Abstract
Interdental papilla construction, especially in the esthetic area, is one of the most challenging tasks. Interdental papilla loss might occur due to several reasons as a consequence of periodontal surgery or trauma. The purpose of this study is to report the reconstruction of lost interdental papilla using hyaluronic acid gel. Hyaluronic acid is a glycosaminoglycan molecule with anti-inflammatory, anti-edematous properties on periodontal tissues invaded by submicrobial flora. It enhances wound healing and accelerates periodontal repair and regeneration. In addition to the field of dentistry, it has been used in other fields such as orthopedics, ophthalmology, and dermatology. It shows growth factor interaction, regulates osmotic pressure, and enhances tissue lubrication, which helps in maintaining the structural and homeostatic integrity of tissues, hence resulting in beneficial effect on lost interdental papilla. This study was aimed to reconstruct the lost interdental papilla by injecting 0.2% hyaluronic acid via nonsurgical approach. It is a noninvasive approach which reduces patient's postoperative discomfort with marked variations in the volume of interdental papilla before and after the procedure. As sufficient information is not available regarding the effectiveness of hyaluronic acid in interdental papilla construction, this study was conducted.
Keywords: Esthetics, hyaluronic acid, periodontal wound healing, regeneration
INTRODUCTION
The ultimate goal of periodontal therapy is to arrest the progress of periodontal disease and regenerate the lost periodontal support.[1] Hyaluronic acid (HA) is a high molecular weight, nonsulphated glycosaminoglycan component which is produced during various phases of cell's life cycle. HA forms a major and critical component of connective tissue. It contributes in tissue hydrodynamics, cell migration, and proliferation, and improves healing properties of the tissue.[2] It acts as barrier to various gram negative bacteria.[3] Its physiological, structural, and biochemical properties prove that it provides elasticity and stability to tissues and is critically beneficial in tissue regeneration.[4] Pharmacologically, the content of HA in the tissues is often broken down by the blood stream or by lymphatic drainage and its turnover rate is found to be 20–30%. After being broken down, it reaches the blood stream and is removed by the liver. Excretion is in very minute quantities in the urine 2–10%. Its plasma half-life is 2–3 days depending upon how it is eliminated.[3] HA is also known as hyaluronan or hyaluronate.[5] HA was discovered in 1934 from cow's eye by Karl Meyer and John Plamer and was first used in 1942 by Endre Balazs as a substitute for egg white in bakery products. The chemical structure of HA shows alternate units of N-acetyl glucosamine and D-glucorounic acid, both of the components linked through glycosidic bonds. Its nonimmunogenic nature increases its use in clinical applications. It is a highly biocompatible polysaccharide molecule with anti-edematous and bacteriostatic properties. HA acts as an antioxidant by scavenging reactive oxygen species, which helps in the regulation of immune response implying its anti-inflammatory properties.[6]
It has also been reported that HA shows osteoinductive properties, which is useful for treatment of periodontal disease.[7] Other beneficial effects have also been seen for the treatment of recurrent apthous ulcer,[8] for treating gingival lesions,[9] and promote healing in extraction socket.[10]
HA has also reported as a diagnostic biomarker of inflammation in gingival crevicular fluid and repair of tissues. Recently, HA has been added as a local chemotherapeutic agent to tissues. It is available in various forms.[11] Low molecular weight HA shows angiogenesis whereas high molecular weight is opposite to it.[12]
Recent investigations have indicated that HA induces mineralization of dental pulp cells through CD44 cell surface glycoprotein and is considered to be a principle ligand for receptor CD44.[13]
Hyaluronic acid was found to have extensive actions in various periodontal therapies such as topically applied in subgingival regions reduces microbial activity, bone regeneration in deep periodontal bony defects, guided bone regeneration, nonsurgical treatment of peri-implantitis pockets, peri-implant maintenance of immediately placed implants, and gingival augmentation in mucogingival surgery. HA may act as a scaffold for other molecules such as Bone morphogenic protien-2 and platelet derived growth factor-BB, used in guided bone regeneration techniques and tissue engineering research.[14] HA when applied to patients with chronic periodontitis showed reduction in bleeding on probing (BOP), probing pocket depth (PPD), and clinical attachment level, and hence, can be used as an adjunct to scaling and root planning.[15]
HA is biocompatible and intrinsically safe to use, with no evidence of cytotoxicity. HA gel, injections, or oral (by mouth) should not be used in patients with allergies.[7]
CASE REPORT
A 24-year-old female reported to the Department of Periodontics with her esthetic concern and complaint regarding the loss of interdental papilla, i.e. black triangle, in the anterior maxillary region [Figure 1]. Patient was concerned for esthetics; class II type of interproximal tissue loss without any bone loss and no extra oral deformities were detected. Patient's oral hygiene was found to be good. A written informed consent was taken from the patient before the procedure, and ethical clearance certificate was obtained from the institution.
Figure 1.
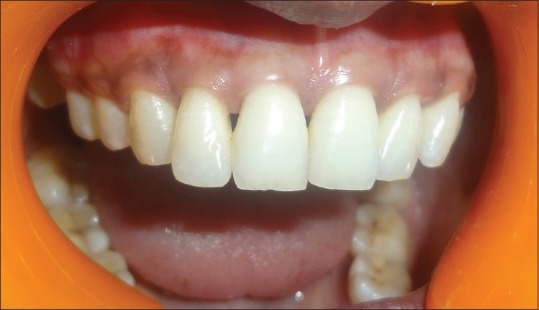
Preoperative: black triangle between maxillary right central incisor and lateral incisor
Nordland and Tarnow[16] proposed a classification using three reference points, i.e., contact point, facial and apical extent of cementoenamel junction (CEJ), and interproximal extent of CEJ (iCEJ), and was classified into the following four classes:
Normal: Interdental papilla occupies embrasure space to the apical part of the interdental contact point.
Class I: Tip of interdental papilla occupies space between the interdental contact point and the most coronal part of CEJ.
Class II: Tip of interdental papilla lies at/or the apical to the iCEJ but coronal to the apical most part of the CEJ on facial aspect.
Class III: Tip of interdental papilla lies at level with or apical to the facial CEJ.
The loss of interdental papillae can be due to many reasons such as gingivitis, oral hygiene procedures with trauma, tooth shape with abnormal anatomy, or improper contours of the restoration.
Inclusion criterion for the study were:
Age range: 20–75 years
Maxillary anterior teeth
Plaque index less than 20%
Teeth should not have caries and no fixed prosthesis or orthodontic appliance
Nonsmoker individuals
No systemic disease that may affect the periodontal status
Avoid consumption of drugs causing gingival overgrowth.
Patient's medical status was noncontributory. Patient was completely informed about the study. After introducing a local anesthetic agent, less than 0.2 ml of the HA gel was injected at the sites 2–3 mm apical to the coronal tip of the papilla [Figure 2]. The patient was instructed not to brush on the day of treatment but maintain oral hygiene, and after 24 hours, using a soft bristle toothbrush at the anterior teeth to start their routine oral hygiene measures and avoid using dental floss at the sites of treatment. The patient was on follow-up for 3 weeks and was recalled for a total of 4 times. The tolerance of the gel was unconditionally good and no intolerance was observed. After first follow-up, 3 weeks later, the treatment patient did not show any improvement, therefore; another shot of 0.2% HA injection was given. Measurements of the black triangle were done using photography.
Figure 2.
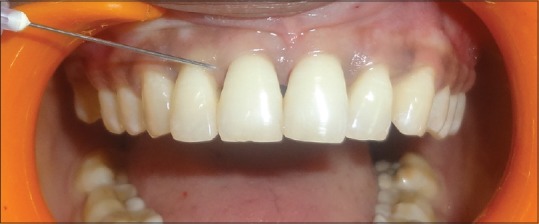
Injecting 2% hyaluronic acid into interdental papilla
After 3 months, photographs were taken and comparison was done using these images. This technique resulted in significant gain in papillary volume and esthetic improvements were notable [Figure 3]. Therefore, the desired result was obtained with the multiple use of HA.
Figure 3.
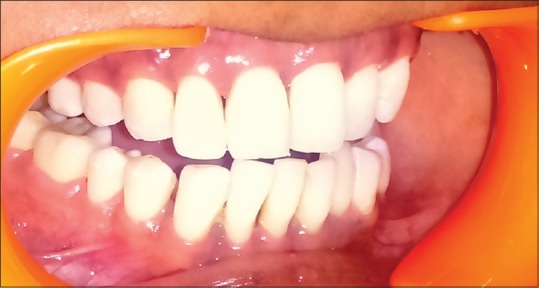
Postoperative: interdental papilla coverage after 3 months
DISCUSSION
Because using 0.2% HA for the reconstruction of lost interproximal tissue gave significant changes, it can be used as a noninvasive method, which will reduce the use of surgical procedures for regenerating soft tissues in upcoming years. This study can be limited by the amount of the interproximal tissue loss, depending upon the size of the black triangle, which might require surgical intervention to gain complete coverage. The main advantage of this study is that it is nontoxic to the patient and there is reduced discomfort after the procedure as compared to surgical procedure done. Furthermore, this study can be elaborated by more number of patients depending upon the size and type of the black triangle. Several studies have been proposed regarding the effects of HA on periodontium. Becker et al.[17] concluded that HA gel is a synthetic material and can be used with no drug interference and is a safe material, which significantly decreases the interdental black triangle in the esthetic zone. It has also been approved by the Food and Drug Associatoion. Vedamurthy[18] reported HA to be a dermal filler and applied it for soft tissue augmentation, observing significant improvements. Monheit et al.[19] discussed the inherent properties of HA that make them ideal for cosmetic surgeries. Prato et al.[20] studied gingival augmentation with an autologous cell HA and reported significant results with the complete coverage. Pendyala et al. found that antioxidant capacity of HA is inversely proportional to the severity of inflammation and can be used as a biomarker in periodontitis. It is acceptable that injecting HA to periodontal wound sites had shown significant effects in periodontal tissue regeneration. Engström et al. reported bone regenerative effects of HA in nonsurgical and surgical groups and showed no statistical difference when evaluated on radiographs in the nonsurgical group; however, there was remarkable decrease in the height of alveolar bone after oral prophylaxis in both the nonsurgical and surgical group. There was also decrease in PPD after surgical treatment and also with (SRP. HA when involved with soft and hard tissues showed negligible effect on the immune system of the patient. Ballini et al. stated enhanced accelerating capacity of new bone formation in the infrabone defects when combined with autologous bone graft.[6]
CONCLUSION
This study indicates possible improvements in regenerating lost interdental papilla and removal of black triangle by injecting HA into the lost papilla using a nonsurgical approach. Because of its property of modulating periodontal wounds, HA is useful in regenerating periodontal tissues. This nonsurgical approach limits the use of surgical procedures for regenerating lost papilla, and hence, it is a noninvasive method and also reduces patient discomfort. Therefore, this study demonstrates HA to be a nonsurgical approach for regenerating lost papilla and gave significant and satisfactory results. To overcome the limitations of using of HA for regenerating lost papilla, this study need to be elaborated with more number of patients depending upon the size of the black triangle.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Akizuki T, Oda S, Komaki M, Tsuchioka H, Tsuchioka H, Kawakatsu N, et al. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J Periodontal Res. 2005;40:245–51. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 2.Mansouri SS, Ghasemi M, Salmani Z, Shams N. Clinical Application of Hyaluronic Acid Gel for Reconstruction of Interdental Papilla at the Esthetic zone. J Islamic Dent Assoc Iran. 2013;25:152–7. [Google Scholar]
- 3.Vangelisti R, Pagnaco A, Era C. Hyaluronic acid in the topical treatment of inflammatory gingivits. A preliminary clinical study. Prevenz Asist Dent. 1993;1:16–20. [Google Scholar]
- 4.Sahayata VN1, Bhavsar NV, Brahmbhatt NA. An Evaluation of 0.2% Hyaluronic Acid Gel (Gengigel®) in the Treatment of Gingivitis: A Clinical and Microbiological Study. Oral Health Dent Manag. 2014;13:779–85. [PubMed] [Google Scholar]
- 5.Bansal J1, Kedige SD, Anand S. Hyaluronic acid: A promising mediator for periodontal regeneration. Indian J Dent Res. 2010;21:575–8. doi: 10.4103/0970-9290.74232. [DOI] [PubMed] [Google Scholar]
- 6.Dahiya P1, Kamal R. Hyaluronic Acid: A Boon in Periodontal Therapy. N Am J Med Sci. 2013;5:309–15. doi: 10.4103/1947-2714.112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdulhameed BS, Ibraheem LM. Periodontal effect of 8% Hyaluronan as an Adjunct to Scaling and Root Planning in the Treatment of Chronic Periodontitis (Comparative Study) J Dent Med Sci. 2014;13:76–81. [Google Scholar]
- 8.Nolon A, Baillie C, Badminton J, Rudralinglam M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 2006;35:461–5. doi: 10.1111/j.1600-0714.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontal. 2003;30:159–64. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 10.Mendes RM1, Silva GA, Lima MF, Calliari MV, Almeida AP, Alves JB, et al. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol. 2008;53:1155–62. doi: 10.1016/j.archoralbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Pogrel MA, Lowe MA, Stern R. Hyaluronan (hyaluronic acid) in human saliva. Arch Oral Biol. 1996;41:667–71. doi: 10.1016/s0003-9969(96)00050-7. [DOI] [PubMed] [Google Scholar]
- 12.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, et al. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–6. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Chen KL, Yeh YY, Lung J, Yang YC, Yuan K. Mineralization Effect of Hyaluronan on Dental Pulp Cells via CD44. J Endod. 2016;42:711–6. doi: 10.1016/j.joen.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Park JK, Yeom J, Oh EJ, Reddy M, Kim JY, Cho DW, et al. Guided bone regeneration by poly (lactic-co-glycolic acid) grafted hyaluronic acid bi-layer films for periodontal barrier applications. Acta Biomater. 2009;5:3394–403. doi: 10.1016/j.actbio.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Rajan P, Baramappa R, Rao NM. Hyaluronic Acid as an adjunct to scaling and root planing in chronic periodontitis. A randomized clinical trail. J Clin Diagn Res. 2014;8:ZC11–4. doi: 10.7860/JCDR/2014/8848.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordland WP, Tarnow DP. A classification system for loss of papillary height. J Periodontol. 1998;69:1124–6. doi: 10.1902/jop.1998.69.10.1124. [DOI] [PubMed] [Google Scholar]
- 17.Becker W, Gabitov I, Stepanov M, Kois J, Smidt AE, Becker B. Minimally invasive treatment for papillae deficiencies in the esthetic zone: A pilot study. Clin Implant Dent Relat Res. 2010;12:1–8. doi: 10.1111/j.1708-8208.2009.00247.x. [DOI] [PubMed] [Google Scholar]
- 18.Vedamurthy M. Soft tissue augmentation: Use of hyaluronic acid as dermal filler. Indian J Dermatol Venereol Leprol. 2004;70:383–7. [PubMed] [Google Scholar]
- 19.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19:141–50. doi: 10.1111/j.1529-8019.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Prato GP, Rotundo R, Magnani C, Soranzo C, Muzzi L, Cairo F. An Autologous Cell Hyaluronic Acid Graft Technique for Gingival Augmentation: A Case Series. J Periodontol. 2003;74:262–7. doi: 10.1902/jop.2003.74.2.262. [DOI] [PubMed] [Google Scholar]


