Abstract
Deficits in the adaptive, flexible control of behavior contribute to the clinical manifestations of schizophrenia. We used functional MRI and an antisaccade paradigm to examine the neural correlates of cognitive control deficits and their relations to symptom severity. Thirty-three chronic medicated outpatients with schizophrenia and 31 healthy controls performed an antisaccade paradigm. We examined differences in recruitment of the cognitive control network and task performance for Hard (high control) versus Easy (low control) antisaccade trials within and between groups. We focused on the key regions involved in ‘top-down’ control of ocular motor structures – dorsal anterior cingulate cortex, dorsolateral and ventrolateral prefrontal cortex. In patients, we examined whether difficulty implementing cognitive control correlated with symptom severity. Patients made more errors overall, and had shorter saccadic latencies than controls on correct Hard vs. Easy trials. Unlike controls, patients failed to increase activation in the cognitive control network for Hard vs. Easy trials. Reduced activation for Hard vs. Easy trials predicted higher error rates in both groups and increased symptom severity in schizophrenia. These findings suggest that patients with schizophrenia are impaired in mobilizing cognitive control when presented with challenges and that this contributes to deficits suppressing prepotent but contextually inappropriate responses, to behavior that is stimulus-bound and error-prone rather than flexibly guided by context, and to symptom expression. Therapies aimed at increasing cognitive control may improve both cognitive flexibility and reduce the impact of symptoms.
Keywords: Schizophrenia, Anterior cingulate cortex, Prefrontal cortex, Antisaccade, Cognitive control, Functional MRI
Highlights
-
•
Patients with schizophrenia fail to mobilize the cognitive control network during a challenging cognitive task.
-
•
This deficit results in behavior that is stimulus-bound and error-prone rather than flexibly guided by context.
-
•
Therapies aimed at increasing cognitive control may improve both cognitive flexibility and reduce the impact of symptoms.
1. Introduction
A key feature of schizophrenia is impaired cognitive control, the ability to mobilize cognitive resources to support task goals in the face of response competition (Fornito et al., 2011). Deficient cognitive control contributes to behavior that is rigid and perseverative rather than flexibly guided by changing contingencies. Here we used functional MRI (fMRI) and an antisaccade paradigm to investigate neural and behavioral adjustments of cognitive control in response to “Hard” versus “Easy” task demands. Antisaccades require cognitive control since one must inhibit the prepotent response of looking toward a visual stimulus (i.e., a prosaccade) and substitute the novel behavior of looking in the opposite direction (Hallett, 1978). Patients with schizophrenia (Manoach et al., 2002), including antipsychotic-naïve first episode patients (Harris et al., 2006), and their first-degree relatives (McDowell et al., 1999, Reilly et al., 2014) consistently make more antisaccade errors (i.e., failures to suppress the prepotent prosaccade) than controls suggesting that the antisaccade deficit is an endophenotype (For reviews see, Gooding and Basso, 2008, Hutton and Ettinger, 2006). Here, we tested the hypothesis that schizophrenia patients fail to modulate cognitive control in response to task difficulty. We expected that this would be manifested as reduced behavioral control and a failure to increase activation in the cognitive control network for Hard (high control) versus Easy (low control) antisaccade trials.
Antisaccades are ideally suited to study the volitional control of behavior since their neural mediation has been extensively characterized by human and monkey neurophysiology, neuroimaging and lesion studies (Connolly et al., 2005, Everling and Munoz, 2000, Munoz and Everling, 2004). The dorsal anterior cingulate cortex (dACC) and lateral prefrontal cortex (PFC) are key regions in the ‘top-down’ control of ocular motor structures (Johnston et al., 2007, Miller and Cohen, 2001). The dACC and lateral PFC are structurally (Selemon and Goldman-Rakic, 1988, Wang et al., 2004) and functionally (Koski and Paus, 2000, Margulies et al., 2007, Tu et al., 2010) connected to the frontal eye field (FEF), which is the main cortical generator of saccades. Lesions of the dACC (Milea et al., 2003) and both the dorsolateral PFC (DLPFC, Pierrot-Deseilligny et al., 2003) and ventrolateral PFC (VLPFC, Hodgson et al., 2007) increase antisaccade errors. In human neuroimaging the dACC, DLPFC and VLPFC all show greater activation for antisaccades than for prosaccades (Dyckman et al., 2007, Matsuda et al., 2004, McDowell et al., 2008). This body of work establishes the dACC, DLPFC and VLPFC as key anatomical components of the cognitive control network for volitional saccades.
In a prior magnetoencephalography (MEG) study, we found that compared with healthy individuals, schizophrenia patients failed to increase preparatory activation in the dACC in response to cues that indicated an impending antisaccade (high control) versus prosaccade (low control) trial and this failure was accompanied by an increased antisaccade error rate (Manoach et al., 2013). These findings suggest that healthy individuals were better able to use the instructional cue to increase cognitive control in anticipation of a more challenging task than patients. The aim of the present study was to extend this finding in several important ways: (i) To determine whether this deficit in mobilizing the control network extends from task preparation to task execution. Patients did not use the instructional cue to increase activation when anticipating a more challenging task, but would they be able to mobilize the cognitive control network when they actually had to perform the more difficult task? This question about task execution was difficult to address with MEG due to saccadic artifact during task performance. (ii) While the previous study contrasted different tasks (i.e., antisaccades and prosaccades), we wanted to determine whether the deficit in mobilizing control extended to situations where the task is the same (antisaccades) and only the difficulty level varies. (iii) We further wanted to take advantage of the superior spatial resolution of fMRI to fully characterize the deficit. (iv) Most importantly, we wanted to understand the clinical relevance of the deficit in cognitive control by examining its relation to symptom severity.
2. Methods
2.1. Participants
Thirty-three outpatients with schizophrenia and 31 healthy controls participated. Patients were recruited from an urban mental health center. Four patients were unmedicated and the rest were maintained on stable doses of atypical antipsychotic medications for at least six weeks. Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV (First et al., 1997) and symptom severity was characterized with the Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987) and the Scale for the Assessment of Negative Symptoms (SANS, Andreasen, 1983).
Healthy control participants, screened to exclude a personal history of mental illness (SCID-Non-patient edition, First et al., 2002) and a family history of schizophrenia spectrum disorder, were recruited from the community by poster and website advertisements. All participants were screened to exclude substance abuse or dependence within the preceding six months and any independent condition that might affect brain function. Patient and control groups did not differ significantly in age, sex, handedness, mean parental education or an estimate of premorbid verbal IQ based on the reading subtest of the Wide Range Achievement Test- III (Wilkinson, 1993) (Table 1). All participants reported normal or corrected-to-normal vision.
Table 1.
Participant characteristics. Means, standard deviations and group comparisons of demographic data.
| Schizophrenia patients (n = 33) |
Healthy controls (n = 31) |
|||||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | t | p | |
| Age | 43 | 12 | 41 | 13 | 0.69 | 0.49 |
| Sex | 7F/26M | 7F/24M | Χ2 = 0.02 | 0.89 | ||
| Parental Education | 14 | 3 | 14 | 3 | 0.39 | 0.69 |
| Laterality Score (Handedness)a | 61 | 45 | 67 | 55 | − 0.49 | 0.63 |
| Estimated Verbal IQb | 98 | 16 | 101 | 28 | 0.52 | 0.61 |
| PANSS Total | 59 | 13 | Mild | |||
| PANSS Positive | 15 | 4 | ||||
| PANSS Negative | 14 | 5 | ||||
| PANSS General | 31 | 7 | ||||
| SANS | 24 | 16 | Minimal | |||
| Age at Onset | 24 | 7 | ||||
| Duration of illness (years) | 19 | 13 | ||||
| CPZ Equivalentsc | 482 | 386 | ||||
Abbreviations: PANSS = Positive and Negative Syndrome Scale; SANS = Scale for the Assessment of Negative Symptoms; CPZ = Chlorpromazine.
Based on the modified Edinburgh Handedness Inventory(Oldfield, 1971, White and Ashton, 1976). Laterality scores of − 100 and + 100 denote exclusive use of left or right hand, respectively.
Based on standard scores on the reading subtest of the Wide Range Achievement Test (WRAT-III; Wilkinson, 1993).
Antipsychotic drug dosage measured in chlorpromazine equivalents (Woods, 2003).
The study was approved by the Partners Human Research Committee and all participants gave written informed consent. In addition to a base rate of pay, participants received five cents for each correct antisaccade response, an incentive intended to enhance attention and motivation.
2.2. Antisaccade paradigm
The task was programmed using MATLAB Psychtoolbox (Mathworks Inc., Natick, MA). It was comprised of a pseudorandom sequence of three types of antisaccade trials that were balanced for right and left stimuli (Fig. 1). Randomly interleaved with the antisaccade trials were intervals of fixation lasting 2, 4, or 6 s, which provided a baseline and introduced “temporal jitter” to optimize the analysis of rapid presentation event-related fMRI data (Buckner et al., 1998, Burock and Dale, 2000, Miezin et al., 2000). The schedule of events was determined using a technique that optimizes the statistical efficiency of event-related designs (Dale, 1999). Participants performed six task runs. Each run lasted 5 min 16 s and generated an average of 64 antisaccade trials and 20 fixation epochs.
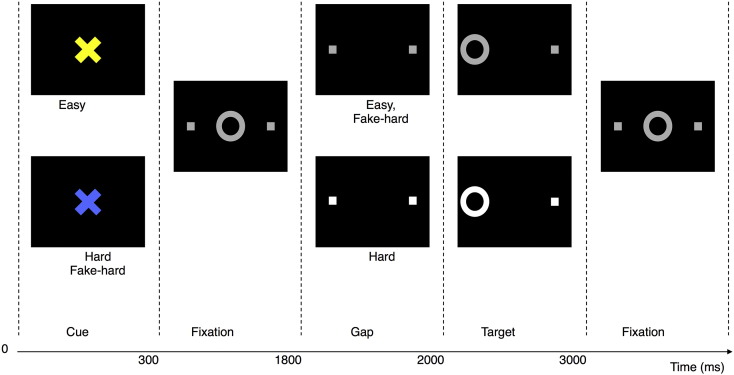
Fig. 1.
Antisaccade paradigm. Schematic and timeline of the three trial types: Easy, Hard and Fake-Hard. Each trial lasted for 4 s and began with a cue informing the participant of either an Easy or Hard trial. The cue was either a blue or a yellow “X” and the mapping of cue color to trial type was counterbalanced across participants. The cue was flanked horizontally by two small white squares with a width of 0.4° that marked the potential locations of stimulus appearance: 10° left and right of center. These squares remained visible for the duration of each run. At 300 ms, the instructional cue was replaced by a white fixation ring at the center of the screen, with a diameter of 1.3°. At 1800 ms, the central fixation ring disappeared (200 ms gap), and at 2000 ms, it reappeared on either the right or left side as the imperative stimulus to which participants were required to respond. Hard trials were distinguished by an increase in luminance of both the peripheral squares that mark the potential locations of stimulus appearance during the gap and the imperative stimulus. Except for the hard cue, Fake-Hard trials were identical to Easy trials. In the trials depicted, the correct response was a saccade away from the stimulus on the left side of the display. An error would involve a saccade toward the stimulus. After 1 s, the fixation ring returned to the center. Participants were instructed to return their gaze to the center and fixate until another trial began. Fixation intervals, which lasted 2, 4, or 6 s, were simply a continuation of the fixation display that constitutes the final second of the previous saccadic trial.
The three types of antisaccade trials were: Hard (40%), Easy (50%) and Fake-Hard (10%). Hard trials introduced a distraction consisting of a luminance change in the peripheral squares during the gap between the offset of the central fixation ring and the appearance of the imperative stimulus. This sudden change attracts attention and thereby disrupts focus on the task at hand. This makes it more difficult to inhibit saccades in response to the imperative stimulus resulting in faster responses and a higher error rate. Fake-Hard trials started with a cue indicating a Hard trial, but were otherwise identical to Easy trials. They were included to isolate the effects of hard vs. easy cues on activation unconfounded by the luminance change. Fake-Hard trials were restricted to be only 20% of trials with a hard cue so that participants would associate the hard cue with increased task difficulty.
Each trial lasted for 4 s and began with a cue informing the participant of either an Easy or Hard trial. The cue was either a blue or a yellow “X” and the mapping of cue color to trial type was counterbalanced across participants. After 300 ms, the cue was replaced by a white fixation ring at the center of the screen, where participants maintained their gaze. At 1800 ms the central fixation ring disappeared for 200 ms and then reappeared on either the right or left side. This was the stimulus to which participants were required to respond by making a saccade in the opposite direction.
Prior to scanning, participants practiced three runs of the task in a mock scanner to familiarize themselves with the task and to become acclimated to the appearance, noise, and confinement of the actual MRI scanner. Participants were instructed, “You may find that the blue/yellow trials are harder than the yellow/blue trials” depending on the counterbalance. They were not told about the Fake-Hard manipulation. They were asked to respond as quickly and accurately as possible by making a saccade away from the stimulus and were told that they would receive a bonus of five cents for each correct trial.
2.3. Eye tracking data collection and analysis
The ISCAN fMRI Remote Eye Tracking (Burlington, MA) recorded eye position during mock and fMRI scanning using a 120-Hz video camera. Eye movement data were scored offline using a partially automated MATLAB (Mathworks, Natick, MA) program to determine for each saccade the directional accuracy with respect to the required response and the latency from the onset of the imperative stimulus. Saccades occurring after the onset of the imperative stimulus were identified as horizontal eye movements with velocities exceeding 47° per second. The onset of a saccade was defined as the point at which the velocity of the eye first exceeded 30° per second. Trials with initial saccades in the direction of the stimulus were scored as errors. The outcome measures were error rate and saccadic latencies for correct trials. Error rates were logit-transformed before analysis to normalize their distribution.
2.4. MRI acquisition
Images were acquired using a 3.0 T Siemens (Erlangen, Germany) Trio whole body high-speed imaging device equipped for echo planar imaging (EPI) and a 12-channel head coil. Head stabilization was achieved with cushioning and participants wore earplugs (29 dB rating) for noise attenuation. A high-resolution structural scan was acquired in the sagittal plane using a 3D rf-spoiled magnetization prepared rapid gradient echo (MPRAGE) sequence (repetition time (TR) = 2530 ms; echo time (TE) = 3.39 ms; flip angle = 7°; FOV = 256 mm; 176 in-plane sagittal slices; voxel size = 1.33 × 1 × 1.33 mm). Functional images were collected using a gradient echo T2*-weighted sequence for Blood Oxygen Level Dependent (BOLD) contrast (TR = 2000 ms; TE = 30 ms; flip angle = 90°; 32 contiguous horizontal slices parallel to the intercommissural plane, voxel size = 3.1 × 3.1 × 3.7 mm, interleaved). The functional sequences included prospective acquisition correction (PACE) for head motion (Thesen et al., 2000) to adjust slice position and orientation in real time during data acquisition.
2.5. Surface-based fMRI analyses
Analyses were conducted using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) and FreeSurfer Functional Analysis Stream (FS-FAST) software. Functional images were intensity-normalized, smoothed using a 3D 8-mm FWHM Gaussian kernel and aligned to the MPRAGE scan for each participant. MPRAGE scans were used to reconstruct inflated (2D) models of individual cortical surfaces using FreeSurfer segmentation, surface reconstruction, and inflation algorithms (Dale et al., 1999, Fischl et al., 1999). For group-level analysis, inflated cortical surfaces were registered to a template brain consisting of the averaged cortical surface of an independent sample of 40 adults from the Buckner laboratory at Washington University (St. Louis, MO) using the Freesurfer surface-based spherical coordinate system. Cortical activation was localized using automated surface-based parcellation software (Fischl et al., 2004). We defined approximate anatomical boundaries for the dACC by dividing the ACC labels into dorsal and rostral segments by drawing a line perpendicular to the intercommissural plane at the anterior boundary of the genu of the corpus callosum (Devinsky et al., 1995); VLPFC was defined by combining the pars opercularis and pars triangularis labels and DLPFC was defined as the rostral middle frontal label.
2.6. Motion
In addition to on-line motion correction (PACE), functional scans were corrected retrospectively for motion using the AFNI algorithm (Cox and Jesmanowicz, 1999), intensity normalized, and smoothed using a 3D 8 mm FWHM Gaussian kernel. To characterize average motion for each participant, total translation (x, y, z) and rotation (pitch, roll, yaw) were averaged across the six runs of the task and compared between groups.
2.7. Activation
Finite impulse response estimates (Burock and Dale, 2000, Miezin et al., 2000) of the event-related hemodynamic responses (HDRs) were calculated for each of the three trial types (Hard, Easy, Fake-Hard) and for error trials for each participant. This involved using a linear model to provide unbiased estimates of the average signal intensity at each time point for each trial type without making a priori assumptions about the shape of the HDR. HDR estimates were computed at 12 timepoints with an interval of 2 s (corresponding to the TR) ranging from 4 s prior to the start of a trial to 18 s after the start. Temporal correlations in the noise were accounted for by prewhitening using a global estimate of the residual error autocorrelation function truncated at 30 s (Burock and Dale, 2000). Registered group data were smoothed with a 2D 4.6 mm FWHM Gaussian kernel. To facilitate comparison with other studies, approximate Talairach coordinates were derived by mapping the surface-based coordinates of activation back to the original structural volume for each of the individuals whose brains were used to create the template brain, registering the volumes to the Montreal Neurological Institute (MNI305) atlas (Collins et al., 1994) and averaging the corresponding MNI305 coordinates. These coordinates were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
To correct for multiple comparisons, 10,000 Monte Carlo simulations were run using the mri_glmfit-sim function in FreeSurfer (Hagler et al., 2006). Simulations of synthesized white Gaussian noise were run using the smoothing, resampling, and averaging parameters of the surface-based functional analysis and a vertex-wise (cluster-forming) probability threshold of p ≤ 0.01. These simulations were used to determine the likelihood that a cluster of a certain size would be found by chance based on cluster-wise probability (CWP) threshold of p ≤ 0.05.
3. Results
3.1. Task performance
Participants made more errors (F(2,61) = 80.27, p < 0.001) and responded faster (F(2,61) = 30.56, p < 0.001) on Hard than either Easy (error: t(63) = 8.88 p < 0.001; latency: t(63) = − 6.94 p < 0.001) or Fake-Hard (error: t(63) = 9.76 p < 0.001; latency: t(63) = − 7.77, p < 0.001) trials (Fig. 2) indicating that the luminance manipulation on Hard trials was effective in making saccadic inhibition more difficult and in shifting the speed accuracy trade-off function in the saccadic system to a riskier position (Agam et al., 2013). Patients made more errors than controls (F(1,62) = 17.41, p < 0.001) regardless of trial type (Group by Trial Type: F(2,61) = 2.52, p = 0.09; Fig. 2A). For latency, there was a significant Group by Trial Type interaction (F(2,61) = 4.93, p = 0.01; Fig. 2B). While patients and controls did not differ significantly in latency overall (F(1,62) = 0.009, p = 0.93), the interaction reflected that patients had disproportionately faster responses than controls for Hard trials compared with Easy or Fake-Hard trials (Hard vs. Easy: HC = 18 ± 42 ms, SZ = 51 ± 42, t(62) = − 3.14, p = 0.003; Hard vs. Fake-Hard: HC = 26 ± 42, SZ = 57 ± 42, t(62) = − 2.91, p = 0.005). We interpret this as indicating that patients had reduced attentional control and were therefore more vulnerable to distraction by the sudden change in the peripheral stimuli during Hard trials than controls. The difference in latency between Hard and Easy trials, which indexes this effect and was larger in patients than controls, significantly correlated with symptom severity only on the SANS (Fig. 2C; r = 0.41, p = 0.02, uncorrected for multiple comparisons), although correlations with other symptom ratings were in the same direction.
Fig. 2.
Antisaccade task performance. (A) Error rate and (B) latency of correct antisaccades for Hard, Easy and Fake-Hard trials. Error bars show standard error of the mean. (C) The relationship between SANS negative symptom severity and the difference in latency for Easy minus Hard trials in schizophrenia patients.
3.2. fMRI data
3.2.1. Motion
The groups did not differ significantly in residual motion measured as mean translation (HC: 0.96 ± 0.72 mm, SZ: 1.18 ± 0.60 mm, t(62) = − 1.34, p = 0.19) or rotation (HC: 0.26 ± 0.15°, SZ: 0.30 ± 0.13°, t(62) = − 1.05, p = 0.30) during the functional scans.
3.2.2. Activation
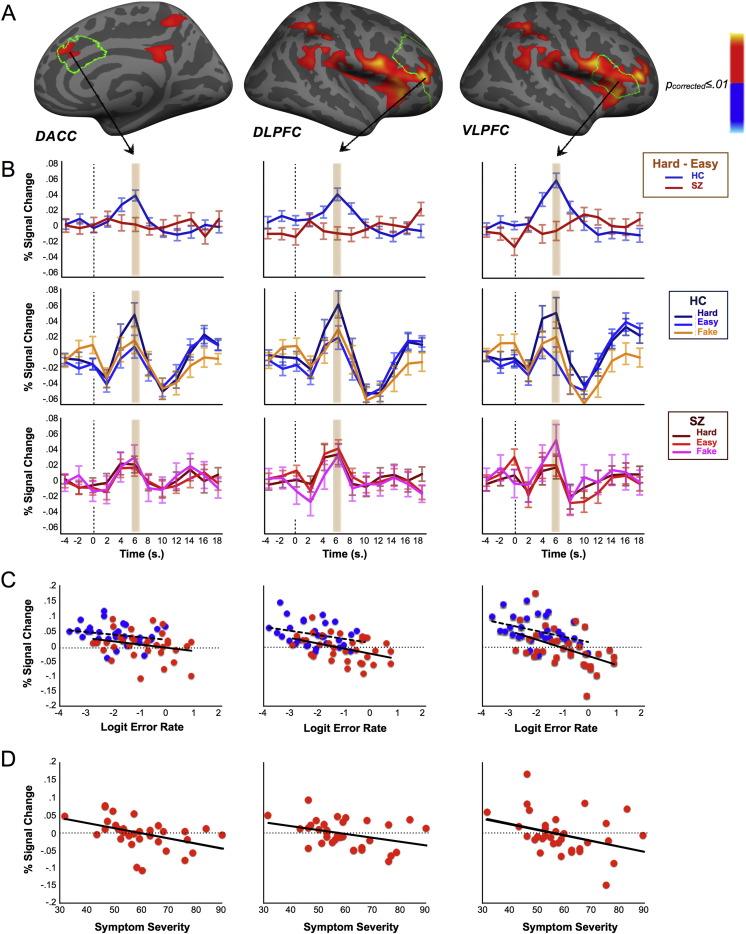
On Hard trials, controls showed significantly stronger activation in cognitive control regions (dACC, DLPFC, VLPFC) than on either Easy or Fake-Hard trials. Patients, in contrast, failed to significantly increase activation in these regions on Hard trials (Supplementary Figs. 1–4; Supplementary Tables 1–2). These group differences reached significance for Hard versus Easy trials in the right dACC (Talairach coordinates: x = 9, y = 36, z = 26; cluster size: 257 mm2; CWP = 0.014; Fig. 3A) and right lateral PFC (including DLPFC and VLPFC; 37, 41, 7; cluster size: 3895 mm2; CWP = 0.0001; Fig. 3A) and for Hard vs. Fake-Hard trials in the right VLPFC (51, 14, 6; cluster size = 559 mm2; CWP = 0.0004 and 46, 29, − 1; cluster size = 458 mm2; CWP = 0.003). (The fact that cluster sizes were larger for the comparison of Hard with Easy trials than the comparison of Hard with Fake-Hard trials likely reflects that there were five times as many Easy than Fake-Hard trials.) That the activation patterns were quite similar for both comparisons indicates that the increased activation on Hard trials in controls reflected increased task demands and not the instructional cue, which was identical for Hard and Fake-Hard trials.
Fig. 3.
Group activation differences in cognitive control regions and relations with errors and symptoms. (A) Statistical maps of group differences at 6 s for Hard vs. Easy trials displayed on the inflated right cortical surface of the template brain at p ≤ 0.01 (Monte Carlo corrected). Greater activation in healthy controls (HC) is depicted in warm colors. Anatomical boundaries for dACC, DLPFC and VLPFC are outlined in green. (B) Hemodynamic responses for significant clusters: Hard vs. Easy (top row); Hard, Easy and Fake-Hard trials vs. fixation in healthy controls (HC; middle row) and patients (SZ; bottom row). The 6 s time point is highlighted. (C) Relations of activation with errors. Scatterplots show the relations between logit transformed error rate and activation for dACC, DLPFC and VLPFC clusters showing group differences. Blue circles and dashed regression lines represent controls, red circles and solid regression lines represent patients. (D) Relations of activation with symptom severity in schizophrenia. Scatterplots show the relations of symptom severity as indexed by PANSS total score with activation for dACC, DLPFC and VLPFC clusters showing group differences.
Increased activation for Hard vs. Easy trials in the right dACC (r = − 0.37, p = 0.002), DLPFC (r = − 0.52, p < 0.001) and VLPFC (r = − 0.56, p < 0.001) clusters that showed significant group differences was associated with a lower error rate (Fig. 3C). The relations between activation and error rate did not differ by group (all p's > 0.49). In patients, activation in these clusters also inversely correlated with symptom severity as indexed by the total PANSS score (sum of negative, positive and general psychopathology symptom scores) (Fig. 3D; dACC: r = − 0.44, p = 0.01; DLPFC, r = − 0.35, p = 0.05; VLPFC; r = − 0.35, p = 0.05, all uncorrected for multiple comparisons).
3.2.3. Antipsychotic medications
Dosage of antipsychotic medications as measured in chlorpromazine equivalents (Woods, 2003) did not significantly correlate with behavioral or neural measures of cognitive control (latency difference for hard vs easy trials: r = − 25, p = 0.23; hard vs easy activation: dACC: r = − 0.16, p = 0.45; DLPFC: r = − 0.03, p = 0.88; VLPFC: r = − 0.11, p = 0.62)
4. Discussion
When confronted with a more challenging version of the same task, healthy individuals increased recruitment of cognitive control network regions (dACC, DLPFC and VLPFC). This increased recruitment is likely adaptive since participants who showed greater increases made fewer errors, regardless of group. Patients with schizophrenia made more errors than controls and failed to increase activation in cognitive control regions for harder trials. These findings suggest that recruitment of the cognitive control network is important for behavioral inhibition and that reduced control impairs performance in schizophrenia.
In addition to being relevant to behavioral inhibition, the ability to implement control predicted symptom severity in schizophrenia. Reduced recruitment of cognitive control regions correlated with total symptom severity. In addition, a behavioral index of reduced control correlated with more severe negative symptoms. Specifically, both groups made more errors and had faster responses on Hard than Easy trials but the latency difference was exaggerated in patients. We interpret the differences between error rate and latency on Hard versus Easy trials to index behavioral control (with larger differences indicating less control) because on Hard trials, the luminance change in the periphery is a distraction that disrupts attention to the task at hand. For this reason, it is necessary to exert greater control to stay focused and inhibit saccades in response to the imperative stimulus. Although patients did not make disproportionally more errors on Hard versus Easy trials, their exaggerated latency difference suggests increased vulnerability to distraction (i.e., reduced cognitive control). Thus, patients showed neural and behavioral deficits in ramping up control on a more challenging task that correlated with more severe symptoms.
There are a number of plausible explanations for the correlations of reduced cognitive control with symptoms. These findings could reflect that cognitive control deficits and symptoms arise from common mechanisms (Lesh et al., 2011). Alternatively, individuals who are more symptomatic may have greater difficulty exerting control on more difficult tasks. A more intriguing possibility is that reduced cognitive control contributes to the expression of symptoms by rendering patients less able to disregard unwanted thoughts and feelings. If this were the case, cognitive-behavioral and mindfulness interventions that enhance control may allow schizophrenia patients to redirect attention away from internal experiences and to the external environment (e.g. task demands) and by so doing, decrease the intensity of symptoms and improve task performance. Recent work provides partial support for this hypothesis (Tabak et al., 2015). Schizophrenia patients endorsed lower levels of mindfulness than controls and mindfulness was related to indices of adaptive function such as emotion regulation, but not to symptoms.
The present findings of difficulty mobilizing control for task execution extend our previous MEG findings that patients fail to use instructional cues to ramp up control in preparation for the onset of a difficult task (Manoach et al., 2013). Specifically, patients failed to significantly increase dACC activity for cues indicating an impending antisaccade versus prosaccade. The temporal resolution of MEG allowed us to definitively attribute this failure to the preparatory period that occurs prior to the onset of the imperative stimulus. During this period the task is known, but the direction of the required movement is not. Conversely, in the present study, we can definitively attribute the reduced recruitment of the control network in schizophrenia to antisaccade task requirements (i.e., response inhibition and generation of a novel saccade in the opposite direction) rather than cue-based preparation, since similar reduced recruitment was seen when Hard trials were compared with Fake-Hard trials, which had an identical cue but easy task demands. More specifically, we can attribute the differential activation to the inhibition requirement of the antisaccade task, since the requirement to generate a novel saccade away from the stimulus was identical for all trial types, but inhibition demands were greater for Hard trials. Together, along with previous studies showing deficits in preparatory (e.g., Zahn et al., 1961) and contextual (e.g. Rodrigue et al., 2016) processing these two studies indicate that behavioral Inhibition deficits in schizophrenia reflect difficulty in mobilizing cognitive resources for both task preparation and inhibiting motor responses.
A limitation to the present study is that all but four patient participants were chronically maintained on antipsychotic medications. For this reason, their symptoms can be considered residual in that they have not responded to dopaminergic medications and may differ from symptoms present early in the course in both character and mechanism. In addition, antipsychotic drugs may have affected hemodynamic responses. We note, however, that antipsychotic dosage as measured by chlorpromazine equivalents did not correlate with neural measures of cognitive control and activation to Easy trials did not differ between patients and controls. What differentiated groups was the failure to increase cognitive control activation on Hard relative to Easy trials. It is more difficult to attribute this pattern to a general blunting effect of antipsychotics on hemodynamic responses.
Our analyses of the relations of symptom severity to behavioral and neural indices of cognitive control were conducted to understand the clinical relevance of cognitive control deficits. Although previous studies have linked cognitive control deficits to increased negative (Thakkar et al., 2011) and disorganization (Barch et al., 2003) symptoms, we did not have a strong a priori basis for expecting correlations with a particular type of symptom since the neural underpinnings of symptoms are not well-understood. Although the relations were consistently in the expected direction (increased severity is associated with reduced cognitive control), given the number of comparisons, these findings would not survive correction for multiple comparisons.
In conclusion, patients fail to optimally mobilize cognitive control when increased inhibition is required and this correlates with worse performance and increased symptom severity. Reduced control may contribute to impaired ability to inhibit prepotent but contextually inappropriate responses leading to behavior that is stimulus-bound and perseverative rather optimally guided by situational demands. Reduced control may also contribute to symptom expression by making it more difficult to disregard internal stimuli. For these reasons, therapies that increase cognitive control may improve function.
Acknowledgements
This work was supported by the National Institute of Mental Health (R01 MH67720; K24 MH099421); National Alliance for Research on Schizophrenia and Depression; Mental Illness and Neuroscience Discovery (MIND) Institute to DSM; MGH Fund for Medical Discovery Clinical Fellowship Award 44EA - MGH and National Heart, Lung, and Blood Institute 5T32HL007901-17 to BB; Swiss National Science Foundation P2ELP2_159891 to FIK and utilized resources provided by Shared Instrumentation Grants 1S10RR023401, 1S10RR019307, and 1S10RR023043. The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.nicl.2016.10.020.
Appendix A. Supplementary data
Supplementary material
References
- Agam Y., Carey C., Barton J.J., Dyckman K.A., Lee A.K., Vangel M., Manoach D.S. Network dynamics underlying speed-accuracy trade-offs in response to errors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C. University of Iowa; Iowa City: 1983. Scale for the Assessment of Negative Symptoms (SANS) [Google Scholar]
- Barch D.M., Carter C.S., MacDonald A.W., 3rd, Braver T.S., Cohen J.D. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J. Abnorm. Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- Buckner R.L., Goodman J., Burock M., Rotte M., Koutstaal W., Schacter D., Rosen B., Dale A.M. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burock M.A., Dale A.M. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum. Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Connolly J.D., Goodale M.A., Goltz H.C., Munoz D.P. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J. Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn. Reson. Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–140. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dyckman K.A., Camchong J., Clementz B.A., McDowell J.E. An effect of context on saccade-related behavior and brain activity. NeuroImage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Everling S., Munoz D.P. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research; New York State Psychiatric Institute, New York: 1997. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Nonpatient ed. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding D.C., Basso M.A. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P.E. Primary and secondary saccades to goals defined by instructions. Vis. Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Harris M.S., Reilly J.L., Keshavan M.S., Sweeney J.A. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol. Med. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Hodgson, T., Chamberlain, M., Parris, B., James, M., Gutowski, N., Husain, M., Kennard, C., 2007. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. [DOI] [PubMed]
- Hutton S.B., Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Johnston K., Levin H.M., Koval M.J., Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koski L., Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp. Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach D.S., Lindgren K.A., Cherkasova M.V., Goff D.C., Halpern E.F., Intriligator J., Barton J.J.S. Schizophrenic subjects show deficient inhibition but intact task-switching on saccadic tasks. Biol. Psychiatry. 2002;51:816–826. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Manoach D.S., Lee A.K., Hamalainen M.S., Dyckman K.A., Friedman J.S., Vangel M., Goff D.C., Barton J.J. Anomalous use of context during task preparation in schizophrenia: a magnetoencephalography study. Biol. Psychiatry. 2013;73:967–975. doi: 10.1016/j.biopsych.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Matsuura M., Ohkubo T., Ohkubo H., Matsushima E., Inoue K., Taira M., Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McDowell J.E., Myles-Worsley M., Coon H., Byerley W., Clementz B.A. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- McDowell J.E., Dyckman K.A., Austin B.P., Clementz B.A. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin F.M., Maccotta L., Ollinger J.M., Petersen S.E., Buckner R.L. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Milea D., Lehericy S., Rivaud-Pechoux S., Duffau H., Lobel E., Capelle L., Marsault C., Berthoz A., Pierrot-Deseilligny C. Antisaccade deficit after anterior cingulate cortex resection. Neuroreport. 2003;14:283–287. doi: 10.1097/00001756-200302100-00026. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Muri R.M., Ploner C.J., Gaymard B., Demeret S., Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Reilly J.L., Frankovich K., Hill S., Gershon E.S., Keefe R.S., Keshavan M.S., Pearlson G.D., Tamminga C.A., Sweeney J.A. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr. Bull. 2014;40:1011–1021. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue A.L., Austin B.P., Dyckman K.A., McDowell J.E. Brain activation differences in schizophrenia during context-dependent processing of saccade tasks. Behav. Brain Funct. 2016;12:19. doi: 10.1186/s12993-016-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L.D., Goldman-Rakic P.S. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak N.T., Horan W.P., Green M.F. Mindfulness in schizophrenia: associations with self-reported motivation, emotion regulation, dysfunctional attitudes, and negative symptoms. Schizophr. Res. 2015;168:537–542. doi: 10.1016/j.schres.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Schall J.D., Boucher L., Logan G.D., Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biol. Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tu P., Buckner R.L., Zollei L., Dyckman K.A., Manoach D.S. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Matsuzaka Y., Shima K., Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport. 2004;15:1559–1563. doi: 10.1097/01.wnr.0000133300.62031.9b. [DOI] [PubMed] [Google Scholar]
- White K., Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson G.J. Jastak Associates; Wilmington, DE: 1993. The Wide Range Achievement Test-Revision 3. [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zahn T.P., Shakow D., Rosenthal D. Reaction time in schizophrenic and normal subjects as a function of preparatory and intertrial intervals. J. Nerv. Ment. Dis. 1961;133:283–287. doi: 10.1097/00005053-196110000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material