Abstract
This study was designed to characterize morphometric sperm subpopulations in normozoospermic men by using different statistical methods and examining their suitability to classify correctly different sperm nuclear morphologies present in human ejaculates. Ejaculates from 21 normozoospermic men were collected for the study. After semen collection and analysis, samples were prepared for morphometric determination. At least 200 spermatozoa per sample were assessed for sperm morphometry by computer-assisted sperm morphometry analysis (CASA-Morph) using fluorescence. Clustering and discriminant procedures were performed to identify sperm subpopulations from the morphometric data obtained. Clustering procedures resulted in the classification of spermatozoa into three morphometric subpopulations (large-round 30.4%, small-round 46.6%, and large-elongated 22.9%). In the second analysis, using discriminant methods, the classification was made independently of size and shape. Three morphological categories according to nuclear size (small <10.90 μm2, intermediate 10.91–13.07 μm2, and large >13.07 μm2) and four categories were defined on 400 canonical cells (100 × 4) from 10 men according to sperm nuclear shape (oval, pyriform, round, and elongated). Thereafter, the resulting classification functions were used to categorize 4200 spermatozoa from 21 men. Differences in the class distribution were observed among men from both clustering and discriminant procedures. It was concluded that the combination of CASA-Morph fluorescence-based technology with multivariate cluster or discriminant analyses provides new information on the description of different morphometric sperm subpopulations in normal individuals, and that important variations in the distribution of morphometric sperm subpopulations may exist between men, with possible functional implications.
Keywords: computer-assisted sperm morphometry analysis, man, sperm subpopulations
INTRODUCTION
Spermatozoa are highly specialized cells that have to function in the complex environment of the female genital tract. Spermatozoa present in ejaculates are heterogeneous, and the existence of sperm subpopulations in mammalian ejaculates is now widely accepted.1 These subpopulations may be an adaptive mechanism to increase the chance of a fertilization.
Sperm subpopulations in semen have been identified in different species on the basis of biochemistry (humans2,3), function (boars4,5), motility (stallions;6 red deer;7,8 dogs;9 bulls;10 rams;11,12,13 and blue foxes14), and morphometry (stallions;15 boars;16,17 red deer;7,18 bulls;17,19 brown bears;20 rams;12,13,17,21,22 Goeldi's monkey;23 and marmosets24). There is increasing evidence that the heterogeneity of these subpopulations has functional relevance. For example, relationships have been found between the sperm subpopulations and fertility,8,25 and the ability to survive cryopreservation.9,26,27 Theoretically, heterogeneity of spermatozoa ensures a greater potential to fertilize an oocyte at some unpredictable interval after ejaculation.28
The introduction of computer-assisted sperm morphometry analysis (CASA-Morph) systems has increased the objectivity and sensitivity of sperm morphological evaluation. The use of morphometric data obtained with this technique has changed the classical approach of considering the whole ejaculate as a single homogeneous population with a normal distribution, by showing the existence of sperm subpopulations.24 Thus, there is a substantial loss of information when traditional statistical procedures are applied to the results, because the real distribution of sperm morphometric forms is not uniform and normal, but rather structured in separate subpopulations.24,29 An association between computerized and statistical techniques allows classifying the overall sperm population of semen samples into homogeneous, separated subpopulations, grouping spermatozoa with similar morphometry characteristics.12 Two different statistical methods, cluster and discriminant analyses, have been used to disclose sperm morphometric subpopulations in different species.29 The aim of this study was to characterize sperm morphometric subpopulations in normozoospermic men by using different statistical methods and examining their ability to classify correctly different sperm nuclear morphologies present in ejaculates. This study may constitute the basis for future analyses of the relationships of sperm quality, freezing capacity, and human male fertility.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all chemicals used were obtained from Sigma-Aldrich Chemical Company (Alcobendas, Madrid, Spain) and were of the highest grade available.
Donors and sample selection
The study was approved by the Institutional Ethics Committee and written informed consent was given by all patients. Semen samples from 21 volunteers, with mean age of 24.2 years (range 21–32 years), were obtained by masturbation after 3–5 days of sexual abstinence. Only men with clinically normal semen parameters, judged from the World Health Organization30 reference values, were included in the study.
After collection, the semen was allowed to liquefy at 37°C for at least 30 min and then was examined within 1 h. Each ejaculate was thoroughly mixed, and aliquots were prepared for sperm morphometric assessment as previously described.31,32 In brief, semen smears were allowed to air dry for a minimum of 2 h, fixed with 2% (v/v) glutaraldehyde in PBS for 3 min, washed thoroughly in distilled water and labeled with Hoechst 33342 as detailed below.
Sperm morphometric determination by computer-assisted sperm morphometry analysis (CASA-Morph)
Semen smears were stained by placing 20 μl of a Hoechst 33342 suspension (20 μg ml−1 in a TRIS-based solution) between the slide and a coverslip, which was then incubated for 20 min in the dark at room temperature.31 The coverslip was then removed and the slide was washed thoroughly with distilled water and allowed to dry. Digital images of the fluorescent sperm nuclei were recorded by means of a setup composed of an epifluorescence microscope (DM4500B, Leica, Wetzlar, Germany; A-UV filter cube, BP340–380 excitation filter, LP425 suppressor filter, dichromatic mirror: DM400) with a 63× plan apochromatic objective, and photographed with a Canon Eos 400D digital camera (Canon Inc., Tokyo, Japan). The camera was controlled by a computer using DSLR Remote Pro software (Breeze Systems, Camberley, UK).
At least 200 sperm cells per sample were randomly captured at least two slides per sample. From each captured image, sperm nuclei morphometry was automatically analyzed by the ImageJ open software (available on-line at http://rsbweb.nih.gov/ij/download.html), with a plug-in module created for this purpose.31 Each sperm nucleus was measured for four primary parameters and four derived parameters for nuclear shape. Primary parameters were Area (A, μm2, as the sum of all pixel areas contained within the boundary), Perimeter (P, μm, as the sum of external boundaries), and Length (L) and Width (W) (μm, the highest and lowest values, respectively, of the Feret diameters, i.e., the projection of the sperm nucleus on the horizontal axis measured at angles of rotation of 0°, 30°, 60°, 90°, 120°, and 150°; Length and Width are not necessarily orthogonal). Derived nuclear shape parameters were Ellipticity (L/W), Rugosity (4πA/P2), Elongation ([L − W]/[L + W]), and Regularity (πLW/4A).
Statistical analysis
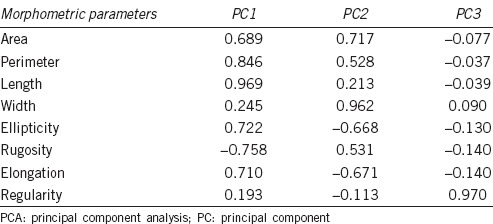
Statistical analyses were performed with the SPSS package, version 15.0 (SPSS Inc., Chicago, IL, USA). Two methods were used to obtain sperm subpopulations based on the morphometric data. The first method was based on two-step cluster procedures.12,17 The first step was to perform a principal component analysis (PCA) of the morphometric data. The purpose of PCA is to derive a small number of linear combinations (principal components) from a set of variables that retain as much of the information in the original variables as possible. This allows the summarizing of many variables in few, jointly uncorrelated, principal components. A preferred result is when there are few principal components accounting for a large proportion of the total variance. To select the number of principal components that should be used in the next step of the analysis, the criterion was used of selecting only those with an eigenvalue (variance extracted for that particular principal component) >1 (Kaiser criterion). The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA. This analysis allows the identification of sperm subpopulations and the detection of the outliers.
The second method was based on a two-step discriminant analysis.33,34 The first step consisted of defining the nuclear size into three categories: small, intermediate, and large. Each threshold was established on the basis of the area values, considering the 25th centile and below for small, 26th–74th centile for intermediate, and 75th centile and above for large. The second step focused on nuclear shape, initially comprising four subjective forms: round, elongated, oval, and pyriform (Figure 1). Oval nuclei were the most frequently represented and may be considered analogous to the normal cells described by the WHO.30 Round, elongated, and pyriform spermatozoa also have a correspondence to the round, narrow-tapered, and pyriform cells described by the WHO.30 For each class, a number of 100 canonical cells/category obtained from 10 men were selected and used for the subsequent calculation of the classification matrix. Different donor samples (10 donors) were used to define canonical cells from those used for the global discriminant analysis (21 donors). Discriminant analysis was performed by the linear stepwise procedure to identify the most useful parameters for the shape classification of these cells. Variables were added one by one to the discriminating functions until it was found that the addition of a new variable did not give a better discrimination. Wilk's lambda was used to test discrimination. Then, the classification matrix was applied to the whole population to establish the proportion of each class (subpopulation) per animal.
Figure 1.
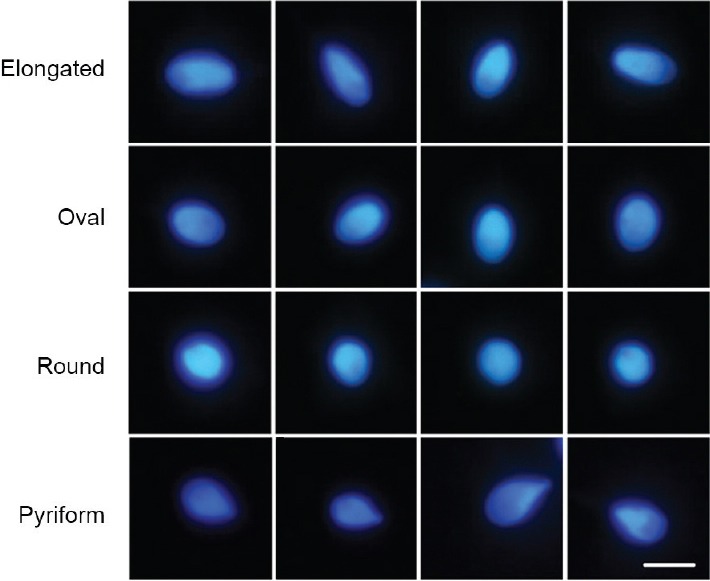
Four examples of each of the sperm categories defined according to sperm nuclear shape. Scale bar for all images, 5 μm.
To study the distributions of subpopulations between men, the Chi-squared test was used. The values obtained were expressed as mean ± standard error of the mean (s.e.m.). The statistical level of significance was set at P < 0.05.
The variability of each parameter at different grouping levels was determined from the coefficients of variation (CV).31 In all the donors, within-man and between-man CVs were calculated for all the morphometric parameters. Within-man CVs were expressed as the mean of individual values.
RESULTS
Spermatozoa in humans are characterized by substantial between-man variability. The highest variability in sperm nuclear morphometry was identified for elongation and area (CV 38.98% and 20.34%, respectively). All morphometric parameters showed a higher degree of variation between individuals than within individuals.
From the two-step cluster procedure, PCA analysis revealed three components with eigenvalues over 1, representing more than 97.4% of the cumulative variance (Table 1). The first factor (PC1) was defined positively by primary (P and L) and secondary (Ellipticity and Elongation) parameters, the second (PC2) by positive primary (A and W) and negative secondary (Ellipticity and Elongation) factors, and the third (PC3) by Regularity.
Table 1.
Results of the PCA performed on the CASA Morph data obtained from 21 normozoospermic men
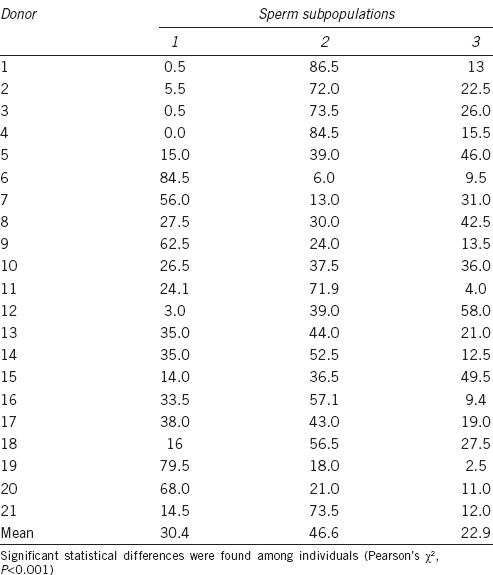
The second step of clustering analysis revealed the existence of three sperm subpopulations (Table 2). Subpopulation 1 (SP1) had positive values for PC2, so this cluster includes large and round spermatozoa. Subpopulation 2 (SP2) had negative values for PC1, so it comprises small and round spermatozoa. Subpopulation 3 (SP3) had positive values for PC1 and negative for PC2, thus comprising large and elongated spermatozoa. Of the total spermatozoa, 29.8%, 47.5%, and 22.7% were included in Subpopulations 1, 2, and 3, respectively. The distribution of sperm subpopulations was completely different among men (P < 0.001, Table 3).
Table 2.
Results of the two-step cluster procedure in men with the morphometric indices (PC) as variables
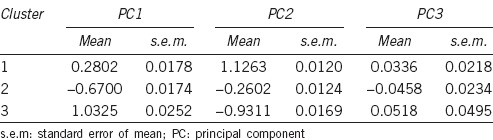
Table 3.
Percentage distribution of sperm subpopulations in the different men
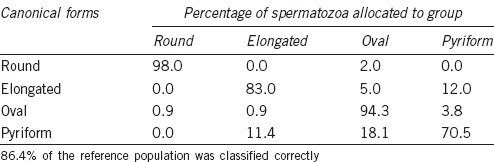
In the second analysis, with discriminant methods, the classification was made independently of size and shape. For shape, the matrix of classification obtained gave the Fisher's discriminant linear functions for each class as listed in Table 4. This matrix was applied to the reference population with a globally correct assignment of 86.4% of cells (Table 5). Round and oval sperm nuclei were more accurately classified (more than 94%) than elongated and pyriform forms. When the allocation matrix for shape was applied to the whole population, oval cells were the most frequently represented (54.1%), while the most infrequent were round and elongated spermatozoa (11.5% and 13.0%, respectively).
Table 4.
Discriminant classification matrix showing Fisher's linear discriminant functions for shape of human sperm cells
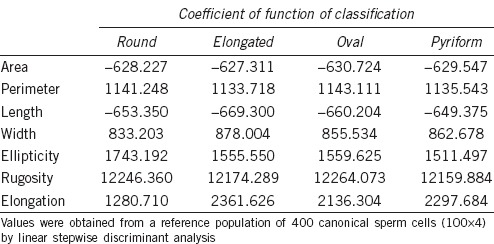
Table 5.
Percentage of sperm heads in each class of the reference population assigned to each class after discriminant analysis
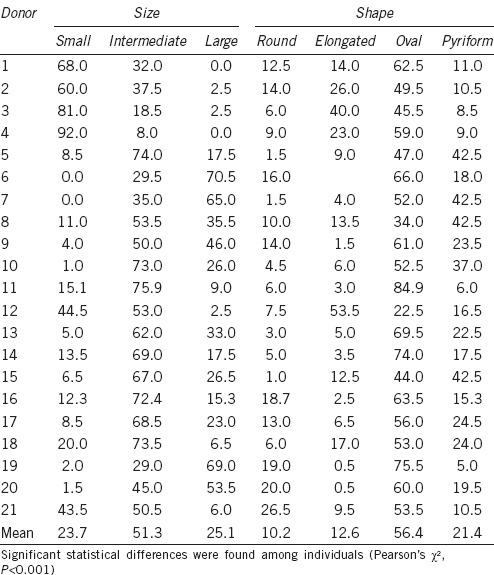
For size, the sperm nuclei were divided into three classes from the area data, considering the small ones as those equal to and below the 25th centile, the large ones as those equal to and above the 75th centile, and the intermediate ones between these limits. The values observed were: small, area <10.90 μm2; intermediate, 10.90 μm2 ≥ area ≤13.07 μm2; and large, area >13.07 μm2. Differences in the class distribution were observed among men (P < 0.001), with some donors having more than 90% of the cells with a small area, while others had 0%. This extent of difference was also present for the other two size classes (Table 6). Moreover, differences in the distribution of the shape classes were found between men (Table 6).
Table 6.
Percentage distribution of sperm subpopulations in different men
DISCUSSION
The subjective evaluation of sperm morphology lacks precise replication, and the coefficients of variation associated with this analysis are very high.35,36 This fact points to the need to establish quantitative criteria for the definition of sperm cell morphology, which has been improved by the introduction of CASA-Morph systems. Morphometry analyzed by these systems has been considered a powerful tool for the selection of human patients for ART.37
A wide range of values for sperm nuclear morphometry parameters was evident here, both within samples and among men. Differences in values both within samples and among individuals are also present in other animal species.31,32 Despite this variability among men, the mean values for the sperm nuclear dimensions are higher than those reported by some,38,39,40,41,42 and lower than those described by others43,44 for the whole sperm head. These results are not surprising because previous work has shown that variation in fixation, staining, or the software used can cause important differences in sperm head morphometr.29
Human spermatozoa are highly heteromorphous, with morphological differences both in the same ejaculate and in different individuals.40,45 The different sperm subpopulations may be considered to work synergistically to increase fertilization success.26,46,47 Morphometric data provided by CASA-Morph systems may be analyzed by traditional statistical procedures although, given the heterogeneity of spermatozoa in the human ejaculates, the study of sperm subpopulations may be more informative.29 A combination of computerized and statistical techniques has allowed classification of the overall sperm population of semen samples into homogeneous, separate subpopulations in different species, by grouping spermatozoa with similar morphometry characteristics.29 However, research into morphometric sperm subpopulations has received little attention in the human species.
In the present study, two alternative statistical procedures were compared to disclose sperm morphometric subpopulations: two-step cluster and two-step discriminant analyses. These methods have been successfully used in different species.7,15,16,17,18,19,20,21,29,33,34,48 From the two-step cluster procedure, different sperm subpopulations were obtained and their distribution varied significantly among men, providing more information than classical analysis of sperm morphometric data that are based on mean values. However, this method provides a classification of spermatozoa mainly based on head size and elongation.17 The two-step discriminant analysis, however, allows a separate and more precise classification of spermatozoa according to their head size and shape, and its use may be more adequate for heteromorphous species,33,34 particularly in those species, as man, in which a previous standard has been defined.30 While measuring sperm head size can be considered an easy task, shape evaluation is commonly evaluated subjectively and expressed in descriptive terms: round, elongated, oval, and pyriform. Here, oval nuclei were the most frequently represented and may be considered analogous to the normal cells described by the WHO.30 However, there was no concordance between our results and those indicated in the WHO manual. Certainly, we are considering only the nuclear shape and size, even separately, and other cell components can contribute to the normal/abnormal definition, but we observed a higher proportion of oval cells with our technique than that indicated by the WHO. This implies that both the technique and the statistical approach used here can be a new model for human sperm morphological evaluation.
It is concluded that the combination of our defined CASA-Morph fluorescence-based technology with multivariate cluster or discriminant analyses provides new information on the description of different morphometric sperm subpopulations in normozoospermic individuals. Important variations in the distribution of morphometric sperm subpopulations may exist among men, with possible functional implications.
AUTHOR CONTRIBUTIONS
JLY and PS conceived and designed the experiments; SVF, CS, PR, TC, AB, and JMB performed the experiments; PS analyzed the data; and JY wrote the paper.
COMPETING INTERESTS
CS is Professor at Valencia University and acts as Scientific Director of Proiser R+D S.L Research and Development Laboratory. Neither he nor the other authors have interests that influenced the results presented in this paper.
ACKNOWLEDGMENTS
The authors would like to thank Neil Macowan for assistance with the English translation. We are especially grateful for the support from the healthy semen donor volunteers that enabled us to perform the present study. This work was supported by the Spanish MINECO (grant AGL2014-52775-P), and the DGA-FSE (grant A40).
REFERENCES
- 1.Holt WV, Van Look KJ. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction. 2004;127:527–35. doi: 10.1530/rep.1.00134. [DOI] [PubMed] [Google Scholar]
- 2.Calamera J, Buffone M, Ollero M, Alvarez J, Doncel GF. Superoxide dismutase content and fatty acid composition in subsets of human spermatozoa from normozoospermic, asthenozoospermic, and polyzoospermic semen samples. Mol Reprod Dev. 2003;66:422–30. doi: 10.1002/mrd.10368. [DOI] [PubMed] [Google Scholar]
- 3.Buffone MG, Doncel GF, Briggiler CI, Vazquez-Levin MH, Calamera JC. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Hum Reprod. 2004;19:139–46. doi: 10.1093/humrep/deh040. [DOI] [PubMed] [Google Scholar]
- 4.Petrunkina AM, Topfer-Petersen E. Heterogeneous osmotic behaviour in boar sperm populations and its relevance for detection of changes in plasma membrane. Reprod Fertil Dev. 2000;12:297–305. doi: 10.1071/rd00087. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Llano B, Yenes-Garcia P, Garcia-Casado P. Four subpopulations of boar spermatozoa defined according to their response to the short hypoosmotic swelling test and acrosome status during incubation at 37 degrees C. Theriogenology. 2003;60:1401–7. doi: 10.1016/s0093-691x(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 6.Quintero-Moreno A, Miro J, Rigau AT, Rodriguez-Gil JE. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology. 2003;59:1973–90. doi: 10.1016/s0093-691x(02)01297-9. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Pastor F, Garcia-Macias V, Alvarez M, Herraez P, Anel L, et al. Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process. Biol Reprod. 2005;72:316–27. doi: 10.1095/biolreprod.104.032730. [DOI] [PubMed] [Google Scholar]
- 8.Ramon M, Soler AJ, Ortiz JA, Garcia-Alvarez O, Maroto-Morales A, et al. Sperm population structure and male fertility: an intraspecific study of sperm design and velocity in red deer. Biol Reprod. 2013;89:110. doi: 10.1095/biolreprod.113.112110. [DOI] [PubMed] [Google Scholar]
- 9.Nunez Martinez I, Maria Moran J, Pena FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl. 2006;27:596–603. doi: 10.2164/jandrol.05153. [DOI] [PubMed] [Google Scholar]
- 10.Muino R, Pena AI, Rodriguez A, Tamargo C, Hidalgo CO. Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls. Theriogenology. 2009;72:860–8. doi: 10.1016/j.theriogenology.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Alvarez O, Maroto-Morales A, Ramon M, Del Olmo E, Jimenez-Rabadan P, et al. Dynamics of sperm subpopulations based on motility and plasma membrane status in thawed ram spermatozoa incubated under conditions that support in vitro capacitation and fertilisation. Reprod Fertil Dev. 2014;26:725–32. doi: 10.1071/RD13034. [DOI] [PubMed] [Google Scholar]
- 12.Yániz JL, Palacín I, Vicente-Fiel S, Sánchez-Nadal JA, Santolaria P. Sperm population structure in high and low field fertility rams. Anim Reprod Sci. 2015;156:128–34. doi: 10.1016/j.anireprosci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Santolaria P, Vicence-Fiel S, Palacín I, Fantova E, Blasco ME, et al. Predictive capacity of sperm quality parameters and sperm subpopulations on field fertility after artificial inseminationin sheep. Anim Reprod Sci. 2015;163:82–8. doi: 10.1016/j.anireprosci.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Soler C, Garcia A, Contell J, Segervall J, Sancho M. Kinematics and subpopulations’ structure definition of blue fox (Alopex lagopus) sperm motility using the ISAS®V1 CASA system. Reprod Domest Anim. 2014;49:560–7. doi: 10.1111/rda.12310. [DOI] [PubMed] [Google Scholar]
- 15.Gravance CG, Liu IK, Davis RO, Hughes JP, Casey PJ. Quantification of normal head morphometry of stallion spermatozoa. J Reprod Fertil. 1996;108:41–6. doi: 10.1530/jrf.0.1080041. [DOI] [PubMed] [Google Scholar]
- 16.Thurston LM, Watson PF, Holt WV. Sources of variation in the morphological characteristics of sperm subpopulations assessed objectively by a novel automated sperm morphology analysis system. J Reprod Fertil. 1999;117:271–80. doi: 10.1530/jrf.0.1170271. [DOI] [PubMed] [Google Scholar]
- 17.Vicente-Fiel S, Palacin I, Santolaria P, Yaniz JL. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F) Anim Reprod Sci. 2013;139:182–9. doi: 10.1016/j.anireprosci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Esteso MC, Fernandez-Santos MR, Soler AJ, Montoro V, Martinez-Pastor F, et al. Identification of sperm-head morphometric subpopulations in Iberian red deer epididymal sperm samples. Reprod Domest Anim. 2009;44:206–11. doi: 10.1111/j.1439-0531.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 19.Rubio-Guillen J, Gonzalez D, Garde JJ, Esteso MC, Fernandez-Santos MR, et al. Effects of cryopreservation on bull spermatozoa distribution in morphometrically distinct subpopulations. Reprod Domest Anim. 2007;42:354–7. doi: 10.1111/j.1439-0531.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez M, Garcia-Macias V, Martinez-Pastor F, Martinez F, Borragan S, et al. Effects of cryopreservation on head morphometry and its relation with chromatin status in brown bear (Ursus arctos) spermatozoa. Theriogenology. 2008;70:1498–506. doi: 10.1016/j.theriogenology.2008.06.097. [DOI] [PubMed] [Google Scholar]
- 21.Maroto-Morales A, Ramon M, Garcia-Alvarez O, Soler AJ, Fernandez-Santos MR, et al. Morphometrically-distinct sperm subpopulations defined by a multistep statistical procedure in ram ejaculates: intra- and inter-individual variation. Theriogenology. 2012;77:1529–39. doi: 10.1016/j.theriogenology.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Marti JI, Aparicio IM, Garcia-Herreros M. Sperm morphometric subpopulations are differentially distributed in rams with different maturity age in cryopreserved ejaculates. Theriogenology. 2011;76:97–109. doi: 10.1016/j.theriogenology.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Valle RR, Arakaki PR, Carvalho FM, Muniz JA, Leal CL, et al. Identification of sperm head subpopulations with defined pleiomorphic characteristics in ejaculates of captive Goeldi's monkeys (Callimico goeldii) Anim Reprod Sci. 2013;137:93–102. doi: 10.1016/j.anireprosci.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Valle RR, Nayudu PL, Leal CL, Garcia-Herreros M. Sperm head morphometry in ejaculates of adult marmosets (Callithrix jacchus): a model for studying sperm subpopulations and among-donor variations. Theriogenology. 2012;78:1152–65. doi: 10.1016/j.theriogenology.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 25.de Paz P, Mata-Campuzano M, Tizado EJ, Alvarez M, Alvarez-Rodriguez M, et al. The relationship between ram sperm head morphometry and fertility depends on the procedures of acquisition and analysis used. Theriogenology. 2011;76:1313–25. doi: 10.1016/j.theriogenology.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Thurston LM, Watson PF, Mileham AJ, Holt WV. Morphologically distinct sperm subpopulations defined by Fourier shape descriptors in fresh ejaculates correlate with variation in boar semen quality following cryopreservation. J Androl. 2001;22:382–94. [PubMed] [Google Scholar]
- 27.Ortega-Ferrusola C, Garcia BM, Rama VS, Gallardo-Bolanos JM, Gonzalez-Fernandez L, et al. Identification of sperm subpopulations in stallion ejaculates: changes after cryopreservation and comparison with traditional statistics. Reprod Domest Anim. 2009;44:419–23. doi: 10.1111/j.1439-0531.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 28.Curry MR. Cryopreservation of semen from domestic livestock. Rev Reprod. 2000;5:46–52. doi: 10.1530/ror.0.0050046. [DOI] [PubMed] [Google Scholar]
- 29.Yániz JL, Soler C, Santolaria P. Computer assisted sperm morphometry in mammals: a review. Anim Reprod Sci. 2015;156:1–12. doi: 10.1016/j.anireprosci.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 30.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 31.Yániz JL, Vicente-Fiel S, Capistrós S, Palacín I, Santolaria P. Automatic evaluation of ram sperm morphometry. Theriogenology. 2012;77:1343–50. doi: 10.1016/j.theriogenology.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Vicente-Fiel S, Palacín I, Santolaria P, Hidalgo CO, Silvestre MA, et al. A comparative study of the sperm nuclear morphometry in cattle, goat, sheep, and pigs using a new computer-assisted method (CASMA-F) Theriogenology. 2013;79:436–42. doi: 10.1016/j.theriogenology.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Soler C, Sancho M, Garcia A, Fuentes M, Nunez J, et al. Ejaculate fractioning effect on llama sperm head morphometry as assessed by the ISAS® CASA system. Reprod Domest Anim. 2014;49:71–8. doi: 10.1111/rda.12226. [DOI] [PubMed] [Google Scholar]
- 34.Buendia P, Soler C, Paolicchi F, Gago G, Urquieta B, et al. Morphometric characterization and classification of alpaca sperm heads using the Sperm-Class Analyzer® computer-assisted system. Theriogenology. 2002;57:1207–18. doi: 10.1016/s0093-691x(01)00724-5. [DOI] [PubMed] [Google Scholar]
- 35.Davis RO, Gravance CG, Casey PJ. Automated morphometric analysis of stallion spermatozoa. Am J Vet Res. 1993;54:1808–11. [PubMed] [Google Scholar]
- 36.Cooper TG, Atkinson AD, Nieschlag E. Experience with external quality control in spermatology. Hum Reprod. 1999;14:765–9. doi: 10.1093/humrep/14.3.765. [DOI] [PubMed] [Google Scholar]
- 37.Soler C, Gabner P, Nieschlag E, de Monserrat JJ, Gutierrez R, et al. Use of the integrated semen analysis system (ISAS®) for morphometrics analysis and its role in assisted reproduction technologies. Rev Int Androl. 2005;3:112–9. [Google Scholar]
- 38.Soler C, Perez-Sanchez F, Schulze H, Bergmann M, Oberpenning F, et al. Objective evaluation of the morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 39.Soler C, De Monserrat JJ, Gutierrez R, Nunez J, Nunez M, et al. Use of the Sperm-Class Analyser® for objective assessment of human sperm morphology. Int J Androl. 2003;26:262–70. doi: 10.1046/j.1365-2605.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 40.Bellastella G, Cooper TG, Battaglia M, Strose A, Torres I, et al. Dimensions of human ejaculated spermatozoa in Papanicolaou-stained seminal and swim-up smears obtained from the Integrated Semen Analysis System (ISAS®) Asian J Androl. 2010;12:871–9. doi: 10.1038/aja.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maree L, du Plessis SS, Menkveld R, van der Horst G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum Reprod. 2010;25:1369–82. doi: 10.1093/humrep/deq075. [DOI] [PubMed] [Google Scholar]
- 42.van der Horst G, Maree L. SpermBlue®: a new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech Histochem. 2009;84:299–308. doi: 10.3109/10520290902984274. [DOI] [PubMed] [Google Scholar]
- 43.Falzone N, Huyser C, Becker P, Leszczynski D, Franken DR. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int J Androl. 2011;34:20–6. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Sanchez F, Demonserrat JJ, Soler C. Morphometric analysis of human sperm morphology. Int J Androl. 1994;17:248–55. doi: 10.1111/j.1365-2605.1994.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 45.Moruzzi JF, Wyrobek AJ, Mayall BH, Gledhill BL. Quantification and classification of human sperm morphology by computer-assisted image analysis. Fertil Steril. 1988;50:142–52. [PubMed] [Google Scholar]
- 46.Aziz N, Said T, Paasch U, Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22:1413–9. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 47.Thilagavathi J, Venkatesh S, Kumar R, Dada R. Segregation of sperm subpopulations in normozoospermic infertile men. Syst Biol Reprod Med. 2012;58:313–8. doi: 10.3109/19396368.2012.706361. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal A, Sharma RK, Nelson DR. New semen quality scores developed by principal component analysis of semen characteristics. J Androl. 2003;24:343–52. doi: 10.1002/j.1939-4640.2003.tb02681.x. [DOI] [PubMed] [Google Scholar]


