Abstract
Personalized medicine promises to revolutionize cancer therapy by matching the most effective treatment to the individual patient. Using a nanoparticle-based system, we predict the therapeutic potency of anticancer medicines in a personalized manner. We carry out the diagnostic stage through a multidrug screen performed inside the tumour, extracting drug activity information with single cell sensitivity. By using 100 nm liposomes, loaded with various cancer drugs and corresponding synthetic DNA barcodes, we find a correlation between the cell viability and the drug it was exposed to, according to the matching barcodes. Based on this screen, we devise a treatment protocol for mice bearing triple-negative breast-cancer tumours, and its results confirm the diagnostic prediction. We show that the use of nanotechnology in cancer care is effective for generating personalized treatment protocols.
 Determining the most effective treatment for each cancer patient is a key challenge in cancer therapy. In this article, the authors show, in a mouse model of breast cancer, that DNA barcoded nanoparticles can be used for pre-screening the efficacy of anticancer drugs.
Determining the most effective treatment for each cancer patient is a key challenge in cancer therapy. In this article, the authors show, in a mouse model of breast cancer, that DNA barcoded nanoparticles can be used for pre-screening the efficacy of anticancer drugs.
In cancer, a key challenge is determining the most effective medicinal treatment for each patient. In the past, after a short chemotherapeutic cycle, biopsies were taken from the patient's tumour and examined histologically for tumour response1. Imaging technologies later enabled the tracking of treatment efficacy, based on tumour regression/progression, vasculature proliferation and metabolic activity2,3. However, even advanced imaging modalities have their restrictions, including overlapping spectral bandwidths of the contrast agents and poor light penetration in tissues4. This limits the assessment of the therapeutic activity at a single-cell level, or at differentiating between the therapeutic activities of multiple drugs inside the tumour. In recent years, genetic signatures and patient-specific biomarkers have advanced the field of personalized medicine5. Yet gaps in our understanding of the complex genetic and epigenetic pathways6 leave room for complementary technologies that probe for drug activity inside the tumour cells.
To prescreen multiple drugs for their anticancer activity, in vitro and in vivo assays were developed7,8. These tests are based on tumour tissue that is biopsied from a patient and then either grown in a dish (in vitro) or implanted into immune-deficient mice (in vivo)7. Subsequently, the cells, or the mice, are treated with various drugs to predict the most efficient treatment. Although these methods are available, controversy regarding the accuracy of the in vitro approach9, as well as the lengthy in vivo implantation process, have hampered wide clinical implementation10.
Nanomaterials are becoming important medical tools, granting therapeutic precision and diagnostic functionality that cannot be attained using methods of larger scale11,12. More than 40 nanomedicines have already been approved for clinical use and experimental nanotechnologies have the potential to revolutionize diagnosis and care13,14.
Therapeutic nanoparticles are effective carriers of a wide range of medicines, including small molecules, nucleic acids and proteins15. These nanoparticles can target diseased tissues, including tumours and metastasis16, as well as track the biodistribution of drugs at real time17,18. More specifically, liposomes, vesicles with an inner aqueous core surrounded by a lipid bilayer, are clinically approved drug carriers11. When injected intravenously, 100 nm liposomes accumulate preferentially in solid tumours, penetrating through defects in the endothelial wall of tumour capillaries19. This phenomenon is known as passive targeting, or as the enhanced permeability and retention effect20. Although not all tumours display permeable vasculature, the enhanced permeability and retention effect has been demonstrated pre-clinically and clinically in metastatic breast21,22 and ovarian cancers23,24, melanoma25,26, AIDS-related Kaposi sarcoma27,28 and other diseases29,30,31.
For diagnostic applications, synthetic DNA has been suggested as a useful probe32,33,34,35,36. A DNA barcode is a sequence of nucleotides that can be decoded using one or more technologies, such as PCR and sequencing. These assays have several benefits, including an extremely sensitive detection threshold (∼30 attomolar), high versatility and a short analysis time compared with other assays36,37,38,39.
Herein, we sought to harness these advantages of nanotechnology, combined with DNA barcodes, to screen anticancer drugs for their therapeutic potency inside the patient's tumour (Fig. 1). Screening active agents inside a patient's body is adapted conceptually from allergy tests, in which minuscule doses of allergens are injected subdermally. Unlike allergy tests, which elicit a visible reaction on the skin, detecting the activity of multiple medicines inside the tumour cells adds another level of complexity to the process. Therefore, we load nanoparticles with anticancer medicines and corresponding DNA barcodes, enabling us to track the drugs that entered each tumour cell and follow their potency more precisely. Using the proposed method, we were able to advise an efficient, personalized therapeutic protocol within less than 72 h.
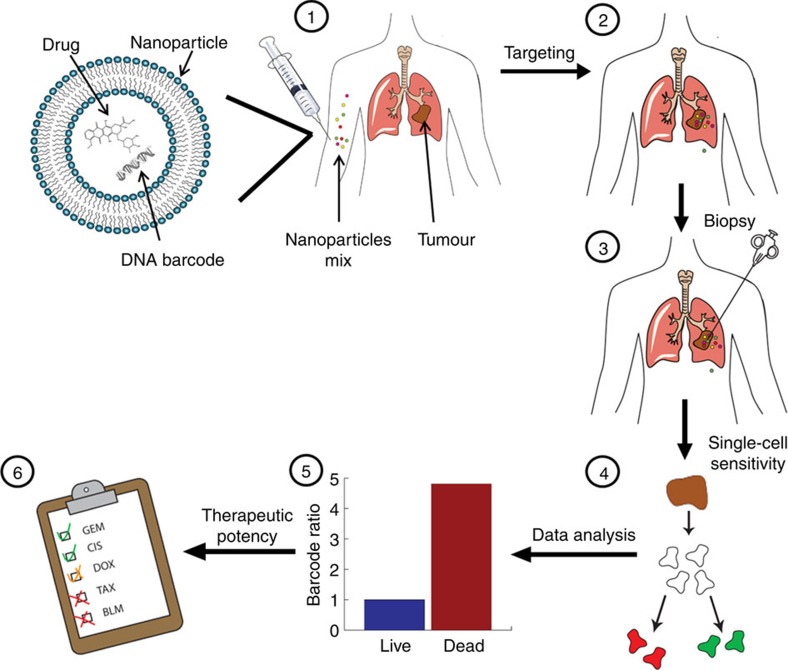
Figure 1. Using nanotechnology to probe the sensitivity of cancer to medicines.
Barcoded nanoparticles (BNPs) were loaded with different drugs and corresponding DNA barcodes. (1) A cocktail of BNPs was injected intravenously. (2) The BNPs targeted the tumour and each of the drugs carried out its therapeutic activity inside different tumour cells. (3) Forty-eight hours later, a biopsy was taken from the tumour, and the biopsied tissue was dissociated into a single-cell suspension. (4) Each of the cells was sorted according to its viability (live/dead). (5) The barcodes were extracted from the live/dead cells and amplified using real-time PCR. The codes were detected by sequencing. The activity of the drugs inside the tumour was analysed by recording the number of each barcode found in the live cells, versus the number of barcodes found inside the dead cells. In this manner, the orthotropic tumour was used as a miniature laboratory, which was diagnosed with the nanoparticles at a cellular level. (6) Based on the screened results, a suggested treatment protocol was devised. In our studies, we found this predictive assay to achieve the best therapeutic results. The overall diagnosis takes less than 72 h.
Results
Barcoded nanoparticles
The nanoscale probes, used for gauging drug activity, were constructed of 100 nm liposomes (Supplementary Fig. 1), loaded with minuscule amounts of anticancer agents and corresponding synthetic DNA strands. DNA offers unlimited barcoding possibilities, with extremely sensitive detection modalities40. We chose to work with 50 to 120 base-pair (bp)-long double-stranded DNA barcodes. At these lengths, DNA is less susceptible to endogenous degradation by DNase41 or to eliciting immune responses34.
The barcode has three regions, which allowed us to detect and quantify it at the single-cell level; a forward primer (20 bp), a code region (10–80 bp) and a reverse primer (20 bp). Varying the primers, or altering the barcode sequence and length, enables detecting the barcode by sequencing, PCR and gel electrophoresis (see the ‘Methods' section, Fig. 2a,d). In addition, the sequences were kept nonspecific, biologically noncoding and were designed not to form major secondary structures (for a full sequence list, see Table 1 in the ‘Methods' section). Each nanoparticle was loaded with approximately 15 barcodes.
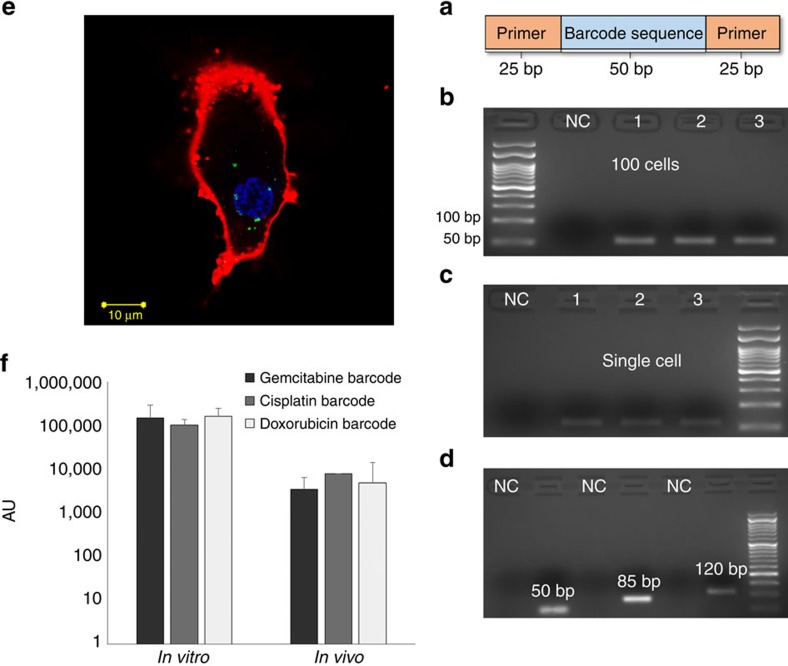
Figure 2. DNA was used as a barcode for labelling and detecting nanoparticles in single cells.
Synthetic DNA strands were embedded in 100 nm liposome together with a corresponding drug. (a) The barcodes were constructed to be in the range of 50 to 120 bp long, and detected using PCR and sequencing. Barcoded nanoparticles were taken up spontaneously by triple-negative breast cancer cells in culture and in tumours. (b) Gel electrophoresis of PCR-amplified barcodes derived from 100 cells, and (c) from a single cell. (d) Different strand lengths of barcodes (50, 85, 120 bp) can be detected within a single-cell suspension. Negative control wells are designated NC, and repeats are numbered 1–3. (e) A confocal microscopy image of uptake of BNPs by a triple-negative breast cancer 4T1 cell labelled for membrane (red), nucleus (blue) and DNA barcodes (green). The single-cell uptake of barcoded nanoparticles is not affected by the cargo (f). Barcoded nanoparticles, all 100 nm in diameter but loaded with different cargo, were added to triple-negative breast cancer cells in culture, or injected intravenously into tumour-bearing mice. To ensure the uptake of multiple particles per cell, a dose 1,000 times higher than that used for the diagnostic procedure was administered. The cells were collected from the dish (after 24 h), or a biopsy was taken from the tumour (after 48 h). The tissue was then dissociated, and 60 individual cells were examined for the presence of each of the different barcodes. Each cell contained a similar number of each of the barcodes, indicating that the internal payload of the nanoparticles does not affect their cellular uptake. The data were calculated as the mean±s.e.m. of n=60.
Table 1. Barcode sequences.
Barcoded nanoparticles detected in a single cancer cell
When the barcoded nanoparticles were added to triple-negative 4T1 breast cancer cells in culture, they were taken in readily by the cells. To detect the barcodes, we washed the cells thoroughly and sorted them, by FACS, to clusters of 1,000, 100, 10 or single cell. The cells were lysed, and the barcodes were extracted and amplified using real-time (RT) –PCR. Both the carrier (liposome) and the barcodes were detected at a single-cell level, using both microscopy and biochemical assays (Fig. 2b,c,e). After the extraction process, the barcodes were also sequenced, to validate the specificity of the process. Free (non-particulate) DNA barcodes were not taken in by the cells; indicating that the barcodes found intracellularly were carried into the cell by the nanoparticle (Supplementary Fig. 2).
Barcoded nanoparticles were loaded with an anticancer drug or with a control compound (see the ‘Methods' section). The number of barcodes found intracellularly corresponded to the concentration of drug found inside the cells. Specifically, HPLC analysis of the drug found in cells, correlated with the RT–PCR quantification of the cellular barcodes (Supplementary Fig. 3a).
Barcoded nanoparticles accumulate in the tumour
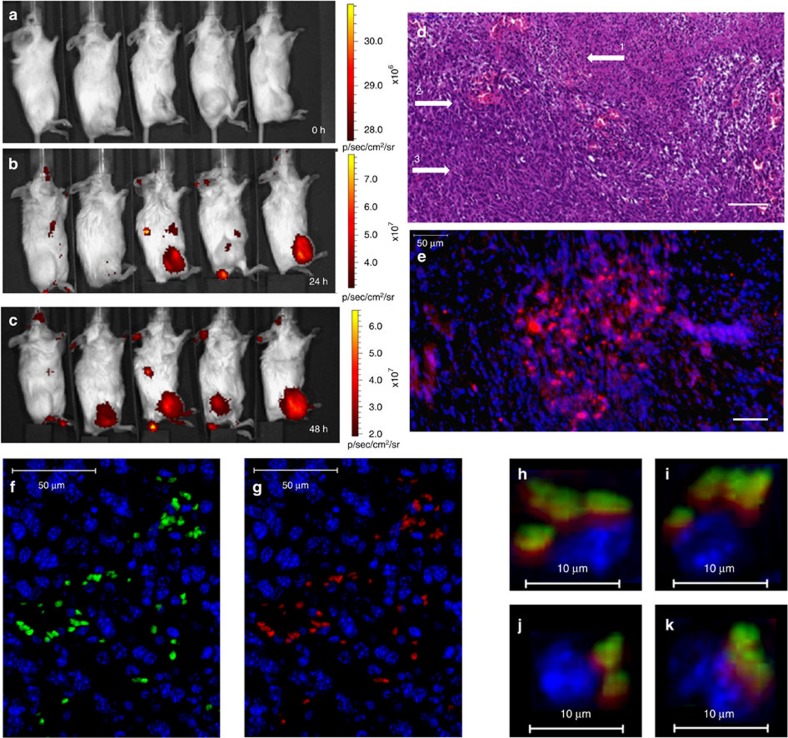
We studied the biodistribution of nanoparticles in BALB/c mice bearing triple-negative breast cancer (TNBC, 4T1) xenografts. Barcoded liposomes, loaded with a diagnostic agent (indocyanine green, ICG), were injected intravenously, and the mice were imaged over a period of 48 h. We compared the preferential accumulation of the liposomes in the tumour site with the free dye (Fig. 3a–c). The free dye was observed in the tumour, spleen and liver, during the first 24 h, while the liposomal accumulation in the tumour increased over a period of 48 h (Supplementary Fig. 4). Histological analysis of the tumour showed that the nanoparticles were taken up by the tumour cells (Fig. 3d–k), which was also confirmed after extracting the barcodes from the tumour cells. To achieve this, the tumours were resected and dissociated enzymatically into single-cell suspension. We were able to detect 0.1% of the injected dose of the barcoded nanoparticles inside the biopsied tumour cells.
Figure 3. Barcoded nanoparticles accumulate in triple-negative breast cancer tumour cells.
Barcoded nanoparticles, containing the diagnostic imaging agent indocyanine green (ICG), were injected intravenously to BALB/c mice bearing a tumour in their hind leg. The animals were imaged over 48 h: before the injection (a), 24 h (b) and 48 h after the injection (c). The fluorescent ICG intensity is presented by the red–yellow scale bar on the right side of each sub-figure. The left mouse on each figure is a healthy control and all the animals to the right are tumour-bearing mice; all animals were injected with 150 μl of barcoded ICG nanoparticles. After 48 h, the tumour was resected and examined histologically. The tumour tissue was stained with haematoxylin and eosin stain (H&E) (d). The tumour is closely packed where three major parts can be observed: necrotic tissue (1), multiple blood vessels that contribute to the EPR effect (2) and cancerous cells (3). To detect the liposomal accumulation in the tumour tissue, an overlay fluorescent image is presented (e); the cell nuclei were stained with DAPI (blue) and the particles with rhodamine (red). To detect the barcoded nanoparticles in single cells within the tumour, the barcode was labelled with fluorescein (f, green) and the particle membrane was labelled with rhodamine (g, red). Co-localization of the barcodes and the particles can be seen inside single cells (h–k). The scale bar in the histology images represents 100 μm, in the fluorescent images 50 μm (e–g) and in the fluorescent single cells 10 μm (h–k).
Barcoded nanoparticles as a diagnostic tool
We used the barcoded nanoparticles to investigate the tumour sensitivity to drugs. All the barcoded nanoparticles had an identical, 100 nm structure, and varied only in their internal content. Three of the barcoded nanoparticles contained clinically approved chemotherapeutics: doxorubicin, cisplatin and gemcitabine. One contained caffeine, as a control compound, and one was a placebo (nanoparticles with a barcode and phosphate-buffered saline, PBS). To ensure the screening had no systemic effect, the total dose of all the injected barcoded nanoparticles combined was below 1/1,000th the therapeutic dose.
A cocktail containing a mixture of all five barcoded nanoparticles was injected intravenously to mice bearing 4T1 xenografts. A core biopsy was taken from the tumour 48 h later. This time frame ensured that the apoptotic cells remain intact for future analysis42. The biopsied tumour tissue was dissociated enzymatically into a single-cell suspension, and then sorted by FACS according to cell viability (live/dead, using a propidium iodide stain). The live and dead cells were collected separately, washed, lysed and the DNA barcodes were extracted. Then, each group's barcodes were amplified using RT–PCR. Barcodes found in the dead cells were characterized as active drugs, whereas barcodes found in the live cells were of nonactive drugs.
During the procedure, the animals did not lose weight or change their behavioural patterns; this can be explained by the low total dose of the administered drug.
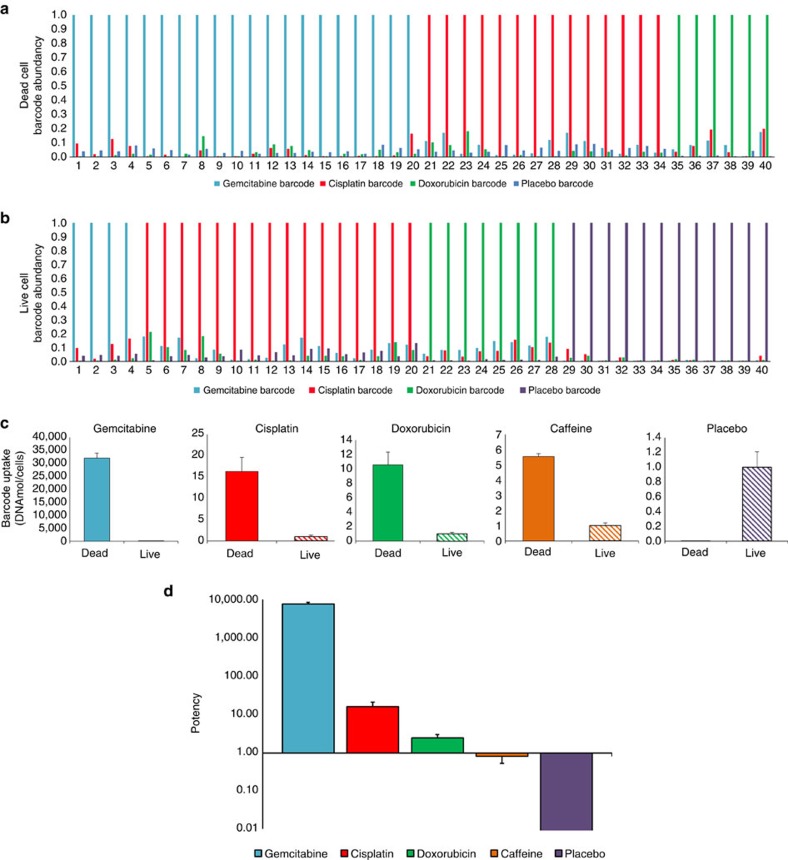
We found a correlation between the drug activity and the presence of a barcode in the live or dead cells of the tumour (Fig. 4). As expected, both doxorubicin and cisplatin showed a positive therapeutic effect, while caffeine showed a minor therapeutic effect43,44. However, the most astonishing results came from gemcitabine and the placebo. Gemcitabine was found to be the most efficient drug based on its barcode distribution. The gemcitabine barcode accumulated 30,000 times more abundantly in the dead cells compared with live cells (Fig. 4c). In contrast, the placebo barcodes accumulated 3,000 times more abundantly in live cells compared with the dead cells.
Figure 4. Predicting the therapeutic potency of multiple drugs using barcoded nanoparticles.
Mice bearing triple-negative breast cancer tumours were administered five different barcoded nanoparticles, simultaneously. The nanoparticles contained either one of the anticancer agents: doxorubicin, gemcitabine or cisplatin, or were loaded with caffeine, or empty (barcode alone). The barcoded nanoparticle cocktail was injected intravenously and accumulated in the tumour cells over a period of 48 h. After 48 h, the tumour tissue was biopsied and dissociated into single cells. The cells were sorted according to their viability (live/dead) and the barcodes in each of these populations were extracted, analysed and quantified using RT–PCR and sequencing. The activity of each of the agents was measured at the single-cell level; data from 80 representative cells are shown (a,b). A noise level below 20% was set as the threshold for determining the activity of a single agent at the single-cell level. In addition, (c) the overall activity of each of the agents in the tumour was determined by analysing the barcode abundance in groups of at least three million live or dead cells. (d) To compare between the potency of the different drugs, a potency scale was plotted. The comparative potency is based on the ratio of barcodes found in the dead cells to those found in live cells. On the basis of this comparative diagnostic scale, a treatment protocol was devised. To ensure statistical significance, each screen was based on at least three million cells extracted from the tumour. The data were calculated as the mean±s.e.m. of n=6, in two independent experimental replicates.
To rank the drugs according to their therapeutic activity, we defined the potency parameter as: BarcodeDead cells/BarcodeLive cells; the greater the ratio, the more potent the drug is in the patient. In accordance, gemcitabine was found to be the most potent drug and the placebo showed no therapeutic activity, Fig. 4d.
To ensure statistical significance and to reduce false positive hits of the drug activity analysis, we sought to have as many tumour cells as possible that contained only one type of barcode. Therefore, we limited the total number of injected barcoded nanoparticles. Our biodistribution analysis showed that 0.1% (which are 1/1,000) of the injected nanoparticles are found in the biopsied tumour cells. Furthermore, we were able to extract approximately 40,000 intact cells per mm3 tumour tissue. Therefore, the maximal number of the injected barcoded nanoparticles can be determined based on the tumour volume, and should not exceed × 40,000 (tumour volume, mm3) × 1,000. For example, for a 500 mm3 tumour, 2 × 1010 barcoded nanoparticles were injected, which correlate to a total liposomal dose of 0.005 mg drug per kg body weight.
Treatment efficacy mirrors the diagnostic procedure
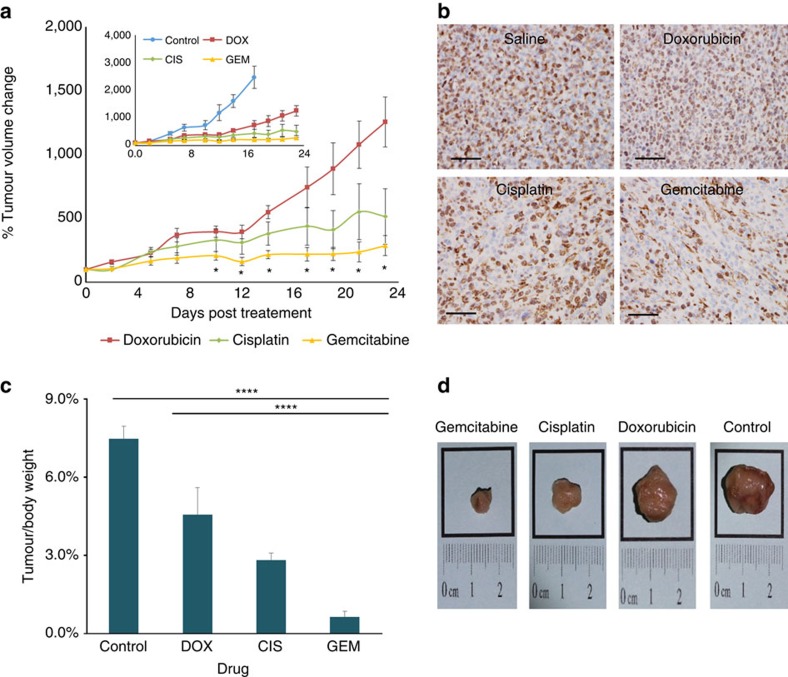
On the basis of the diagnostic analysis, we devised a treatment protocol. We administered gemcitabine, cisplatin, doxorubicin or saline to four groups of mice bearing TNBC tumours (six mice per group). The therapeutic outcome mirrored the prediction (Fig. 5). The tumour size of gemcitabine-treated mice was smallest and gemcitabine attenuated the growth of the tumours relative to doxorubicin, cisplatin and the control treatments (Fig. 5a,c,d). All the groups responded in a similar manner to the potency parameter; this was also exemplified by the Kaplan–Meier survival curve (Supplementary Fig. 5). Twenty-four days after beginning the treatment, the tumours were resected for tissue analysis. Interestingly, the histological examination demonstrated that tumours that showed clinical response to therapy were also less cellular and had lower mitotic counts. An immunohistochemical analysis (using anti-Ki67 antibody) demonstrated a lower proliferation index in tumours that received gemcitabine (40%) in comparison with those treated with doxorubicin (65%), cisplatin (85%) or saline (90%; Fig. 5b). In summary, the histological evaluation of gemcitabine-treated mice had a reduced proliferative state in comparison with the other groups, indicating improved prognoses also at the tissue level (Fig. 5b).
Figure 5. Treating mice according to the barcode analysis.
Based on the barcoded nanoparticle drug screen, a treatment protocol was devised. Mice bearing triple-negative breast cancer tumours were administered doxorubicin, gemcitabine, cisplatin or saline (control). Tumour growth (a) was recorded and postmortem resection (c) and histology (b) of each of the groups was performed. Each group received a therapeutic weekly dose of chemotherapy—doxorubicin (5 mg kg−1), cisplatin (6 mg kg−1) or gemcitabine (125 mg kg−1), while the control group was administered saline. The tumours were resected 23 days after starting the treatment. Tissue slides (b) were immunohistochemically stained with rabbit monoclonal anti-Ki67 antibody to compare the proliferation rate of each group. These show a reduction in the proliferation rate in the gemcitabine-treated tumours compared with the other groups. The data were calculated as the mean±s.e.m. of n=6 per group; *P<0.01; ****P<0.0001. Differences between the two means were tested using an unpaired, two-sided Student's t-test. The efficacy of the treatment is also shown in the tumour size (d), thereby indicating the potency of the treatment. Before introducing the treatment, all the mice had the same average tumour size.
Discussion
Personalized medicine aims to tailor treatments to accommodate each patient's unique disease presentation45,46. Since patients respond differently to medication; choosing the best drug for the individual patient, at the right time, is crucial for the success of the treatment. Common assays of personalized medicine involve genetic signatures and patient-specific biomarkers. However, gaps in our understanding of genetics and epigenetic pathways leave room for complementary diagnostic technologies that predict drug activity inside the tumour.
Our approach is to probe the patient's tumour for its sensitivity to anticancer medications, before beginning a treatment cycle. For this, we developed DNA-barcoded nanoparticles loaded with minuscule doses of various therapeutic agents. The nanoparticles were shown to target the tumour cells after being injected intravenously (Fig. 3), and the barcodes were used as mediators to correlate which drug is most therapeutically efficient. Double-stranded DNA offers unlimited barcoding possibilities, a relatively long half-life in vivo (Fig. 4)41, and quantitative detection modalities that far exceed analytical methods used for detecting a drug in tissue40. In addition, DNA cannot penetrate a cell without a vehicle, thereby ensuring that the cellular presence of a barcode was mediated by the nanoparticle. The barcode enabled us to assess the therapeutic sensitivity at various levels of detection, reaching even a single cell (Fig. 2b,c,e). Our analysis showed that approximately 0.1% of the intravenously injected nanoparticles are taken up by the tumour cells.
Theoretically, hundreds of different drugs/anticancer agents can be screened with the technology. The noise of this screen can rise due to uptake of several particles by an individual cell. To optimize the signal-to-noise ratio of the technology, there is a need to both minimize the number of particles per cell and to maximize the number of analysed cells. In fact, we found that injecting less than 4 × 107 barcoded nanoparticles per cubic millimetre tumour, results in a single nanoparticle per cell. This takes into account the biodistribution of the nanoparticles and our ability to source approximately 40,000 intact cells from each cubic millimetre of dissociated tumour. To improve the predictive confidence, and to increase tumour-cell heterogeneity, we analysed three million cells from each tumour. Furthermore, after sorting the cells according to their viability and barcode type, we insisted on having at least 100,000 cells per efficacy subgroup (such as dead cells with gemcitabine or live cells with caffeine).
The disease model we chose to evaluate the technology in was TNBC. Owing to an absence of hormone receptors such as oestrogen and progesterone, nor overexpressed HER2, triple-negative tumours lack a clear medicinal treatment modality. TNBC also has poor prognosis, low survival and high recurrence rates. TNBC treatments usually involve systemic chemotherapy infusions47.
We used the barcoded nanoparticles to screen three clinically approved chemotherapeutics: doxorubicin, cisplatin and gemcitabine, all prescribed in advanced breast cancer48,49,50,51,52. Among the three, gemcitabine was found to be the most efficient drug, based on its barcode distribution in dead tumour cells. After gemcitabine, both doxorubicin and cisplatin showed a positive therapeutic effect as expected. The placebo barcode showed no therapeutic activity (Fig. 4d). As a diagnostic tool, the barcode distribution in cells correlated well with the therapeutic potency (live/dead, Fig. 4c), reducing false-positives. The rationale behind primarily finding barcodes of potent drugs in dead cells is that once a cell begins apoptotic processes it will not take up additional nanoparticles. Live cells, which took up ineffective drugs or placebo particles, were found alive in the tumour.
Then we tested the accuracy of the diagnostic prediction in a treatment mode. Tumour-bearing animals were treated with each of the drugs and the tumour progression was tracked. While nanoparticulate drugs enabled diagnostic prescreening at the single-cell level, the treatment was performed using an intravenous infusion of the free drugs (simulating the common clinical route of administration). Mice bearing breast cancer tumours were administered gemcitabine, cisplatin, doxorubicin or saline. The parameters of final tumour size, progression rate and proliferative state mirrored the intratumoral cellular prediction (Fig. 5).
To conclude, this study describes the development of a diagnostic nanotechnology that prescreens multiple drugs inside the tumour, with emphasis on cellular sensitivity. From a clinical perspective, our aim is to provide physicians with a new tool that matches each patient with the most potent medicine, during the different stages of disease to improve cancer care.
Methods
Liposome fabrication
Liposomes were composed of 55 mole% hydrogenated soybean phosphatidylcholine (HSPC; Avanti Polar Lipids, Alabaster, AL, USA), Mw 762 g mol−1; 5 mole% polyethylene glycol distearoyl-phosphoethanolamine (m2000PEG DSPE; Avanti), Mw 2,805 g mol−1; and 40 mole% cholesterol (Sigma-Aldrich, Revohot, Israel), Mw 386.65 g mol−1. Loading concentration of the lipid was 50 mM in a 5% w/v aqueous dextrose solution (5% DEX). To form liposomes, lipids were dissolved in pure ethanol, warmed to 65 °C and added to 1 ml of the 5% DEX. The liposomes were downsized by stepwise extrusion, using 400, 200, 100 and 50 nm pore-size polycarbonate membranes (GE Water and Process Technology, Osmonics) in a Lipex Extruder (Northern Lipids, Canada). After extrusion, the size was measured, using dynamic light scattering (ZetaSizer ZSP, Malvern Instruments, UK). The polydispersity index was between 0.04 and 0.08 and particle size 110±10 nm. In addition, the nanoparticles were characterized qualitatively using CryoTEM (‘Supplementary Methods').
Drug-loaded barcoded nanoparticles
Desalted dsDNA oligos (Integrated DNA Technologies, IDT, Leuven, Belgium) ranging 50–120 bp (Table 1) were annealed at 60 °C and diluted to a concentration of 100 μM. The barcodes were inserted into the liposomes during the lipid hydration process by adding them to the aqueous phase at a DNA/lipid ratio of 1:20, respectively. At that point the liposomes were extruded to reach the final size. Non-encapsulated DNA was removed by dialysis, using a 106 MW cutoff membrane (Spectrum Laboratories, Inc., USA).
Drug-loaded barcoded liposomes were prepared either by active (doxorubicin and gemcitabine) or passive (cisplatin, caffeine) loading. The barcodes were loaded as described above. Doxorubicin and gemcitabine were loaded into barcoded nanoparticles using an ammonium sulfate gradient, reaching a final concentration of 1 mg ml−1 according to Haran et al.53. Cisplatin was loaded in an NaCl environment according to Peleg-Shulman et al.54. Caffeine was dissolved using DMSO/ethanol (1/1, v/v) together with the lipids and then hydrated using 5% dextrose. The non-encapsulated drugs were removed by dialysis. When injected intravenously, the dose of all the drugs combined is held below 0.005 mg drug per kg body weight.
Cellular uptake
In vitro experiments were conducted on 4T1 (triple-negative breast cancer, ATCC) murine cell lines and MDA-MB-231 (human breast cancer, ATCC) in culture (Roswell Park Memorial Institute medium; Sigma-Aldrich, Rehovot, Israel). Twenty-four hours after seeding the cells, 10% of the volume was replaced with the barcoded liposome solution and incubated for 24 h at 37 °C.
To test the barcode uptake, the cells were washed thoroughly with PBS, detached with trypsin, centrifuged at 400g for 5 min, and the DNA was extracted using a modified Bligh and Dyer assay55. Specifically, a chloroform:methanol:sample, ratio of 1:1:1 (v/v) mixture was prepared. The sample was centrifuged at 400g for 5 min, achieving phase separation, where the upper aqueous phase contained both the genetic and the synthetic dsDNA barcodes.
PCR and RT–PCR
dsDNA barcodes were amplified by PCR in a thermocycler (LabCycler SensoQuest PCR, SensoQuest, Germany) and quantified by RT–PCR thermocycler (BioRad CFX96, Bio-Rad Laboratories Ltd., Israel). Cycling times: 5 min at 95 °C, (15 s at 95 °C, 45 s at 63 °C) × 40 cycles. Then, the solution was loaded on a 3% (w/w) agarose gel and separated for 25 min at 100 V (Wide Mini-Sub Cell GT Cell and PowerPac Basic Power Supply, Bio-Rad Laboratories Ltd., Israel). To estimate DNA band size, a DNA ladder was used (50 bp DNA ladder RTU, GeneDireX, Hy Laboratories, Israel). To quantify the barcodes, real-time PCR was used. Following DNA extraction, the strands were amplified and analysed using TaqMan Probe (qPCRBIO Probe Mix Lo-Rox+PrimeTime Mini qPCR Assay; IDT). Each barcode was independently amplified using specific probe and primers. Cycling times: 5 min at 95 °C, (15 s at 95 °C, 45 s at 63 °C) × 40 cycles. After PCR and DNA purification using a QIAquick PCR Purification Kit (Qiagen, CA, USA), the samples were sequenced (HyLabs, Jerusalem, Israel).
Cell sorting
The cells were sorted using a flow-activated cell sorter (FACS; FACSARIA III, BD Biosciences, San Jose, CA, USA), and separated according to: (i) cell count—1,000, 100, 10 cells and a single cell; and (ii) viability condition—dead cells were labelled using propidium iodide (PI) assay56. Single cells were selected after defining a gate in FSC/SSC dot plot. To sort live/dead cells, a subpopulation in the single cells population was chosen. The dead cell's gate was defined for PI positive staining.
Barcode encapsulation and uptake
The cells were seeded in optical plates (60 μ-Dish 3.5 cm, high‘−'ibidi‘', Madison, WI, USA) for 24 h, and then exposed for an additional 24 h to liposomes encapsulating fluorescently labelled dsDNA barcodes (labelled with 6-flourescin amitide—IDT). Then, the cells were washed thoroughly and the cell membrane was stained with DID (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt, Biotium, USA) or Hoechst (Life Technologies, USA) for nuclei labelling. The cells were imaged using confocal microscopy (LSM-700, Zeiss, Germany) equipped with Axiovision software, and +cell observer (Zeiss). The images were analysed using Imaris software (Bitplane, Zurich, Switzerland).
Tumours
All the animal studies were approved by, and complied with, the institutional and ethical committee at the Technion. Animal well-being was monitored daily by the researchers and staff veterinarians.
A total 106 4T1 cells, suspended in 100 μl PBS, were injected subcutaneously to 10-week-old BALB/c female mice (Harlan Laboratories Inc., Jerusalem, Israel). About 14 days later (allowing the primary tumour to reach 5 mm in diameter) barcoded liposomes were injected intravenously at a barcode concentration of 500 nM. After 48 h, the mouse was killed and its organs (+tumour) were sourced. In addition, a core biopsy was taken from live mice using an adjustable coaxial Temno biopsy device equipped with an 11-cm long, 20 G needle (San Diego, CA, USA). The average tissue biopsy volume was 40 mm3 (corresponding to approximately 40 million cells)57,58.
Single-cell suspension
The tumour was held on ice-cold PBS until the beginning of tissue dissociation, always within less than 1 h from excising the tissue. Then, the tissue was dissociated enzymatically using 25 mg ml−1 hyaluronidase (Sigma-Aldrich, Rehovot, Israel), 25 mg ml−1 collagenase-3, 50 mg ml−1 collagenase-4 (Worthington Biochemical, Lakewood, NJ, USA) in 2 ml sample, 37 °C, and incubation of 40. The dissociation continues physically using a GentleMacs machine (Miltenyi Biotec, Teterow, Germany) based on mouse tumour dissociation protocol. Single-cell suspension was obtained by passing the suspension in 70 μm cell strainer (BD Biosciences, San Jose, CA, USA). The average number of cells that are sorted is about 5 million cells.
Live and dead assay
After dissociating the tumour tissue into a single-cell suspension, the cells were counted and stained using PI. The PI intercalates with the DNA in intact dead cells that have sufficient permeable membrane. We found that intact dead cells with barcodes inside them are most effectively sourced from the tumour within 48 h after the administration of barcoded nanoparticles.
DNA extraction from primary tumour or tissue
After sorting the cells, they were centrifuged (400g, 7 min). The supernatant was discarded and the modified Bligh and Dyer assay was performed (see above). The upper (aqueous phase) was concentrated using a Speedvac Concentrator (UNIVAPO 100H, UNIEQUIP, Fraunhofer, Germany) and the DNA barcodes were analysed using RT–PCR and sequencing.
Bio-distribution of ICG-liposomes in subcutaneous 4T1 tumours
The experiment was carried out in 10-week-old female BALB/c mice (Harlan Laboratories Inc.). Murine breast cancer cell line, 4T1 (PerkinElmer, MA, USA), was injected subcutaneously into the rear right flank (5 × 105 cells in 100 μl of PBS). Three weeks after the injection, 150 μl of 120 nm ICG liposomes or free ICG dye (0.09 mg ml−1) were injected intravenously through the mice's tail vein. The bio-distribution of particles or free dye was monitored under In Vivo Imaging System for a period of 72 h.
In vivo therapeutic efficiency
Four groups (n=6 each) were divided as follows: control, cisplatin, doxorubicin and gemcitabine. Hundred microlitres of 106 cells ml−1 of 4T1 (triple negative breast cancer) were injected subcutaneously to 10-week-old BALB/c female mice. The mice were weighed and the tumour dimensions were taken three times a week. The tumour volume was measured with a caliper (Digital-Messschieber mit Rolle und Datenausgang, MIB -Messzeuge GmbH, Germany and calculated as length/2 × (width)2. Therapeutic treatment began when the tumour volume reached ∼500 mm3, approximately 10 days after the initial tumour cells injection. All the therapeutic groups received a single dose each week as follows: doxorubicin 5 mg kg−1, cisplatin 6 mg kg−1 and gemcitabine 125 mg kg−1. The control group received 100 μl of saline.
Fluorescence tissue microscopy
The slides of the paraffin-embedded tissue were scanned using a Pannoramic Midi Virtual Microscope (3D Histech, Hungary) equipped with a Turrets Chroma 49000/2/4-ET reflector and T-400/495/565 beam-splitter. The cell nuclei were stained with DAPI, DNA barcodes with fluorescein, and nanoparticles with rhodamine. The images were analysed using Pannoramic Viewer software (3D Histech).
Histology
After killing the mice, the tumour was extracted and kept in 10% natural buffer formalin at room temperature. Later, the tissue was paraffin embedded. The slides from the tumours were stained with haematoxylin and eosin. In addition, the tissue slides were immunohistochemically stained with rabbit monoclonal anti-Ki67 antibody (SP6; Thermo Scientific, Fremont, CA, USA, diluted 1:300 in PBS) to determine the proliferation index.
The slides of the paraffin-embedded tissue were scanned and analysed using a Panoramic Midi virtual microscope (3D Histech) with a × 20 objective magnification, 30 ms DAPI exposure time, 700 ms EGFP and CY3 exposure time.
Statistics
Differences between two means were tested using an unpaired, two-sided Student's t-test.
Data availability
All data that support the findings of this study are available from the corresponding author upon request.
Additional information
How to cite this article: Yaari, Z. et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 7, 13325 doi: 10.1038/ncomms13325 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1-5 and Supplementary Methods
Acknowledgments
This work was supported by the EU-FP7 Marie Curie Program (No. 333797), Israel Science Foundation (No. 1778/13), Israel Cancer Association (No. 20150116), Israel Ministry of Economy (No. 52752), Israel Ministry of Science, Technology and Space (3–11878), GIF (No. 1-2328-1139. 10/20), ERC (2015-STG-680242), Mallat Family Foundation and the Alon Fellowship and a Taub Fellowship. We gratefully thank the staff of the Technion's Lorry I. Lokey Interdisciplinary Center for Life Sciences and Engineering and the Russell Berrie Nanotechnology Institute, for their expert technical assistance. In addition, we wish to thank Dr E. Suss Toby, M. Holdengreber and O. Schwartz from the Bioimaging Center at the Technion Faculty of Medicine, for their assistance with imaging and image analysis. Furthermore, we gratefully thank the Pre-Clinical Research Authority at the Technion for assistance with in vivo experiments. Specially we thank Dr R. Shofti, Dr D. Levin Ashkenazi, N. Amit and V. Zlobin. We also thank E. Kesselman for assisting in acquiring CryoTEM images of the liposomes. We thank Professor Ayelet Fishman, Professor Roee Amit and Professor Yuval Shoham for granting us access to their instruments used in this study. We thank Ms Shirley Pattison for editing this manuscript.
Footnotes
Author contributions Z.Y. designed and performed the experimental work, analysed the data and wrote the manuscript. D.d.S. conducted in vitro experiments and created Fig. 1. A.Z. conducted in vivo experiments and tissue microscopy analysis. E.G. conducted in vivo experiments and fabricated ICG liposomes. A.K. and R.T. conducted in vitro and in vivo experiments. E.B. conducted FACS experiments. N.D. assisted in acquiring and analysis of in vitro microscopy. D.H. analysed the histological samples. J.S. and M.G. analysed the data, wrote and edited the manuscript. A.S. proposed the hypothesis, designed the experimental procedures, approved the data analysis, participated in the in vivo trials and wrote the manuscript.
References
- Symmans W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007). [DOI] [PubMed] [Google Scholar]
- Thomas E. et al. The use of alternate, non-cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized trial. J. Clin. Oncol. 22, 2294–2302 (2004). [DOI] [PubMed] [Google Scholar]
- Buzdar A. U. et al. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J. Clin. Oncol. 17, 3412–3417 (1999). [DOI] [PubMed] [Google Scholar]
- Roxbury D. et al. Hyperspectral microscopy of near-infrared fluorescence enables 17-chirality carbon nanotube imaging. Sci. Rep. 5, 14167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C. & Piccart M. J. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat. Rev. Cancer. 7, 545–553 (2007). [DOI] [PubMed] [Google Scholar]
- van't Veer L. J. & Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature 452, 564–570 (2008). [DOI] [PubMed] [Google Scholar]
- Abelson S., Shamai Y., Berger L., Skorecki K. & Tzukerman M. Niche-dependent gene expression profile of intratumoral heterogeneous ovarian cancer stem cell populations. PLoS ONE 8, e83651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortazar P. & Johnson B. E. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J. Clin. Oncol. 17, 1625–1631 (1999). [DOI] [PubMed] [Google Scholar]
- Burstein H. J. et al. American Society of Clinical Oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J. Clin. Oncol. 29, 3328–3330 (2011). [DOI] [PubMed] [Google Scholar]
- Torchilin V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 13, 813–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark W. J. Nanoparticles in biological systems. Angew. Chem. Int. Ed. 50, 1242–1258 (2011). [DOI] [PubMed] [Google Scholar]
- Peer D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007). [DOI] [PubMed] [Google Scholar]
- Lammers T., Aime S., Hennink W. E., Storm G. & Kiessling F. Theranostic nanomedicine. Acc. Chem. Res. 44, 1029–1038 (2011). [DOI] [PubMed] [Google Scholar]
- Khandare J., Calderon M., Dagia N. M. & Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chem. Soc. Rev. 41, 2824–2848 (2012). [DOI] [PubMed] [Google Scholar]
- Theek B., Rizzo L. Y., Ehling J., Kiessling F. & Lammers T. The theranostic path to personalized nanomedicine. Clin. Transl. Imaging 2, 66–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Erez R., Segal E., Shabat D. & Satchi-Fainaro R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew. Chem. Int. Ed. 48, 2949–2954 (2009). [DOI] [PubMed] [Google Scholar]
- Peer D. Immunotoxicity derived from manipulating leukocytes with lipid-based nanoparticles. Adv. Drug Deliv. Rev. 64, 1738–1748 (2012). [DOI] [PubMed] [Google Scholar]
- Carmeliet P. & Jain R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000). [DOI] [PubMed] [Google Scholar]
- Matsumura Y. & Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986). [PubMed] [Google Scholar]
- Schroeder A. et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 12, 39–50 (2012). [DOI] [PubMed] [Google Scholar]
- Harries M., Ellis P. & Harper P. Nanoparticle albumin-bound paclitaxel for metastatic breast cancer. J. Clin. Oncol. 23, 7768–7771 (2005). [DOI] [PubMed] [Google Scholar]
- Gibson J. M., Alzghari S., Ahn C., Trantham H. & La-Beck N. M. The role of pegylated liposomal doxorubicin in ovarian cancer: a meta-analysis of randomized clinical trials. Oncologist 18, 1022–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano C. et al. Clinical trials with pegylated liposomal Doxorubicin in the treatment of ovarian cancer. J. Drug Deliv. 2013, 898146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundra V., Li W. & Mahato R. I. Nanoparticle-mediated drug delivery for treating melanoma. Nanomedicine (Lond) 10, 2613–2633 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bathula S. R., Yang Q. & Huang L. Targeted nanoparticles deliver siRNA to melanoma. J. Invest. Dermatol. 130, 2790–2798 (2010). [DOI] [PubMed] [Google Scholar]
- Udhrain A., Skubitz K. M. & Northfelt D. W. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi's sarcoma. Int. J. Nanomed. 2, 345–352 (2007). [PMC free article] [PubMed] [Google Scholar]
- Cooley T. et al. A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi's sarcoma. Oncologist 12, 114–123 (2007). [DOI] [PubMed] [Google Scholar]
- Wong A. D., Ye M., Ulmschneider M. B. & Searson P. C. Quantitative analysis of the enhanced permeation and retention (EPR) effect. PLoS ONE 10, e0123461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaza Y. M. & Alemany C. Nanoparticle albumin-bound-Paclitaxel in the treatment of metastatic urethral adenocarcinoma: the significance of molecular profiling and targeted therapy. Case Rep. Urol. 2014, 489686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. & Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz J. & Willner I. DNA origami: nanorobots grab cellular control. Nat. Mater. 11, 276–277 (2012). [DOI] [PubMed] [Google Scholar]
- Douglas S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettegowda C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakmann S. DNA-based barcodes, nanoparticles, and nanostructures for the ultrasensitive detection and quantification of proteins. Angew. Chem. Int. Ed. 43, 5730–5734 (2004). [DOI] [PubMed] [Google Scholar]
- Condon A. Designed DNA molecules: principles and applications of molecular nanotechnology. Nat. Rev. Genet. 7, 565–575 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giljohann D. A. & Mirkin C. A. Drivers of biodiagnostic development. Nature 462, 461–464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goluch E. D. et al. A bio-barcode assay for on-chip attomolar-sensitivity protein detection. Lab Chip 6, 1293–1299 (2006). [DOI] [PubMed] [Google Scholar]
- Brakmann S. DNA-based barcodes, nanoparticles, and nanostructures for the ultrasensitive detection and quantification of proteins. Angew. Chem. Int. Ed. 43, 5730–5734 (2004). [DOI] [PubMed] [Google Scholar]
- Fan R. et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat. Biotechnol. 26, 1373–1378 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir Y. et al. Universal computing by DNA origami robots in a living animal. Nat. Nanotechnol. 9, 353–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span L. F. et al. The dynamic process of apoptosis analyzed by flow cytometry using Annexin-V/propidium iodide and a modified in situ end labeling technique. Cytometry 47, 24–31 (2002). [PubMed] [Google Scholar]
- Freedman N. D., Park Y., Abnet C. C., Hollenbeck A. R. & Sinha R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 366, 1891–1904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakoff S. J. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 16, 53–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan G. Redefining clinical trials: the age of personalized medicine. Cell 148, 1079–1080 (2012). [DOI] [PubMed] [Google Scholar]
- Ng P. C., Murray S. S., Levy S. & Venter J. C. An agenda for personalized medicine. Nature 461, 724–726 (2009). [DOI] [PubMed] [Google Scholar]
- Winkeljohn D. L. Triple-negative breast cancer. Clin. J. Oncol. Nurs. 12, 861–863 (2008). [DOI] [PubMed] [Google Scholar]
- de Ruijter T. C., Veeck J., de Hoon J. P., van Engeland M. & Tjan-Heijnen V. C. Characteristics of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 137, 183–192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H. & Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann. Oncol. 23, (Suppl 6): vi40–vi45 (2012). [DOI] [PubMed] [Google Scholar]
- Shastry M. & Yardley D. A. Updates in the treatment of basal/triple-negative breast cancer. Curr. Opin. Obstet. Gynecol. 25, 40–48 (2013). [DOI] [PubMed] [Google Scholar]
- Carlson R. W. et al. NCCN practice guidelines for breast cancer. Oncology (Williston Park) 14, 33–49 (2000). [PubMed] [Google Scholar]
- NCCN. Guidelines for patients, Stage IV breast cancer (Version 1.2014). https://www.nccn.org/patients/guidelines/stage_iv_breast/index.html..
- Haran G., Cohen R., Bar L. K. & Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta 1151, 201–215 (1993). [DOI] [PubMed] [Google Scholar]
- Peleg-Shulman T., Gibson D., Cohen R., Abra R. & Barenholz Y. Characterization of sterically stabilized cisplatin liposomes by nuclear magnetic resonance. Biochim. Biophys. Acta 1510, 278–291 (2001). [DOI] [PubMed] [Google Scholar]
- Bligh E. G. & Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- Riccardi C. & Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 1, 1458–1461 (2006). [DOI] [PubMed] [Google Scholar]
- Sidow A. & Spies N. Concepts in solid tumor evolution. Trends Genet. 31, 208–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottoriva A. et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-5 and Supplementary Methods
Data Availability Statement
All data that support the findings of this study are available from the corresponding author upon request.