Abstract
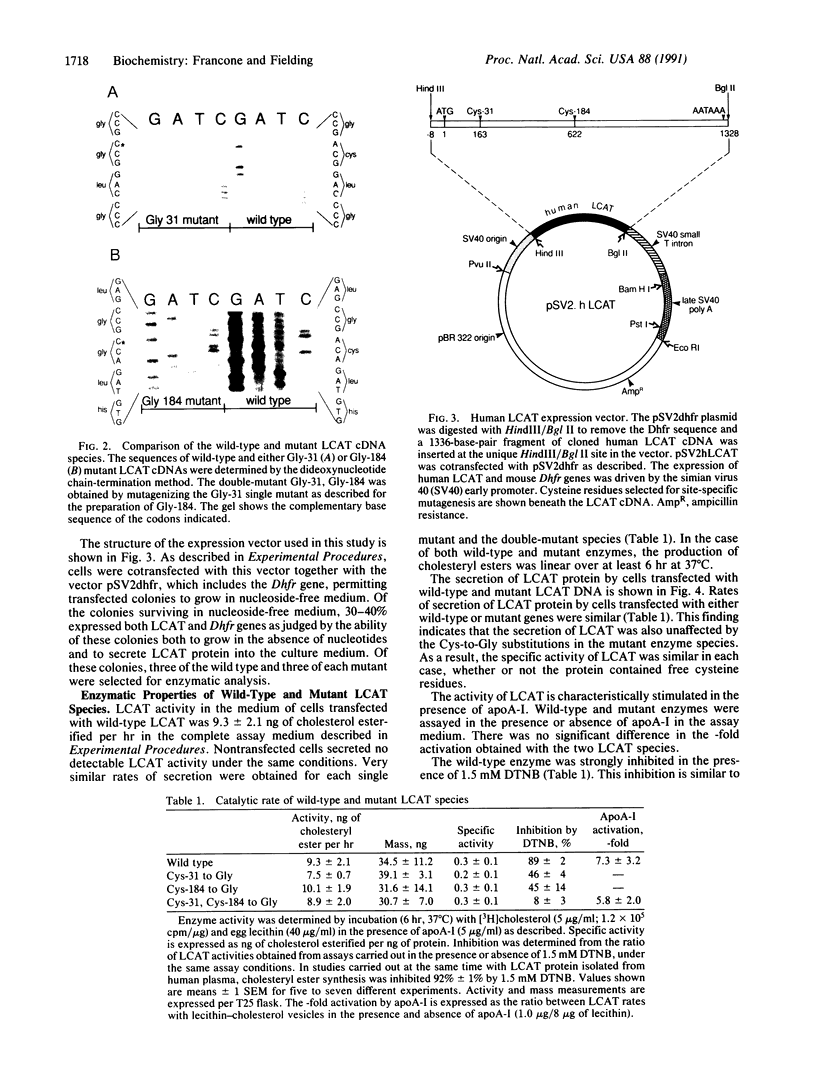
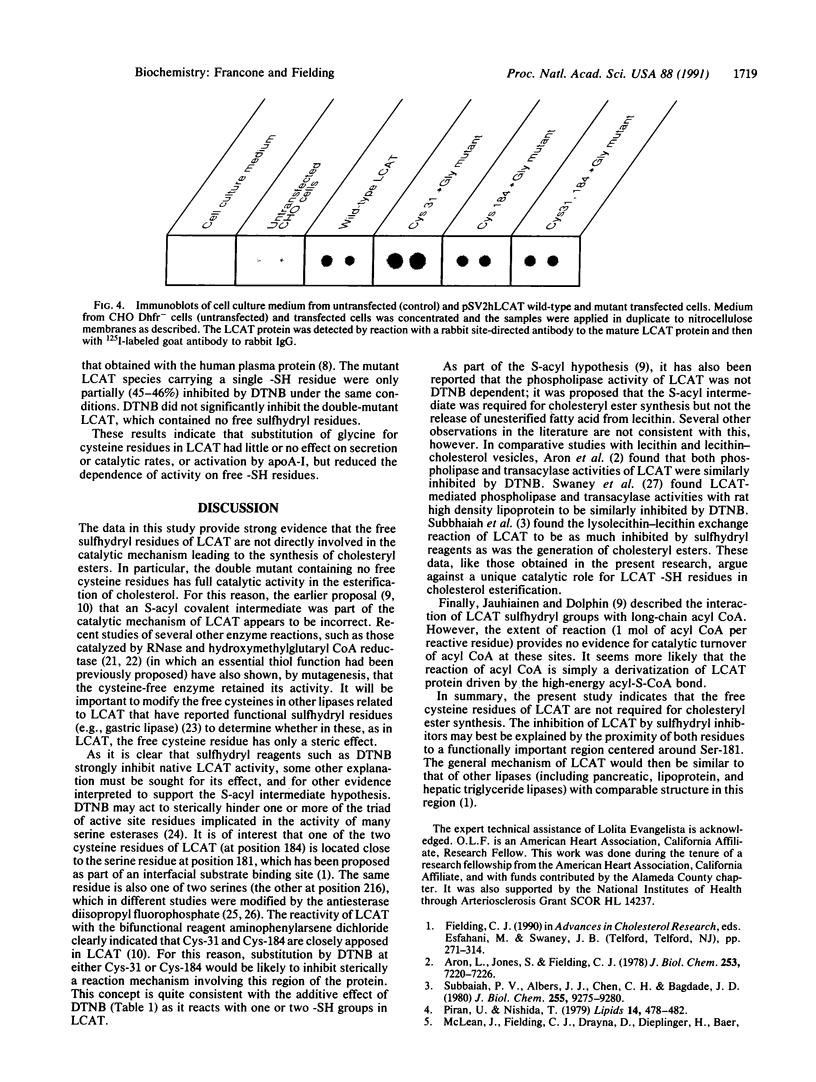
Native lecithin-cholesterol acyltransferase (LCAT; phosphatidylcholine-sterol acyltransferase; phosphatidylcholine:sterol O-acyltransferase, EC 2.3.1.43) protein, and LCAT in which either or both of the enzyme free cysteines had been replaced with glycine residues by site-directed mutagenesis, has been expressed in cultured Chinese hamster ovary cells stably transfected with the human LCAT gene. The mass of LCAT secreted, determined by immunoassay, did not differ in the native and mutant species. LCAT specific activity was also unchanged in the mutant species. In particular, the cysteine-free double mutant, in which Cys-31 and Cys-184 had both been replaced, was fully active in the synthesis of cholesteryl esters. This result is not consistent with a catalytic role for LCAT free cysteine residues. The classical inhibitor of LCAT activity, 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB), which strongly (89%) inhibited the native enzyme, had partial (45%) inhibitory activity with mutant enzyme species containing a single -SH residue, while the double mutant was not significantly inhibited by DTNB. These data are interpreted to suggest that Cys-31 and Cys-184 are vicinal both to each other and to the "interfacial binding site" at residues 177-182, and that DTNB exerts its effect by steric inhibition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron L., Jones S., Fielding C. J. Human plasma lecithin-cholesterol acyltransferase. Characterization of cofactor-dependent phospholipase activity. J Biol Chem. 1978 Oct 25;253(20):7220–7226. [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 1988 Jan 12;27(1):25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui J. Z., Wohl R. C., Kézdy F. J., Scanu A. M. Identification of the active-site serine in human lecithin: cholesterol acyltransferase. Arch Biochem Biophys. 1988 Mar;261(2):330–335. doi: 10.1016/0003-9861(88)90348-7. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Francone O. L., Gurakar A., Fielding C. Distribution and functions of lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in plasma lipoproteins. Evidence for a functional unit containing these activities together with apolipoproteins A-I and D that catalyzes the esterification and transfer of cell-derived cholesterol. J Biol Chem. 1989 Apr 25;264(12):7066–7072. [PubMed] [Google Scholar]
- Gargouri Y., Moreau H., Pieroni G., Verger R. Human gastric lipase: a sulfhydryl enzyme. J Biol Chem. 1988 Feb 15;263(5):2159–2162. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Jr, Goerke J., Guo L. S., Williams M. C., Havel R. J. Unilamellar liposomes made with the French pressure cell: a simple preparative and semiquantitative technique. J Lipid Res. 1980 Nov;21(8):981–992. [PubMed] [Google Scholar]
- Jauhiainen M., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. An elucidation of the catalytic mechanism. J Biol Chem. 1986 May 25;261(15):7032–7043. [PubMed] [Google Scholar]
- Jauhiainen M., Stevenson K. J., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. The vicinal nature of cysteine 31 and cysteine 184 in the catalytic site. J Biol Chem. 1988 May 15;263(14):6525–6533. [PubMed] [Google Scholar]
- Jordan-Starck T. C., Rodwell V. W. Role of cysteine residues in Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl-CoA reductase. Site-directed mutagenesis and characterization of the mutant enzymes. J Biol Chem. 1989 Oct 25;264(30):17919–17923. [PubMed] [Google Scholar]
- Kanaya S., Kimura S., Katsuda C., Ikehara M. Role of cysteine residues in ribonuclease H from Escherichia coli. Site-directed mutagenesis and chemical modification. Biochem J. 1990 Oct 1;271(1):59–66. doi: 10.1042/bj2710059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J., Fielding C., Drayna D., Dieplinger H., Baer B., Kohr W., Henzel W., Lawn R. Cloning and expression of human lecithin-cholesterol acyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2335–2339. doi: 10.1073/pnas.83.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J., Wion K., Drayna D., Fielding C., Lawn R. Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression. Nucleic Acids Res. 1986 Dec 9;14(23):9397–9406. doi: 10.1093/nar/14.23.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. B., Yüksel U., Gracy R. W., Lacko A. G. The catalytic center of lecithin:cholesterol acyltransferase: isolation and sequence of diisopropyl fluorophosphate-labeled peptides. Biochem Biophys Res Commun. 1987 Feb 27;143(1):360–363. doi: 10.1016/0006-291x(87)90673-5. [DOI] [PubMed] [Google Scholar]
- Piran U., Nishida T. Utilization of various sterols by lecithin-cholesterol acyltransferase as acyl acceptors. Lipids. 1979 May;14(5):478–482. doi: 10.1007/BF02533465. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke K. T., Norum K. R. Determination of lecithin: cholesterol acyltransfer in human blood plasma. Scand J Clin Lab Invest. 1971 Feb;27(1):21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- Subbaiah P. V., Albers J. J., Chen C. H., Bagdade J. D. Low density lipoprotein-activated lysolecithin acylation by human plasma lecithin-cholesterol acyltransferase. Identity of lysolecithin acyltransferase and lecithin-cholesterol acyltransferase. J Biol Chem. 1980 Oct 10;255(19):9275–9280. [PubMed] [Google Scholar]
- Swaney J. B., Orishimo M. W., Girard A. Enzymatically induced alterations in the structure of rat serum lipoproteins. J Lipid Res. 1987 Aug;28(8):982–992. [PubMed] [Google Scholar]
- Yang C. Y., Manoogian D., Pao Q., Lee F. S., Knapp R. D., Gotto A. M., Jr, Pownall H. J. Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme. J Biol Chem. 1987 Mar 5;262(7):3086–3091. [PubMed] [Google Scholar]