Abstract
Corynebacterium pseudotuberculosis (C. pseudotuberculosis) is a causative organism of caseous lymphadenitis (CLA) in sheep and acute disease in buffaloes known as oedematous skin disease (OSD). Human affected with the disease show liver abscess and abscess in the internal lymph nodes. The vaccination against CLA up till now occurs by using formalin inactivated whole cells of biovar 1 (sheep strain). Combined vaccine composed of formalin inactivated whole cells of sheep strain and recombinant phospholipase D (rPLD) and another vaccine composed of formalin inactivated whole cells (buffalo origin) and rPLD were prepared in Biotechnology center for services and Researches laboratory at Cairo university and applied for protection against CLA. Both vaccines induced complete protection (100%) against challenge with virulent biovar 1 or biovar 2. Also vaccination against OSD was performed by two types of vaccines. Vaccine-1 was composed of formalin inactivated whole cell biovar 1 combined with rPLD and the second vaccine was composed of formalin inactivated whole cells of biovar 2 combined with rPLD. No lesions developed in vaccinated and non vaccinated buffaloes challenged with C. pseudotuberculosis biovar revealing that biovar 1 C. pseudotuberculosis is not infective for buffaloes. Buffaloes vaccinated with the second vaccine and control non vaccinated animals challenged with biovar 2 (buffalo origin) resulted in development of OSD in all animals. This indicates that OSD results due to production of toxin (s) other than PLD. Discovering this toxin (s) is of value in formulation of a future vaccine against OSD.
Keywords: Corynebacterium pseudotuberculosis, Caseous lymphadenitis, Oedematous skin disease, Vaccination, Recombinant phospholipase D
1. Introduction
Corynebacterium pseudotuberculosis is a Gram positive facultative intracellular organism causing caseous lymphadenitis (CLA) in sheep and goats. The disease characterized by chronic suppurative inflammation and significant economical losses to the sheep industries worldwide (Paton et al., 2003, Dorella et al., 2006).
In human, it is of major occupational impact. Human affected with the disease show liver abscess and abscess in the internal lymph nodes.
C. pseudotuberculosis causes a characteristic acute disease in buffaloes known as Oedematus skin disease (OSD). The disease is endemic in Egypt and is characterized by the development of diffused swellings in the skin of the hind quarter, fore limbs, belly and brisket regions. These lesions usually affect the lymph nodes which become enlarged and inflamed and filled with pus. At first is filled with serous fluid then turned to sac of pus due to contamination with other bacteria as staphylococci, streptococci, E. coli and Pseudomonas. The disease is characterized by high morbidity and low mortality and the badly treated cases died. OSD is a terrible disease for farmers and veterinarians; it causes severe economical losses due to the necessary surgical intervention, expensive medication, reduction in the milk production and lowering of work activity of the affected animals (Selim, 2001). In human infections caused by C. pseudotuberculosis most commonly occur by the occupational exposure, ingestion of raw milk from goat and cow’s milk (Peel et al., 1997).
The majority of studies have employed formalin inactivated toxoid vaccines derived from phospholipase D (PLD) rich C. pseudotuberculosis culture supernatants and these have conferred varying levels of protective immunity in both sheep and goats (Narin et al., 1977 and Eggleton et al., 2005). In the present investigation two combined vaccines prepared from formalin inactivated whole cells of biovar 1 (sheep origin) in each dose, the second vaccine is composed of inactivated whole cells of biovar 2 (buffalo origin) in combination with rPLD. These vaccines will be used for vaccination of sheep and buffaloes and protective efficacy was assessed by virulent strains locally isolated from sheep with CLA or buffaloes with OSD.
2. Materials and methods
2.1. Animals
2.1.1. Sheep
Eight sheep approximately 8–10 months old were tested by ELISA to exclude the positive reactors. Negative ELISA (free of CLA) animals were divided into 4 groups. Group 1 vaccinated with bacterins (biovar 1) and rPLD and second group vaccinated with heterologon bacterin (biovar 2) and rPLD. Animals of group 1 in addition to 2 control non vaccinated animals were challenged with virulent biovar 1 and second vaccinated group with 2 control animals were challenged with virulent biovar 2 (isolated from buffaloes with OSD).
2.1.2. Buffaloes
Eight buffaloes approximately 4–6 months old previously tested with ELISA for exclusion of positive reactors. Negative ELISA animals were divided into 4 groups. The first group were vaccinated with vaccine composed of formalin inactivated whole cell of biovar 1 (sheep origin) with rPLD. Vaccinated animals and control 2 animals were challenged with virulent biovar 2 isolate obtained from buffaloes with OSD.
2.2. Formalin whole cell bacterin
Two types of bacterins were prepared. The first bacterin was prepared from C. pseudotuberculosis biovar 1 (sheep origin) and the second bacterin was prepared from biovar 2 (buffalo origin). Preparations were performed according to Brogden et al. (1990) with modification. Each dose (2 ml of bacterin 1) contained 20 mg of killed bacteria. In bacterin 2 each dose (4 ml) contained 100 mg of killed bacteria (biovar 2).
2.3. Vaccine formulations
2.3.1. Vaccine 1
It is composed 20 mg bacterin combined (sheep origin) with rPLD (50 μg) in volume 1 ml. then combined vaccine was mixed with 1 ml of oil adjuvant to bacterin a dose of 2 ml.
2.3.2. Vaccine 2
It is composed of bacterin prepared from biovar 2 (buffalo origin) and proceeded as in vaccine 1.
2.3.3. Vaccine 3
It is composed of bacterin prepared from biovar 1 (sheep isolate), but with concentration of 100 mg killed whole cells in a volume 2 ml. To bacterin 500 μg of rPLD were added in a volume of 2 ml. To 2 ml suspension of combined bacterin and rPLD 2 ml of oil adjuvant were added to obtain a final volume of 4 ml.
2.3.4. Vaccine 4
It is composed of bacterin prepared from biovar 2 (buffalo origin) and proceeded as in vaccine 3.
2.4. Schedule of vaccination
Group 1 and group 2 of sheep were vaccinated with combined vaccine 1 and 2 respectively by S/C inoculation of 2 ml dose of vaccine in middle third of the neck. All the vaccinated animals were revaccinated after 3 weeks of the first vaccination with the same vaccines.
After 3 weeks of the last vaccination, the vaccinated group 1 animals and the control non vaccinated sheep were challenged with virulent biovar 1 sheep isolate with 2 ml suspension contain 2 × 106 CF distributed intradermally as 1 ml in both sides of the neck, the second group of sheep were vaccinated and challenged by the same manner as with vaccine 1.
The buffaloes of the first and second groups of animals were vaccinated by subcutaneous injection of 2 ml in the neck and the other 2 ml in the hind limb in one side of the animal with vaccine 3 and 4, respectively and poster dose was given after three and six weeks in the same manner. The control animals were inoculated in the same manner with 4 ml saline adjuvant mixture.
All vaccinated buffaloes of group, and its control were challenged with 5 ml of 48 h brain heart broth which contained 5 × 106 of living cells inoculated intradermally in hairless areas as axillary and groin folds. The first group of animals with its control non vaccinated was challenged with virulent sheep isolate biovar 1 and the second group of buffaloes with its control non vaccinated was challenged with virulent C. pseudotuberculosis isolated from buffaloes (biovar 2).
2.5. Antibodies assayed with ELISA
Blood samples were obtained from sheep before vaccination and at weekly intervals till the end of experiment (20 weeks). In case of buffaloes blood samples were collected directly before vaccination and one week after the first dose of vaccination, then 1 week after the second booster dose, then 3 weeks and 3 months after challenge. Antibodies were assayed in 96 ELISA plates as previously described by Selim et al. (2016).
2.6. Post mortem and clinical observation
The primary vaccination sites and site of challenge were inspected at weekly intervals for any signs of local reaction. All sheep were euthanized and necropsied 20 weeks post primary vaccination and the external prescapular and prefemoral lymph nodes were examined in addition to the internal lymph nodes for abscesses. Swabs were collected from abscesses developed in control challenged sheep and buffaloes for isolation of C. pseudotuberculosis.
3. Results
3.1. Serological analysis
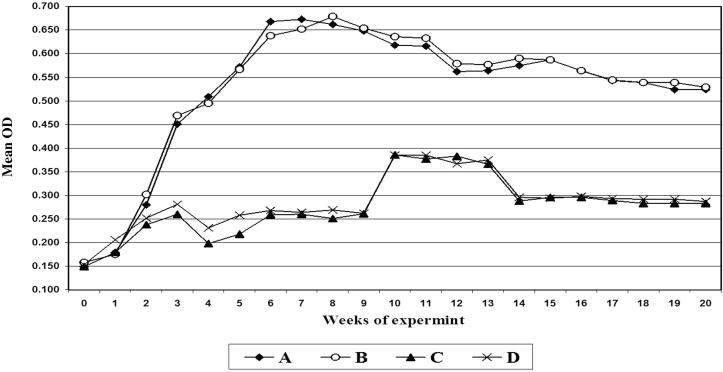
All vaccinated sheep had an antibody response to rPLD antigen, whereas control sheep remained negative throughout this serum period. Antibodies appeared in sera of unvaccinated control animals after exposure to challenge. In vaccinated groups antibodies increased after second booster dose of vaccination, but after exposure to challenge, a slight decrease in OD of examined animals occurred, but the level of antibodies persist higher than the cut off value till 20 weeks the time of slaughtering the animals as shown in Fig. 1.
Figure 1.
Antibody titer of the serum samples of sheep collected at time intervals post immunization. A: Mean OD in sera of sheep vaccinated with bacterin (sheep) + rPLD; B: Mean OD in sera of sheep vaccinated with bacterin (buffalo) + rPLD; C: Control sheep challenged with sheep C. pseudotuberculosis D: Control sheep challenged with buffalo C. pseudotuberculosis.
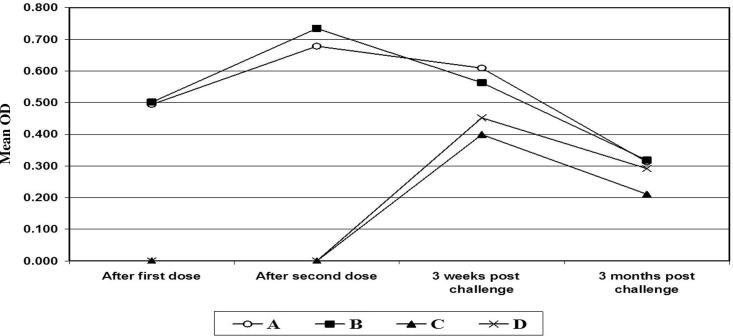
In case of buffaloes all vaccinated animals showed increase in antibody levels, while control non vaccinated showed no titers of antibodies. The highest magnitude of antibodies developed after booster vaccination but after challenge a slight decrease was observed and level of antibodies revered to negative phase after 3 months of challenge as shown in Table 1 and Fig. 2.
Table 1.
Mean OD of ELISA assay in sera of buffaloes vaccinated with combined bacterin + rPLD “sera dil 1:100”.
| Weeks of experiment | Buffaloes vaccinated with combined bacterin (sheep) + rPLD | Buffaloes vaccinated with combined bacterin (buffalo) + rPLD | Control buffaloes |
|
|---|---|---|---|---|
| Challenged with sheep C. pseudotuberculosis | Challenged with buffalo C. pseudotuberculosis | |||
| After 1st dose | 0.494 | 0.501 | – | – |
| After 2nd dose | 0.678 | 0.734 | – | – |
| 3 weeks post challenge | 0.609 | 0.563 | 0.399 | 0.452 |
| 3 months post challenge | 0.313 | 0.318 | 0.211 | 0.294 |
Cut off value: 0.350.
Figure 2.
Antibody titer of the serum samples of sheep collected at time intervals post immunization. A: Mean OD in sera of buffaloes vaccinated with bacterin (sheep) + rPLD; B: Mean OD in sera of buffaloes vaccinated with bacterin (buffaloes) + rPLD; C: Control buffaloes challenged with sheep C. pseudotuberculosis D: Control buffaloes challenged with buffalo C. pseudotuberculosis.
3.2. Challenge
Buffaloes vaccinated with bacterin (sheep origin) and rPLD and the control buffaloes challenged with C. pseudotuberculosis (sheep origin) developed a swelling at site of inoculation which spontaneously disappeared, through 15 days. Meanwhile, vaccinated buffaloes with bacterin (sheep origin) and rPLD and challenged with virulent C. pseudotuberculosis of buffalo origin developed redness, swelling of site of inoculation which extends to the belly region.
Control non vaccinated and challenged buffaloes with C. pseudotuberculosis (buffalo origin) developed swelling at the site of inoculation and extend to the regional lymph node. On the other hand control non vaccinated buffaloes challenged with C. pseudotuberculosis (sheep origin) developed a slight inflammatory medium at site of inoculation which disappears spontaneously.
All sheep were examined for external and internal abscesses. Mean number of abscesses for each group was recorded (Table 2) of group 1 sheep vaccinated with vaccine (A) (bacterin (biovar1) + rPLD) and challenged with homologous virulent strain and group 2 vaccinated with vaccine (B) composed of bacterin (biovar 2) + rPLD). No lesion could be observed either in external or internal lymph nodes. While control group non vaccinated animals challenged with virulent biovar1 developed 12 score lesions. Out of 15 lesions score in typically discussed sheep will CLA. Hence 12/15 represent 80% susceptibility and 20% naturally protected animals. At the same time sheep vaccinated with vaccine (B) which was composed of bacterin (biovar 2) plus rPLD, developed the same in the form abscess in a percent of 13/15 i.e. 86.6% infection and 13.4% presence of natural immunity. C. pseudotuberculosis (nitrate negative) were isolated from group 1 vaccinated animals and C. pseudotuberculosis from abscess in group 2 vaccinated sheep. These isolates were of nitrate positive reactors.
Table 2.
Score of lesions detected during post mortem examination of external and internal lymph nodes of sheep post challenge.
| Groups of sheep | Mean score lesions | Total score of internal lymph nodes | Total score for each group | % of protection | % of infection | |||
|---|---|---|---|---|---|---|---|---|
| External lymph nodes | ||||||||
| RPS | LPS | RPF | LPF | |||||
| Control groups | ||||||||
| 1-Challenged with biotype 1 | 3/3 | 3/3 | 3/3 | 0/3 | 3/3 | 12/15 | 20 | 80 |
| 2-Challenged with biotype 2 | 3/3 | 3/3 | 3/3 | 1/3 | 3/3 | 13/15 | 13.4 | 86.6 |
| Vaccinated group with vaccine (A) | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/15 | 100 | 0 |
| Vaccinated group with vaccine (B) | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/15 | 100 | 0 |
Score: 0 = Normal of slightly enlarged LN (0/3).
1 = Enlarged lymph node (1/3).
2 = Enlarged and congested or hemorrahagic (2/3).
3 = Enlarged lymph node with focal abscess or caseation (3/3).
RPS: Right prescapular lymph node.
LPS: Left prescapular lymph node.
RPF: Right prefemoral lymph node.
LPF: Left prefemoral lymph node.
Internal lymph node one unit (Inguinal, tracheobronchial thoracic, mesenteric, 2 popliteals).
3.3. Clinical findings in vaccinated buffaloes
All buffaloes either vaccinated or control non vaccinated group challenged with C. pseudotuberculosis strain (biotype 1) showed a slight enlargement at site of bacterial inoculation, which disappeared gradually through 15 days. Another clinical features were observed in the case of vaccinated buffaloes with bacterin (biotype 2) + rPLD or control non vaccinated animals and challenged will field strain of C. pseudotuberculosis isolated from buffaloes diseased with OSD. All animals developed swellings at site of inoculation which extend to the skin of belly region associated with redness of skin, in more aged lesions ulcers were developed at site of inoculation. The severity of lesions were the same either in vaccinated or non vaccinated control group, which indicate the production of unknown toxin produced by the challenging C. pseudotuberculosis strain (biotype 2 buffalo origin) and that could not be neutralized by antibodies developed against bacterin or PLD composing the vaccines.
4. Discussion
In the present investigation, this is the first report describing immunizations of sheep with bacterins prepared from nitrate positive C. pseudotuberculosis strain isolated from buffaloes (biovar 2) and challenged with virulent biovar 2 isolate. No significant difference in protection efficacy of both vaccines could be observed in challenged sheep either challenged with virulent biovar 1 or biovar 2. All vaccinated sheep were completely protected against challenge. At the same time control non vaccinated groups either challenged with biovar 1 (sheep origin) or biovar 2 (buffalo origin) showed the development of chronic symptoms of CLA characterized by production of caseous abscess either in external or internal lymph nodes.
These results indicated that C. pseudotuberculosis either of biovar 1 or biovar 2 induced the same lesion which can be attributed to the same virulence factor i.e. PLD possessed by both biovars. Vaccination with a combination of rPLD and killed whole-cells resulted in complete protection against challenge (Fontaine et al., 2006). Our findings are in consistence with our finding about complete protection of combined rPLD and killed whole cells. The results of our study would tend to support the findings of pervious authors (Cameron, 1972 and Cameron and Fuls, 1973).
Interestingly, both PLD and the formalin inactivated whole cell vaccines prevented dissemination of challenge bacteria beyond the site of inoculation to essentially equivalent extents. Cell mediated immunity can be attributed to rPLD which activate macrophages in vaccinated animals. The role of rPLD in activation of macrophages has been shown in immune model (El-Enbaawy et al., 2005).
In the present investigation, ELISA was used for detection of antibodies in vaccinated or challenged animals. rPLD was used for coating well in ELISA plates. ELISA test using rPLD antigen proved to be a highly sensitive test for detection of antibodies in early infection. Test could detect antibodies after one week of first dose of vaccination. On results in using ELISA and rPLD for diagnosis are consistent with those reported by Menzies et al. (1994) and Fontaine et al. (2006). Our data derived from testing serum of naturally CLA positive and truly CLA negative animals, indicate that PLD-ELISA has high specificity and sensitivity for early diagnosis of CLA and of value in aiding in the diagnosis and control of CLA infection.
Vaccination with combined rPLD and formalin inactivated whole cell of C. pseudotuberculosis clearly decreased the prevalence and number of abscesses that formed secondary to C. pseudotuberculosis infection. No abscess developed either external or internal lymph nodes in all vaccinated sheep. If compared with control non vaccinated sheep abscess developed in external and internal lymph nodes in a percent of 80–87%.
In case of buffaloes vaccinated either with vaccine combined of formalin whole cell inactivation of biovar 1 (sheep origin) and its control non vaccinated buffaloes challenged with virulent biovar 1, no lesion could observed in both group of buffaloes except the development of localized swelling at site of inoculation, which disappeared spontaneously through 15 days. These results indicate that sheep (biovar 1) strains are not infections to buffaloes which they could induce CLA in sheep. In second group of buffaloes vaccinated with combined whole cells of biovar 2 and rPLD and challenged with virulent strain of biovar 2 and its control non vaccinated buffaloes.
All animals developed signs of OSD as swelling at site of challenge and may extend to the belly region. Ulceration at site of challenge inoculation occurred in some experiment animals. These results indicate that development of OSD lesions in both vaccinated and non vaccinated group of buffaloes resulted from the production of toxin(s) other than PLD. The production of toxins other than PLD could be reported in previous studies through which buffalo isolates (biovar 2) induced acute hemorrhagic congestions at site of inoculation while guinea pigs infected with sheep isolates (biovar 1) resulted in development of abscesses at site of inoculation. Our conclusion declaring this toxin(s) produced by C. pseudotuberculosis biovar 2, other than PLD, will be of value in future development of vaccine against OSD.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No.: RGP-176..
Footnotes
Peer review under responsibility of King Saud University.
References
- Brogden K.A., Chedid L., Cutlip R.C., Lehmkul H.D., Sacks J. Effect of muramyl dipeptide on immunogenicity of C. pseudotuberculosis whole cell vaccines in mice and lambs. Am. J. Vet. Res. 1990;51(2):200–202. [PubMed] [Google Scholar]
- Cameron C.M. Immunity to C. pseudotuberculosis. J. South Afr. Vet. Med. Assoc.1972;43(4):343–349. [PubMed] [Google Scholar]
- Cameron C.M., Fuls W.J.P. Studies on enhancement of immunity to C. pseudotuberculosis. Onderstepoort J. Vet. Res. 1973;40(3):105–114. [PubMed] [Google Scholar]
- Dorella F.A., Pacheco L.G.C., Oliveira S.C., Miyoshi A., Azevedo V. Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 2006;37:201–218. doi: 10.1051/vetres:2005056. [DOI] [PubMed] [Google Scholar]
- Eggleton D.G., Haynes J.A., Middleton H.D., Cox J.C. Immunization against ovine caseous lymphadenitis: correlation between C. pseudotuberculosis toxoid content and protective efficacy in combined Clostridial Corynebacterial vaccines. Aust. Vet. J. 2005;68:322–325. doi: 10.1111/j.1751-0813.1991.tb03088.x. [DOI] [PubMed] [Google Scholar]
- El-Enbaawy M.I., Saad M.M., Selim S.A. Humoral and cellular immune responses of a murine model against Corynebacterium pseudotuberculosis antigens. Egypt. J. Immunol. 2005;12(2):13–19. [PubMed] [Google Scholar]
- Fontaine M.C., Baird G., Connor K.M., Rudge K., Sales J., Donachie W. Vaccination confers significant protection of sheep against infection with a virulent United Kingdom strain of Corynebacterium pseudotuberculosis. Vaccine. 2006;24:5986–5996. doi: 10.1016/j.vaccine.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Menzies P.I., Muckle C.A., Li Y., Hwang Y.T., Songer J.G. Evaluation of an enzyme linked immunosorbent assay using E.coli recombinant phospholipase D antigen for the diagnosis of C. pseudotuberculosis infection. Small Ruminant Res. 1994;13(2):193–198. [Google Scholar]
- Narin, M.E., Robertson, J.P., Quade, N.C., 1977. The control of caseous lymphadenitis in sheep by vaccination, pp. 159–161.
- Paton M.W., Walker S.B., Rose L.R., Watt G.F. Prevalence of caseous lymphadenitis and usage of caseous lymphadenitis vaccines in sheep flocks. Aust. Vet. J. 2003;81:91–95. doi: 10.1111/j.1751-0813.2003.tb11443.x. [DOI] [PubMed] [Google Scholar]
- Peel M.M., Palmer G.G., Stacpoole A.M., Kerr T.G. Human lymphadenitis due to C. pseudotuberculosis: report of ten cases from Australia and review. Clin. Infect. Dis. 1997;24:185–191. doi: 10.1093/clinids/24.2.185. [DOI] [PubMed] [Google Scholar]
- Selim S.A. Oedematous skin disease of buffalo in Egypt: a review. J. Vet. Med. B. 2001:241–258. doi: 10.1046/j.1439-0450.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Selim S.A., Mohamed F.H., Hessain A.M., Moussa I.M. immunological characterization of diphtheria toxin recovered from Corynebacterium pseudotuberculosis. SJBS. 2016;23(1):282–287. doi: 10.1016/j.sjbs.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]