et al., 2010) they stated that rat exposure to lead acetate caused hepatotoxicity characterized by engorgement of blood vessels along with sinusoidal hemorrhage, infiltration, dilatation of central veins and vacuolar degeneration of hepatocytes. In the present study, lead reached the liver via the portal vein that the liver is the first organ exposed to internally absorbed nutrients and other xenobiotics so lead accumulated in the liver tissue caused severe alterations characterized by congested and dilated portal veins and degeneration in hepatic cells with moderate ballooning, severe inflammation, apoptotic cells and mild fibrosis. Most orally ingested lead is excreted, but a portion is absorbed and is transferred to the blood where lead binds to hemoglobin in the erythrocytes so lead is carried through the circulatory system by erythrocytes, virtually all tissues in the body can become exposed to the toxic metal, particularly hematopoietic and immune system (Goering, 1993, Gidlow, 2004, Lawrence and McCabe, 1995). In the spleen, phagocytes (macrophages and polymorphonuclear cells) are responsible for slowing the propagation of an invading pathogen, while an antigen-specific adaptive immune response (antibody- or cell-mediated) is being established. Lead was reported to inhibit macrophage function (Kowolenko et al., 1988, Mauel et al., 1989) possibly by overloading macrophages with cellular debris and inhibiting macrophage production of nitric oxide (Tian and Lawrence, 1995). In the context of adaptive humoral and cellular immune responses, lead increased both B-cell and T-cell in vitro proliferation (Lawrence, 1981a–c; Warner and Lawrence, 1986, Razani et al., 1999). In the present study, administration of lead resulted in severe changes in the spleen represented by severe lymphoid necrosis, moderate diffusion of white pulp into the red pulp, diminished lymphoid follicles and appearance of large macrophages might be due to the production of debris of dead cells.
Captopril is an angiotensin-converting enzyme inhibitor that inhibits the ACE that catalyzes the conversion of angiotensin I to the vasoconstrictor peptide, angiotensin II. It is generally recommended for the treatment of hypertension, congestive heart failure, acute myocardial infarction and renal complications of diabetes mellitus. It also has beneficial experimental effects in hindering the progression of chronic renal failure, diabetic nephropathy and development of atherosclerosis (Chobanian et al., 1990, Omata et al., 1996). Also there is increasing evidence that the broader pharmacological properties of ACE inhibitors encompass the anti-oxidant ability through scavenging free radical because of its terminal-SH group in a variety of organ systems (Ghazi-Khansari et al., 2006, Wang et al., 2000). The anti-oxidant activity of captopril possibly ameliorates the oxidative stress. The –SH group in the structure is a crucial requirement for free radical scavenging activity but not the proline part (Chopra et al., 1992). The present study proved that captopril could reduce the hepatotoxicity induced by lead that liver sections of mice received captopril before lead treatment showed healthy liver sections revealed by healthy hepatocytes and regeneration process with minimum ballooning in hepatocytes, inflammation, fibrosis and no apoptotic cells as recorded by pathological scoring system, also the present results demonstrated the antifibrotic effect of captopril that it diminished the collagenous fibers compared with the previous groups, these findings agreed with Amirshahrokhi et al. (2010) who suggested that captopril had protective effect against hepatic fibrosis because of the ability of captopril to reduce BDL-induced production of the proinflammatory cytokine – TNF-α which plays a major role in the development of hepatic fibrosis. Also it increases the hepatic content of IL-10 in BDL rats. Interleukin-10 is an anti-inflammatory cytokine and has an important role in the prevention of hepatic inflammation and fibrosis. Moreover, captopril has protective effects against spleen toxicity, it protected lymphoid follicles from severe necrosis, diminishing and decrease red pulp expansion.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No (RG-1435-030).
Footnotes
Peer review under responsibility of King Saud University.
References
- Allouche L., Hamadouche M., Touabti A., Khennouf S. Effect of long-term exposure to low or moderate lead concentrations on growth, lipid profile and liver function in albino rats. Adv. Biol. Res. 2011;5:339–347. [Google Scholar]
- Amirshahrokhi K., Ghazi-khansari M., Farani A., Karimian G. Effect of captopril on TNF-α and IL-10 in the livers of bile duct ligated rats. Iran. J. Immunol. 2010;7:247–251. [PubMed] [Google Scholar]
- Andreoli S.P. Captopril scavenges hydrogen peroxide and reduces, but does not eliminate, oxidant-induced cell injury. Am. J. Physiol. 1993;264:120–127. doi: 10.1152/ajprenal.1993.264.1.F120. [DOI] [PubMed] [Google Scholar]
- Bagchi D., Prasad R., Das D. Direct scavenging of free radicals by captopril, an angiotensin converting enzyme inhibitor. Biochem. Biophys. Res. Commun. 1989;158:52–57. doi: 10.1016/s0006-291x(89)80175-5. [DOI] [PubMed] [Google Scholar]
- Chiras D. Jones and Bartlett Publishers; Sudbury: 2009. Environmental Science; p. 394. [Google Scholar]
- Chobanian A., Haudenschild C., Nickersonand C., Drago R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990;15:327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- Chopra M., Beswick H., Clapperton M., Dargiean H., Smith W. Antioxidant effects of angiotension-converting enzyme (ACE) inhibitors:free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl- and nonsulfhydryl-containing ACE inhibitors. J. Cardiovasc. Pharmacol. 1992;9:330–340. doi: 10.1097/00005344-199203000-00005. [DOI] [PubMed] [Google Scholar]
- Drury R., Wallington E. Carleton's Histological Technique. Oxford University Press; 1980. Preparation and fixation of tissues; pp. 41–54. [Google Scholar]
- Ghazi-Khansari M., Mohammadi-Bardbori A., Hosseini M. Using Janus Green B to study paraquat toxicity in rat liver mitochondria: role of ACE inhibitors. Ann. N. Y. Acad. Sci. 2006;1090:98–107. doi: 10.1196/annals.1378.010. [DOI] [PubMed] [Google Scholar]
- Gidlow D. Lead toxicity. Occup. Med. 2004;54:76–81. doi: 10.1093/occmed/kqh019. [DOI] [PubMed] [Google Scholar]
- Goering P. Lead-protein interactions as a basis for lead toxicity. Neurotoxicol. 1993;14:45–60. [PubMed] [Google Scholar]
- Gupta C. Basic and Clinical Principles. second ed. Elsevier; 2007. Veterinary toxicology. [Google Scholar]
- Ha H., Kim K. Amelioration of diabetic microalbuminuria and lipid peroxidation by captopril. Yonsei Med. J. 1992;33:217–223. doi: 10.3349/ymj.1992.33.3.217. [DOI] [PubMed] [Google Scholar]
- Jankeer M., El-Nouri A. Histological study of the liver and kidney of albino mice Mus musculus exposed to lead. J. Raf. Sci. 2009;20:42–51. [Google Scholar]
- Khattab M., Mostafa A., Al-Shabanah O. Effects of on cardiac captopril on cardiac and renal damage and metabolic alterations in the nitric oxide-deficient hypertensive rat. Kidney Blood Press. Res. 2005;28:243–250. doi: 10.1159/000088829. [DOI] [PubMed] [Google Scholar]
- Kiran B., Prabhakara Rao Y., Noble T., Weddington K., McDowellV Sharada R., Rajanna B. Lead-induced alteration of apoptotic proteins in different regions of adult rat brain. Toxicol. Lett. 2009;184:56–60. doi: 10.1016/j.toxlet.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology – a systematic review. BMC Vet. Res. 2013;9:123–138. doi: 10.1186/1746-6148-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojsova S., Jendeková L., Zicha J., Kunes J., Andriantsitohaina R., Pechánová O. The effect of different antioxidants on nitric oxide production in hypertensive rats. Physiol. Res. 2006;55:S3–S16. doi: 10.33549/physiolres.930000.55.S1.3. [DOI] [PubMed] [Google Scholar]
- Kowolenko M., Tracy L., Mudzinski S., Lawrence D. Effect of lead on macrophage function. J. Leukocyte Biol. 1988;3:357–364. doi: 10.1002/jlb.43.4.357. [DOI] [PubMed] [Google Scholar]
- Lawrence D., McCabe M. Immune modulation by toxic metals. In: Goyer R.A., Klaassen C.D., waalkes M.P., editors. Metal Toxicology. Academic Press; San Diego: 1995. pp. 305–337. [Google Scholar]
- Lynpatrick N. Lead toxicity Part-II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity – lead. Altern. Med. Rev. 2006;6 [PubMed] [Google Scholar]
- Mauel J., Ransijn A., Buchmuller Y. Lead inhibits intracellular killing of leishmania parasites and extracellular cytolysis of target cells by macrophages exposed to macrophage activating factor. J. Leukocyte Biol. 1989;45:401–409. doi: 10.1002/jlb.45.5.401. [DOI] [PubMed] [Google Scholar]
- Moreira R., Moreira J. Os efeitos do chumbo sobre o organism humano e seu significado para a saude. Pan Am. J. Public Health. 2004;15:119–129. doi: 10.1590/s1020-49892004000200007. [DOI] [PubMed] [Google Scholar]
- Mudipalli A. Lead hepatotoxicity and potential health effects. Indian J. Med. Res. 2007;126:518–527. [PubMed] [Google Scholar]
- Muselin F., Trif A., Brezovan D., Stancu A., Snejana P. The consequences of chronic exposure to lead on liver, spleen, lungs and kidney architectonics in rats. Lucrari Stiintifice Medicina Veterinara. 2010;2:123–127. [Google Scholar]
- Omata K., Kanazawa M., Sato T., Abe F., Saitoand T., Abe K. Therapeutic advantages of angiotensin converting enzyme inhibitors in chronicrenal disease. Kidney Int. 1996;49:S57–62. [PubMed] [Google Scholar]
- Pitot C., Dragan P.Y. Chemical Carcinogenesis. McGraw Hill; New York: 1996. Casarett and Doull’s toxicology; pp. 201–260. [Google Scholar]
- Plumlee K. first ed. Mosby; U.S.A.: 2004. Metals and Minerals in Clinical Veterinary Toxicology; pp. 193–230. [Google Scholar]
- Razani S., Edwards B., Sopori M. Lead stimulates lymphocyte proliferation through enhanced T Cell–B-cell interaction. J. Pharm. Exp. Therap. 1999;288:714–719. [PubMed] [Google Scholar]
- Sultana N., Arayne M., Quraish R. In vitro interactions of captopril with h (2)-receptor antagonists. Pak. J. Pharm. Sci. 2007;20:132–139. [PubMed] [Google Scholar]
- Suradkar S., Vihol P., Patel J., Ghodasara D., Joshi B., Prajapati K. Patho-morphological changes in tissues of Wistar rats by exposure of lead acetate. Vet. World. 2010;3:82–84. [Google Scholar]
- Tian L., Lawrence D. Lead inhibits nitric oxide production in vitro by murine splenic macrophages. Toxicol. Appl. Pharmacol. 1995;132:156–163. doi: 10.1006/taap.1995.1096. [DOI] [PubMed] [Google Scholar]
- Wang R., Ibarra-Sunga L., Pick Verlinski R., Uhal B. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L143–151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- Warner G., Lawrence D. Stimulation of murine lymphocyte responses by cations. Cell Immunol. 1986;101:425–439. doi: 10.1016/0008-8749(86)90155-3. [DOI] [PubMed] [Google Scholar]
- Westlin W., Mullane K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation. 1988;77:I30–I39. [PubMed] [Google Scholar]