Abstract
The aim of this study was to compare the sperm nuclear and acrosomal morphometry of three species of domestic artiodactyls; cattle (Bos taurus), sheep (Ovis aries), and pigs (Sus scrofa). Semen smears of twenty ejaculates from each species were fixed and labeled with a propidium iodide-Pisum sativum agglutinin (PI/PSA) combination. Digital images of the sperm nucleus, acrosome, and whole sperm head were captured and analyzed. The use of the PI/PSA combination and CASA-Morph fluorescence-based method allowed the capture, morphometric analysis, and differentiation of most sperm nuclei, acrosomes and whole heads, and the assessment of acrosomal integrity with a high precision in the three species studied. For the size of the head and nuclear area, the relationship between the three species may be summarized as bull > ram > boar. However, for the other morphometric parameters (length, width, and perimeter), there were differences in the relationships between species for sperm nuclei and whole sperm heads. Bull sperm acrosomes were clearly smaller than those in the other species studied and covered a smaller proportion of the sperm head. The acrosomal morphology, small in the bull, large and broad in the sheep, and large, long, and with a pronounced equatorial segment curve in the boar, was species-characteristic. It was concluded that there are clear variations in the size and shape of the sperm head components between the three species studied, the acrosome being the structure showing the most variability, allowing a clear distinction of the spermatozoa of each species.
Keywords: artiodactyls, computer-assisted sperm morphometry analysis, fluorescence microscopy, image analysis, sperm morphometry
INTRODUCTION
Sperm morphometric assessment may be applied to the prediction of potential fertility and freezability of semen, and as a tool in biological studies. Traditional computer-assisted sperm morphometry analytical methods (CASA-Morph1) have the limitation that the cellular components of spermatozoa across species react differently with dyes and fixatives from different preparation methods.2 Therefore, preparation and staining protocols have been adapted for use in a particular species,3 making it difficult to compare sperm morphometry directly between species by traditional CASA-Morph methods. However, the recent development of the CASA-Morph fluorescence-based method, combining fluorescence microscopy and image analysis with open-access software, allows a reduction in factors with potential effect on morphometric results.1,4 By the use of CASA-Morph, spermatozoa from different species may be successfully stained with the same fluorescence probes for a direct comparison between species.5,6
With classical methods of staining and CASA-Morph, it is impossible in many species to discriminate between sperm head components, nucleus, and acrosome. In fact, the assessment of acrosomal morphometry has traditionally only been performed in a few species, such as the humans7,8 and the canine,9 but not in others such as the artiodactyls. In a recent study, we successfully applied the CASA-Morph system to the simultaneous assessment of nuclear and acrosomal sperm morphometry in sheep.10 In this work, we have applied this newly developed method to compare sperm nuclear and acrosomal morphometry of three species of domestic artiodactyls; cattle (Bos taurus), sheep (Ovis aries), and pigs (Sus scrofa).
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all chemicals used were obtained from Sigma-Aldrich Chemical Company (Alcobendas, Madrid, Spain) and were of the best grade available. The diluents were prepared with Milli-Q water (Millipore Ibérica S.A., Barcelona, Spain).
Semen collection and handling
All animal procedures were performed in accordance with the Spanish Animal Protection Regulation RD223/1988, which conforms to the European Union Regulation 86/609. Semen was collected with the aid of an artificial vagina from adult Rasa Aragonesa rams and Friesian bulls, or manually collected from Pietrain boars. All ruminant males were kept at insemination or research centers, whereas boars were on commercial farms. All the animals were fed a standard diet with water ad libitum. Sperm concentration was determined in duplicate by a computer-assisted sperm analyzer (CASA-Conc) (ISAS®, Version 1.0, PROISER, Valencia, Spain) in diluted semen in a Burker chamber. In ruminant males, the semen from each ejaculate was diluted in long-life ultra-heat-treated (UHT) milk (0.7% w/v fat) with antibiotics11 to 800 × 106 spermatozoa ml−1, kept in sterile glass tubes, and stored in a refrigerator at 15°C. The percentage (%) of motile spermatozoa (MS) was measured in duplicate by the ISAS® CASA-Mot in a diluted semen sample (100 × 106 spermatozoa ml−1) in a prewarmed (37°C) slide. Spermatozoa with an average path velocity (VAP) <10 μm s−1 were considered immotile. The criterion for inclusion in the study was 70% MS because only these samples are typically used in the industry. Within the first 24 h of storage, the semen samples were carefully mixed and diluted to 100 × 106 spermatozoa ml−1 with a TRIS-based solution12 immediately before processing for sperm morphometric analysis. In boars, semen was diluted in Bio’dil® (Genes Diffusion, Douai, France) to 50 × 106 spermatozoa ml−1, kept in sterile plastic bottles and stored in a refrigerator at 15°C.
Fluorescence imaging and computer-assisted sperm morphometry analysis (CASA-Morph)
Semen smears were allowed to air dry for a minimum of 2 h and processed as previously described.10 Briefly, smears were fixed with 96% (v/v) ethanol for 15 min and washed carefully in distilled water. For staining, 20 μl of a PI/PSA mixture (5 mg PI ml−1 and 50 mg PSA ml−1 in PBS at pH 7.4) was placed between the slide and a coverslip, which was then incubated for 20 min in dark at room temperature (RT). The coverslip was then removed and the slide was washed with distilled water and allowed to dry. Fluorescence intensity standards (PS-Speck Green 505/515 and Red 633/660 Microscope Image Calibration Kit, Molecular Probes, Madrid, Spain) were mounted onto separate slides and used as fluorescence standards.
Digital images of the fluorescence-labeled sperm nuclei, acrosome, and head (nucleus + acrosome) were recorded in a setup composed of an epifluorescence microscope (DM4500B, Leica, Wetzlar, Germany) with a 63× plan apochromatic objective and equipped with the appropriate filter sets. Images were photographed with a Canon Eos 400D Digital Camera (Canon Inc., Tokyo, Japan). The camera was controlled by a computer using DSLR Remote Pro software (Breeze Systems, Camberley, UK).
From each captured image, sperm head, nuclear and acrosomal morphometry were analyzed in the gray-scale, red and green channels, respectively, by using ImageJ open software (available on-line at http://rsbweb.nih.gov/ij/download.html). Each sperm head nucleus was measured for four parameters: area (A, μm2, as the sum of all pixel areas contained within the boundary), Perimeter (P, μm, as the sum of external boundaries), Length (L), and Width (W) (μm, the highest and lowest values, respectively, of the Feret diameters, i.e., the projection of the sperm nucleus on the horizontal axis measured at angles of rotation of 0°, 30°, 60°, 90°, 120°, and 150°). The area of the head occupied by the acrosome (%), the acrosomal area (μm2), and perimeter (μm) were also measured. At least 200 sperm cells per sample were randomly captured on two slides per sample. The same person carried out capture and morphometric analysis on all slides. The measurements of each individual spermatozoon from each ejaculate were saved in an Excel (Microsoft Corporation, Redmond, WA, USA)-compatible database by the software for further analysis.
Statistical analysis
The values obtained were expressed as the mean ± standard deviation (s.d.). The parameters of nucleus, acrosome, and head sperm morphometry were analyzed by using the SPSS package, version 15.0 (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed with parametric and nonparametric tests, as previously described.13 Briefly, normality of the distributions and variance homogeneity of the median value score for each set were checked from the Kolmogorov–Smirnov and Levene tests, respectively. For variables that were normally distributed, differences in the sperm morphometric parameters between and within species were evaluated by analysis of variance (ANOVA), followed by the Tukey's a posteriori test. For nonnormally distributed populations, the Kruskal–Wallis test, followed by the Mann–Whitney a posteriori test, was used for comparison of sperm morphometric parameters. The statistical level of significance was set at P < 0.05.
Semen samples were processed to obtain three slides per male and per treatment. The variability of each morphometric parameter was estimated from the coefficients of variation (CV). The variability in the slides within each ejaculate was determined to test if preparative variability could hinder the intended between-species analysis (CVslide). An acceptable CVslide value was defined to be not higher than 5%. For all the animals, within-animal and between-animal CVs were calculated for all morphometric parameters. Within-animal CVs were expressed as the mean of individual values.
RESULTS
The analyses of between-slide (within-ejaculate) variability showed that all the primary morphometric parameters exhibited CV values below 5% (P < 0.001). Thus, the variability associated with these factors should not interfere with the variability from the study.
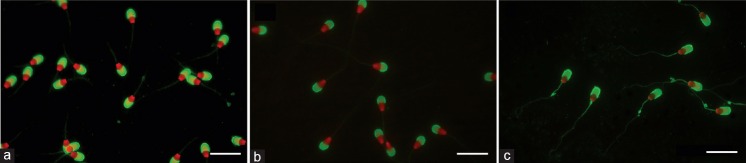
The use of the PI/PSA combination and CASA-Morph fluorescence-based method allowed the capture (Figure 1), morphometric analysis, and differentiation of most sperm nuclei, acrosomes, and whole heads, with a high precision in the three species studied. The use of the dual-band images for the study of nuclear morphometry in cattle and pigs was not adequate, as the high staining intensity of the free margin of the acrosomes hampered proper recognition of the sperm nuclei limits. Therefore, a proper analysis in these species required the use of two images per microscopic field, one from a rhodamine filter set for the sperm nuclei and the second from a double bandpass filter (FITC/TxRed dual-band filter cube) for the acrosome and whole sperm head. Some spermatozoa lacked acrosomes or had acrosomes with a lower staining intensity or with clear damage, allowing ease in the assessment of the acrosomal status.
Figure 1.
Ram (a), bull (b), and boar (c) spermatozoa stained red/green with propidium iodide/Pisum-sativum agglutinin. Scale bar, 10 μm.
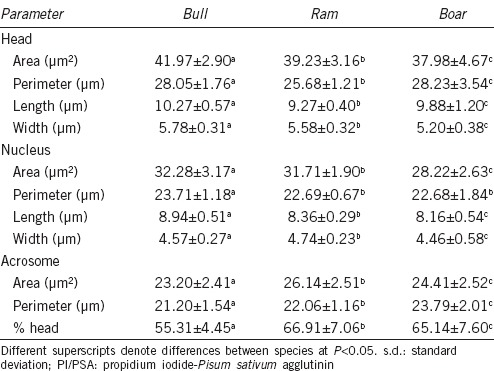
Table 1 contains data that refer to the morphometric traits of spermatozoa from twenty animals in the three species studied. For the head A and W and the nuclear A and L, the relationship between the three species may be described as follows for size: bull > ram > boar. However, head P and L were larger in the boar than in the ram, as this species has the larger nuclear W (Table 1). Bull sperm acrosomes were clearly smaller than those in the other species and covered a smaller proportion of the sperm head. The acrosomal morphology was species-characteristic: small and short in the bull; large and broad in the ram; large, long, and showing a pronounced equatorial segment curve in the boar (Figure 1).
Table 1.
Values (mean±s.d.) for the sperm nuclear morphometric parameters in the three species of domestic artiodactyls (PI/PSA staining)
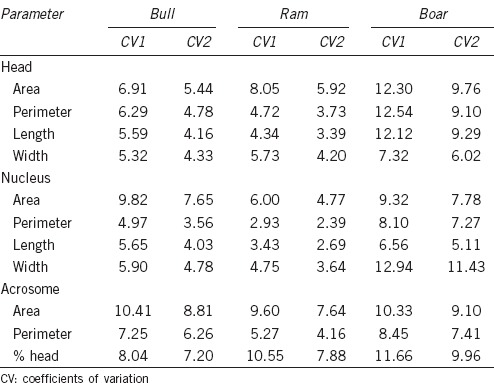
Spermatozoa in these species were characterized by substantial between-animal variability (Table 2). The highest CV in sperm dimensions was identified in the boar for most sperm parameters.
Table 2.
Means of between-male (CV1) and within-male (CV2) coefficients of variation (%) of sperm morphometric parameters for the three species of domestic artiodactyls
DISCUSSION
In this work, a comparison of the sperm head, nuclear, and acrosomal morphometry in three species of domestic artiodactyls has been performed for the first time. Classical methods of staining and CASA-Morph have only allowed assessment of the acrosomal morphometry in a few species, such as the human and canine, but not in artiodactyl species. This is because most of these processing techniques stain all the sperm parts, the background, and some intercellular components. This unspecific staining makes it difficult to study the sperm components separately. To circumvent this difficulty, the CASMA-F method, using the PI/PSA fluorochromes, was recently applied to the ram, allowing a precise evaluation of sperm head, nucleus, and acrosome morphometry. In this study, the method has been adapted to cattle and pig species, allowing between-species comparison.
The mean values for sperm head dimensions for the different species in the present study were higher than those described by others. Staining of the free border of the acrosome, the apical ridge described by Saacke and Marshall,14 in samples processed with PI/PSA combination, but not with traditional staining, may explain why head spermatozoa was larger with the CASA-Morph fluorescence-based method.
The results of sperm nuclear morphometry were in agreement with those described in a previous study with CASA-Morph.5 The bovine species, even though having larger sperm nuclear areas, displayed clearly smaller acrosomes than those of the ovine and porcine. Although for a given species the acrosomal area may be related to the binding ability of the spermatozoa to the zona pellucida, it has not been demonstrated that inter-species variations are related to differences in zona-binding or zona-penetrating capacities.
The results from the current study have shown that acrosomal size and shape allow a clear distinction between spermatozoa of the three species studied. This finding may be useful, for example, in studies aiming to compare the sperm migratory efficiency between species with pooled semen samples.
In our study, CVs between animals were higher than those observed within animals for all parameters and methods. Similar results were obtained for the sperm nucleus with CASA-Morph fluorescence based in the three species studied, and for the sperm head, nucleus, and acrosome in the ram. Results are also in agreement with those obtained in previous studies with conventional CASA-Morph methods in the ram15,4 and in the boar.16 There is only one study obtaining opposite results in the ram.17
CONCLUSIONS
The CASA-Morph fluorescence-based method allows the simultaneous assessment of sperm nuclear, acrosomal, and head size in bulls, rams, and boars. There are significant variations in the size and shape of the sperm head components between the three species studied, the acrosome being the structure that shows most variability, allowing a clear distinction of the spermatozoa of each species.
AUTHOR CONTRIBUTIONS
JLY and PS conceived and designed the experiments; SC, COH and SVF performed the experiments; PS and JLY analyzed the data; and JLY wrote the paper.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors thank Neil Macowan for assistance with the English translation and the CITA (DGA) for their help with procuring semen samples. This work was supported by the Spanish MINECO (grant AGL2014-52775-P) and the DGA-FSE (grant A40).
REFERENCES
- 1.Yániz JL, Soler C, Santolaria P. Computer assisted sperm morphometry in mammals: a review. Anim Reprod Sci. 2015;156:1–12. doi: 10.1016/j.anireprosci.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Harasymowycz J, Ball L, Seidel GE. Evaluation of bovine spermatozoal morphologic features after staining or fixation. Am J Vet Res. 1976;37:1053–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Thurston LM, Watson PF, Holt WV. Sources of variation in the morphological characteristics of sperm subpopulations assessed objectively by a novel automated sperm morphology analysis system. J Reprod Fertil. 1999;117:271–80. doi: 10.1530/jrf.0.1170271. [DOI] [PubMed] [Google Scholar]
- 4.Yániz JL, Vicente-Fiel S, Capistrós S, Palacín I, Santolaria P. Automatic evaluation of ram sperm morphometry. Theriogenology. 2012;77:1343–50. doi: 10.1016/j.theriogenology.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Vicente-Fiel S, Palacín I, Santolaria P, Hidalgo CO, Silvestre MA, et al. A comparative study of the sperm nuclear morphometry in cattle, goat, sheep, and pigs using a new computer-assisted method (CASMA-F) Theriogenology. 2013;79:436–42. doi: 10.1016/j.theriogenology.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Vicente-Fiel S, Palacin I, Santolaria P, Yaniz JL. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F) Anim Reprod Sci. 2013;139:182–9. doi: 10.1016/j.anireprosci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Soler C, De Monserrat JJ, Gutierrez R, Nunez J, Nunez M, et al. Use of the sperm-class analyser (R) for objective assessment of human sperm morphology. Int J Androl. 2003;26:262–70. doi: 10.1046/j.1365-2605.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Falzone N, Huyser C, Becker P, Leszczynski D, Franken DR. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int J Androl. 2011;34:20–6. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 9.Nunez-Martinez I, Moran JM, Pena FJ. Do computer-assisted, morphometric-derived sperm characteristics reflect DNA status in canine spermatozoa? Reprod Domest Anim. 2005;40:537–43. doi: 10.1111/j.1439-0531.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 10.Yániz JL, Capistros S, Vicente-Fiel S, Soler C, Núnez de Murga J, et al. Study of nuclear and acrosomal sperm morphometry in ram using a computer-assisted sperm morphometry analysis fluorescence (CASMA-F) method. Theriogenology. 2014;82:921–4. doi: 10.1016/j.theriogenology.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Yaniz J, Marti JI, Silvestre MA, Folch J, Santolaria P, et al. Effects of solid storage of sheep spermatozoa at 15 degrees C on their survival and penetrating capacity. Theriogenology. 2005;64:1844–51. doi: 10.1016/j.theriogenology.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Yániz JL, Mateos JA, Santolaria P. TRIS buffer improves fluorescence yield of ram spermatozoa when evaluating membrane integrity. Microsc Res Tech. 2012;75:520–3. doi: 10.1002/jemt.21086. [DOI] [PubMed] [Google Scholar]
- 13.Vicente-Fiel S, Palacín I, Santolaria JP, Fantova E, Quintin-Casorran F, et al. In vitro assessment of sperm quality from rams of high and low field fertility. Anim Reprod Sci. 2014;146:15–20. doi: 10.1016/j.anireprosci.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Saacke RG, Marshall CE. Observations on the acrosomal cap of fixed and unfixed bovine spermatozoa. J Reprod Fertil. 1968;16:511–4. doi: 10.1530/jrf.0.0160511. [DOI] [PubMed] [Google Scholar]
- 15.Sancho M, Perez-Sanchez F, Tablado L, de Monserrat JJ, Soler C. Computer assisted morphometric analysis of ram sperm heads: evaluation of different fixative techniques. Theriogenology. 1998;50:27–37. doi: 10.1016/s0093-691x(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Herreros M, Aparicio IM, Baron FJ, Garcia-Marin LJ, Gil MC. Standardization of sample preparation, staining and sampling methods for automated sperm head morphometry analysis of boar spermatozoa. Int J Androl. 2006;29:553–63. doi: 10.1111/j.1365-2605.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 17.Maroto-Morales A, Ramon M, Garcia-Alvarez O, Soler AJ, Esteso MC, et al. Characterization of ram (Ovis aries) sperm head morphometry using the sperm-class analyzer. Theriogenology. 2010;73:437–48. doi: 10.1016/j.theriogenology.2009.10.003. [DOI] [PubMed] [Google Scholar]