Abstract
Background
Modified Gingyo-san (MGS) is empirically used to treat various respiratory infections. MGS has been reported to have antiinflammatory and antiviral activities; however, it is not known if it has an antibacterial activity. Therefore, in this study, we aimed to investigate the antimicrobial activity of MGS against respiratory pathogens.
Methods
MGS, which is sold as an over-the-counter drug in Japan, was used for the study. Antimicrobial activity was evaluated using the disk diffusion method. Growth inhibitory activity was evaluated by measuring colony-forming units of the pathogens in the presence of MGS.
Results
MGS inhibited the growth of Bacillus subtilis, Streptococcus pneumoniae, and Streptococcus pyogenes, which are gram-positive bacteria. Although the growth of most gram-negative bacteria was not inhibited by MGS, interestingly, the growth of Haemophilus influenzae was inhibited. MGS did not show any activity against Candida albicans or bacteriophage φX174.
Conclusions
In addition to the antiinflammatory and antiviral activities of MGS, which have already been reported, the data obtained from this study indicates that MGS has an antibacterial activity.
Keywords: Modified Gingyo-san, Respiratory pathogen, Chinese herbal medicine
Background
Respiratory infections are mainly caused by viruses or bacteria. Particularly, Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes are the major causative bacteria of respiratory infections. In several cases of respiratory infections, the aforementioned bacteria cause severe invasive infections [1–3]. Furthermore, these bacteria are developing resistance to the currently used antimicrobial agents, which can result in various clinical concerns [4]. This indicates the urgent need for developing novel antimicrobial agents.
Until recently, the use of traditional medicines was focused mainly in alternative medicine. In traditional Chinese medicine, herbal medicines are used for the treatment of various respiratory infections. However, the use of such medicines is based on experience and is not supported by basic scientific evidence. These medicines have, however, been applied in many clinical settings. In recent years, many researchers have made efforts to establish basic scientific evidences for the use of several herbal medicines used in traditional Chinese medicine because of evidence-based treatment. For example, it has been reported that Sho-sei-ryu-to and Ma-o-to have antiviral activities against the influenza virus [5–7]. Gingyo-san (GS) has also been reported to have antiviral activity against influenza virus and an immunomodulating activity [8–11]. Modified Gingyo-san (MGS) and GS are sold as over-the-counter (OTC) drugs. MGS is used to treat sore throats, coughs, and headaches. GS and MGS are composed of the crude drugs shown in Table 1. The individual components of MGS have been studied by several researchers. The roots of glycyrrhiza and platycodon, which are included in MGS, have been reported to have antiinflammatory and antitussive activities [12, 13]. In addition, schizonepeta spike and forsythia fruit have antibacterial activities against Propionibacterium acnes and thus, they are used for treating acne [14–16].
Table 1.
Formulations of modified Gingyo-san and Gingyo-san
| Content | Modified Gingyo-san (MGS)aAmount (g)b | Gingyo-san (GS) Amount (g)b |
|---|---|---|
| Lonicerae Flos | 4.26 | 30.0 |
| Forsythiae Fructus | 4.26 | 30.0 |
| Glycyrrhizae Radix | 2.56 | 15.0 |
| Platycodi Radix | 2.56 | 18.0 |
| Menthae Herba | 2.56 | 18.0 |
| Arctii Fructus | 2.14 | 18.0 |
| Schizonepetae Spica | 1.7 | 12.0 |
| Fermented soybean | 2.14 | 12.0 |
| Lophatherum Herba | 1.7 | 15.0 |
| Antelope Horns | 0.13 | - |
aextract
bdaily dose
Therefore, MGS has a potential use in the treatment of various symptoms of infectious diseases. In this study, we evaluated the direct effects of MGS on pathogenic bacteria to establish a basic evidence of its antibacterial activity.
Methods
Microbial strains and culture conditions
The microbial strains used in this study are listed in Table 2. All the microbes, except the streptococci and H. influenzae, were cultured using Mueller-Hinton broth or agar (Oxoid Ltd., Hampshire, UK). The streptococci and H. influenzae were cultured using blood agar or Todd-Hewitt broth (Oxoid Ltd.) and chocolate agar or brain heart infusion broth (Oxoid Ltd.) supplemented with 15 μg/mL of nicotinamide adenine dinucleotide solution and 15 μg/mL of hemin solution, respectively.
Table 2.
Bacterial, fungal and viral strains used in this study
| Microorganism | Description |
|---|---|
| Gram-positive bacteria | |
| Staphylococcus aureus JCM2874 | Quality control strain for susceptibility test, Methicillin-susceptible |
| Staphylococcus aureus N315 | Methicillin-resistant S. aureus |
| Streptococcus pneumoniae ATCC49619 | Quality control strain for susceptibility test, penicillin-susceptible |
| Streptococcus pneumoniae 19F | Clinical isolate, penicillin-resistant S. pneumoniae, serotype 19F |
| Streptococcus pyogenes JCM5674 | Type strain |
| Enterococcus faecalis ATCC29212 | Quality control strain for susceptibility test |
| Bacillus subtilis ATCC6633 | Control strain for various assays |
| Gram-negative bacteria | |
| Escherichia coli ATCC25922 | Quality control strain for susceptibility test |
| Escherichia coli C | Host of bacteriophage φX174 |
| Pseudomonas aeruginosa ATCC27853 | Quality control strain for susceptibility test |
| Haemophilus influenzae ATCC49247 | Quality control strain for susceptibility test |
| Serratia marcescens ATCC13880 | Type strain |
| Fungi | |
| Candida albicans ATCC10231 | Quality control strain for various assays |
| Bacteriophage | |
| φX174 | Virulent phage for E. coli C |
Disk diffusion method
MGS was obtained from ISKRA Industry (Tokyo, Japan). The disk diffusion antimicrobial test was conducted as follows. Briefly, 8-mm paper disks were impregnated with MGS suspension to obtain 8 mg of MGS/disk. The bacteria were suspended in 0.75% agar containing Mueller-Hinton broth and poured into suitable petri dishes. The disks were placed on the set agar and the plates were incubated at 35 °C overnight, after which the zones of growth inhibition were measured.
Evaluation of growth inhibitory activity
A single colony of bacteria was inoculated into the appropriate broth medium and incubated at 37 °C overnight. The culture was diluted with the broth (1:100), with or without MGS, and incubated at 37 °C with shaking. A 100-μL of the culture was sampled at 0, 1, 2, 4, and 6 h after incubation and diluted with phosphate-buffered saline (PBS). Serial dilutions were then plated on the appropriate agar plates and incubated at 37 °C overnight. Afterwards, the numbers of grown colonies were counted. All the experiments were performed at least twice on independent days. It was also confirmed that all the experiments showed similar results.
Plaque assay
MGS was mixed with bacteriophage φX174. The mixtures were diluted with PBS and further mixed with Escherichia coli C and 0.75% agar. The mixtures were then poured onto nutrient agar and incubated at 37 °C for 12 h, after which the plaques were counted.
Cell proliferation assay
Cell proliferation activities with or without MGS were performed using CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, MI, USA). Monolayer human lung epithelial cell lines (A549 cells) were grown using Eagle’s minimal essential medium, to which 10% fetal calf serum had been added, in 96-well plates. Aliquots of MGS were then added and the plates were incubated at 37 °C for 3 h under 5% CO2. After incubation, the numbers of living cells were determined using the kit according to the manufacturer’s instructions [17].
Statistical analysis
We assessed statistical significance of differences for growth in the presence or absence of MGS. We performed Student’s and Welch’s t-tests using JMP software (SAS Institute Inc., NC, USA). P < 0.05 were judged as significant difference.
Results and discussion
Antimicrobial activity of MGS
The disk diffusion method is used as a screening test for the antimicrobial activities of drugs and natural products [18, 19]. The susceptibility disk method was therefore used to determine whether MGS has antimicrobial activity (Table 3). Zones of growth inhibition were obtained in the experiments involving S. pneumoniae and S. pyogenes but not in those involving the other pathogens. This data indicates that MGS has a direct antibacterial activity against S. pneumoniae and S. pyogenes.
Table 3.
Inhibitory zone of modified Gingyo-san containing paper diska
| Strain | Inhibitory zone (mm)b |
|---|---|
| Staphylococcus aureus JCM2874 | - |
| Staphylococcus aureus N315 | - |
| Streptococcus pneumoniae ATCC49619 | 10.0 |
| Streptococcus pneumoniae 19F | 10.0 |
| Streptococcus pyogenes JCM5674 | 8.5 |
| Enterococcus faecalis ATCC29212 | - |
| Bacillus subtilis ATCC6633 | - |
| Escherichia coli ATCC25922 | - |
| Pseudomonas aeruginosa ATCC27853 | - |
| Haemophilus influenzae ATCC49247 | - |
a8 mg/disk
b- , inhibitory zone not appeared
Growth inhibitory activity of MGS against respiratory pathogens
The susceptibility disk test is suitable for screening the antimicrobial activities of drugs; however, not all drugs that have antimicrobial activities produce zones of growth inhibition, which may be due to the chemical properties of the drugs [20]. Therefore, to validate the growth inhibitory effect of MGS, bacterial colony-forming units (CFUs) in cultures were monitored over time in the presence or absence of MGS. The number of CFUs of B. subtilis ATCC6633, which is normally used for testing the antibacterial activity of drugs, decreased in a dose-dependent manner after the addition of MGS to the culture medium (Fig. 1). MGS is usually administered with approximately 100 mL of water; thus, after administration, the concentration of MGS in the oral cavity reaches approximately 24 mg/mL. Since MGS was used at a concentration equivalent to its usually administered dose, the data obtained indicates that MGS has a direct antibacterial effect at its normal dose.
Fig. 1.
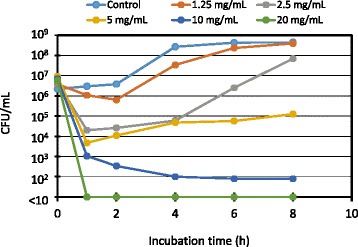
Antibacterial effect of modified Gingyo-san against Bacillus subtilis ATCC6633. This experiment was performed twice on independent occasions and similar results were obtained. The data shown is representative of the results obtained
The antimicrobial activity of MGS was analyzed at a concentration was 20 mg/mL. The growth of S. aureus, S. pneumoniae, S. pyogenes, and Enterococcus faecalis was significantly inhibited by the addition of MGS to the respective culture media (Fig. 2). MGS also inhibited the growth of antimicrobial-resistant strains such as methicillin-resistant S. aureus (MRSA) and penicillin-resistant S. pneumoniae (PRSP) (Fig. 2). Therefore, these results indicate that MGS has growth inhibitory effects against gram-positive bacteria. However, the growth of E. coli and Pseudomonas aeruginosa was not inhibited (Fig. 2). In addition, MGS did not affect other gram-negative bacteria such as Acinetobacter baumannii, Serratia marcescens, and Klebsiella pneumoniae (data not shown).
Fig. 2.
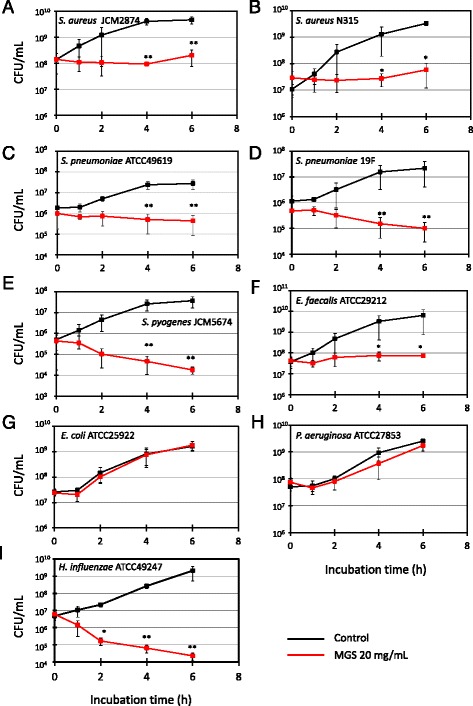
Antibacterial effects of modified Gingyo-san against several bacterial strains. a S. aureus JCM2874; b S. aureus N315 (MRSA); c S. pneumoniae ATCC49619; d S. pneumoniae 19F; e S. pyogenes JCM5674; f E. faecalis ATCC29212; g E. coli ATCC25922; h P. aeruginosa ATCC27853; and i H. influenzae ATCC49247. Each experiment was performed three times on independent occasions and similar results were obtained. The P value was calculated by Welch’s t-test. ** P < 0.01, *P < 0.05
However, interestingly, the growth of H. influenzae, which is a major causative pathogen of respiratory infections, was significantly inhibited in spite of it being a gram-negative bacterium (Fig. 2). It has been reported that among gram-negative bacteria, H. influenzae has a different cell surface structure [21]. In addition, the chromosomal efflux pumps of H. influenzae are fewer than those of other gram-negative bacteria [22]. The aforementioned factors might therefore be related to the observed bactericidal effect of MGS on H. influenzae in this study. These findings indicate that MGS can comprehensively inhibit the growth of respiratory bacterial pathogens. Moreover, MGS showed inhibitory effects against MRSA and PRSP, which suggests that MGS could be a very useful antibiotic for the treatment of respiratory infections.
Effects of MGS on Candida albicans and bacteriophage φX174
The inhibitory effects of MGS on eukaryotes and viruses were analyzed using Candida albicans and bacteriophage φX174 as the eukaryotic and viral models, respectively (Fig. 3). The data obtained indicated that MGS does not inhibit the growth of C. albicans or bacteriophage φX174. In addition, MGS did not show any antiproliferative effects against the A549 cells (Fig. 4). These findings indicate that MGS might not have any direct effect on eukaryotes or viruses.
Fig. 3.
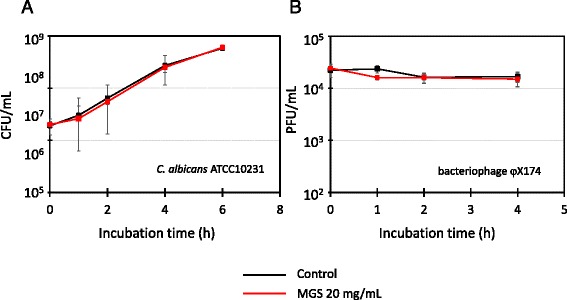
Antifungal and antibacteriophage effects of modified Gingyo-san. a C. albicans ATCC10231 and b bacteriophage φX174. Each experiment was performed 3 times on independent occasions and similar results were obtained
Fig. 4.
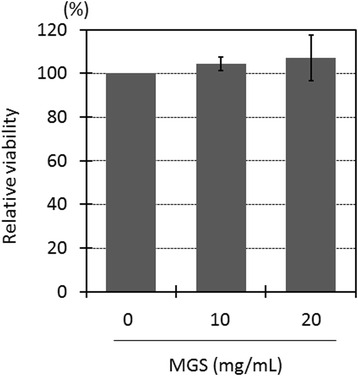
Effect of modified Gingyo-san on a eukaryotic cell. Proliferation activity was evaluated. Each experiment was performed 3 times on independent occasions. The P value was calculated by student’s t-test
Conclusions
Our study indicated that MGS has a direct antibacterial effect against respiratory bacterial pathogens. These findings together with those from previous reports show that, GS has in vivo antiviral activity against the influenza virus and that MGS could be a useful antibacterial agent. Therefore, MGS may be effective for use as a pastille and a gargle. Moreover, future studies need be conducted to clarify whether the metabolites of MGS as well have effects on bacteria.
Acknowledgements
We appreciate Ms. Yui Anzai and Ms. Reina Kinugawa for their technical assistance. We also thank Iskra Industry Co., Ltd. for providing the MGS used in the study.
Funding
Not applicable.
Availability of data and materials
Data are all contained within the paper.
Authors’ contributions
TY, TW, HI and NN designed the study. TY, TW, HN and KK carried out the experimental work. TY, TW, KK, HN, HI and NN analyzed and interpreted the results. TY, TW, and NN drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable
Abbreviations
- GS
Gingyo-san
- MGS
The modified Gingyo-san
References
- 1.Sakai F, Chiba N, Ono A, Yamagata Murayama S, Ubukata K, Sunakawa K, Takahashi T. Molecular epidemiologic characteristics of Streptococcus pneumoniae isolates from children with meningitis in Japan from 2007 through 2009. J Infect Chemother. 2011;17:334–40. doi: 10.1007/s10156-010-0180-3. [DOI] [PubMed] [Google Scholar]
- 2.Ubukata K, Chiba N, Morozumi M, Iwata S, Sunakawa K, Working Group of Nationwide Surveillance for Bacterial Meningitis Longitudinal surveillance of Haemophilus influenzae isolates from pediatric patients with meningitis throughout Japan, 2000–2011. J Infect Chemother. 2013;19:34–41. doi: 10.1007/s10156-012-0448-x. [DOI] [PubMed] [Google Scholar]
- 3.Wajima T, Murayama SY, Sunaoshi K, Nakayama E, Sunakawa K, Ubukata K. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and noninvasive disease. J Med Microbiol. 2008;57:1383–8. doi: 10.1099/jmm.0.2008/002642-0. [DOI] [PubMed] [Google Scholar]
- 4.Morozumi M, Chiba N, Okada T, Sakata H, Matsubara K, Iwata S, Ubukata K. Antibiotic susceptibility in relation to genotype of Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae responsible for community-acquired pneumonia in children. J Infect Chemother. 2013;19:432–40. doi: 10.1007/s10156-012-0500-x. [DOI] [PubMed] [Google Scholar]
- 5.Chang JS, Yeh CF, Wang KC, Shieh DE, Yen MH, Chiang LC. Xiao-Qing-Long-Tang (Sho-seiryu-to) inhibited cytopathic effect of human respiratory syncytial virus in cell lines of human respiratory tract. J Ethnopharmacol. 2013;147:481–7. doi: 10.1016/j.jep.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Nagai T, Kataoka E, Aoki Y, Hokari R, Kiyohara H, Yamada H. Alleviative effects of a Kampo (a Japanese herbal) medicine “Maoto (Ma-Huang-Tang)” on the early phase of influenza virus infection and its possible mode of action. Evid Based Complement Alternat Med. 2014;2014:187036. doi: 10.1155/2014/187036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai T, Yamada H. In vivo anti-influenza virus activity of Kampo (Japanese herbal) medicine “sho-seiryu-to”--stimulation of mucosal immune system and effect on allergic pulmonary inflammation model mice. Immunopharmacol Immunotoxicol. 1998;20:267–81. doi: 10.3109/08923979809038544. [DOI] [PubMed] [Google Scholar]
- 8.Hung CM, Yeh CC, Chong KY, Chen HL, Chen JY, Kao ST, Yen CC, Yeh MH, Lin MS, Chen CM. Gingyo-san enhances immunity and potentiates infectious bursal disease vaccination. Evid Based Complement Alternat Med. 2011;2011:238208. doi: 10.1093/ecam/nep021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Davis SM, Utsunomiya T, Pollard RB, Suzuki F. Antiviral effect of gingyo-san, a traditional Chinese herbal medicine, on influenza A2 virus infection in mice. Am J Chin Med. 1999;27:53–62. doi: 10.1142/S0192415X99000082. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa M, Yamamura J, Li Z, Sato H, Hitomi N, Tatsumi Y, Shiraki K. Antipyretic activity of gingyo-san, a traditional medicine, in influenza virus-infected mice. Chem Pharm Bull (Tokyo) 1998;46:1444–7. doi: 10.1248/cpb.46.1444. [DOI] [PubMed] [Google Scholar]
- 11.Yeh CC, Lin CC, Wang SD, Hung CM, Yeh MH, Liu CJ, Kao ST. Protective and immunomodulatory effect of Gingyo-san in a murine model of acute lung inflammation. J Ethnopharmacol. 2007;111:418–26. doi: 10.1016/j.jep.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Akamatsu H, Komura J, Asada Y, Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991;57:119–21. doi: 10.1055/s-2006-960045. [DOI] [PubMed] [Google Scholar]
- 13.Ishimaru N, Maeno T, Suzuki M, Maeno T. Rapid effects of Kikyo-to on sore throat pain associated with acute upper respiratory tract infection. J Complement Integr Med. 2014;11:51–4. doi: 10.1515/jcim-2013-0052. [DOI] [PubMed] [Google Scholar]
- 14.Akamatsu H, Asada Y, Horio T. Effect of keigai-rengyo-to, a Japanese kampo medicine, on neutrophil functions: a possible mechanism of action of keigai-rengyo-to in acne. J Int Med Res. 1997;25:255–65. doi: 10.1177/030006059702500503. [DOI] [PubMed] [Google Scholar]
- 15.Higaki S, Hasegawa Y, Morohashi M, Takayoshi Y. The correlation of Kampo formulations and their ingredients on anti-bacterial activities against Propionibacterium acnes. J Dermatol. 1995;22:4–9. doi: 10.1111/j.1346-8138.1995.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 16.Higaki S, Nakamura M, Morohashi M, Yamagishi T. Propionibacterium acnes biotypes and susceptibility to minocycline and Keigai-rengyo-to. Int J Dermatol. 2004;43:103–7. doi: 10.1111/j.1365-4632.2004.01887.x. [DOI] [PubMed] [Google Scholar]
- 17.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 18.Ashraf A, Sarfraz RA, Anwar F, Shahid SA, Alkharfy KM. Chemical composition and biological activities of leaves of Ziziphus mauritiana L. native to Pakistan. Pak J Bot. 2015;47(1):367–76. [Google Scholar]
- 19.Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J Food Drug Anal. 2015;23(1):109–15. doi: 10.1016/j.jfda.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klancnik A, Piskernik S, Jersek B, Mozina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81:121–6. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Phillips NJ, John CM, Reinders LG, Gibson BW, Apicella MA, Griffiss JM. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990;19(11):731–45. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- 22.Trepod CM, Mott JE. Identification of the Haemophilus influenzae tolC gene by susceptibility profiles of insertionally inactivated efflux pump mutants. Antimicrob Agents Chemother. 2004;48:1416–8. doi: 10.1128/AAC.48.4.1416-1418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are all contained within the paper.


