Abstract
The Andean puma (Puma concolor) has not been widely studied, particularly in reference to its semen characteristics. The aim of the present study was to define the morphometry of puma sperm heads and classify their subpopulations by cluster analysis. Samples were recovered postmortem from two epididymides from one animal and prepared for morphological observation after staining with the Hemacolor kit. Morphometric data were obtained from 581 spermatozoa using a CASA-Morph system, rendering 13 morphometric parameters. The principal component (PC) analysis was performed followed by cluster analysis for the establishment of subpopulations. Two PC components were obtained, the first related to size and the second to shape. Three subpopulations were observed, corresponding to elongated and intermediate-size sperm heads and acrosomes, to large heads with large acrosomes, and to small heads with short acrosomes. In conclusion, puma spermatozoa showed no uniform sperm morphology but three clear subpopulations. These results should be used for future work in the establishment of an adequate germplasm bank of this species.
Keywords: principal component analysis, puma, sperm morphometry, subpopulation, wild animal
INTRODUCTION
The Andean puma (Puma concolor) is a vulnerable species and so it is difficult to obtain samples for its study, although it is very important to define convenient strategies for its conservation. Moreover, animals in captivity have shown very low reproductive success, even with in vitro techniques.1 Hence, any effort we can make to define good protocols for the maintenance of the germplasm of this species would be of great interest. Similar efforts have been made in other endangered species such as the Spanish brown bear2 with very promising results. References to puma sperm characteristics are very scarce and limited to general descriptions. Even in the most complete work that we have had access to, only ejaculates from two animals were assessed after electroejaculation. The authors classified the abnormal spermatozoa into different types (which means some definition of “normal” must have been assumed, even though not given in the paper), perhaps using the criteria for some other cat species.3
The purpose of this work was to determine the sperm morphometric characteristics of the Puma, looking for possible subpopulations in structure.
MATERIALS AND METHODS
The study was done in May 2015 with one puma obtained from culling to reduce the population of wild animals by selective slaughter, by one shot in the head, without great loss of blood, close to the SouthAmerican Camelids Research Center (CICAS), la Raya of National University of San Antonio Abad del Cusco. The animal weighed about 60 kg, corresponding to a 7 to 10-year-old male. Epididymides were removed and transported at 15°C to the center where the samples were analyzed <5 h after the death of the animal.
Spermatozoa were collected from the epididymal tail after slicing in Tris-based medium (Tris 3.025 g; citric acid 1.7 g; fructose 1.25 g; bi-distilled water 100 ml). After through mixing by inverting the tube, a drop of 5 μl was placed on a slide to confirm the presence of spermatozoa. For the study, 10 smears were air-dried and stained with Hemacolor (Merck, Darmstadt, Germany) and mounted with Eukitt® (O Kindler GmbH and Co, Freiburg, Germany).
Morphometric analysis was done using an ISAS® v1 CASA-Morph system composed of a UOP-UB200i/Proiser microscope using a 100× bright field objective, video camera Proiser 782C, and the morphology software module (Proiser R+D, S.L., Paterna, Spain). Resolution of the analyzed images was of 0.084 μm per pixel in both axes. Images of almost 250 spermatozoa from each epididymis were captured and analyzed, obtaining 13 morphometric parameters: sperm head length (L, μm), width (W, μm), area (A, μm2) and perimeter (P, μm), Ellipticity (L/W), Elongation ([L − W]/[L + W]), Regularity (πLW/4A) and Rugosity (4πA/P2), medium gray level and acrosome (% of total head area) and sperm midpiece width (μm), insertion distance (the distance between head and midpiece axes, μm), and insertion angle (°).
Because morphometric sperm variables are interrelated, principal component analysis (PCA) was performed on the morphometric data. To select the number of principal components that should be used in the next step of our analysis, we followed the criterion of selecting only those with an eigenvalue (variance extracted for that particular principal component) >1 (Kaiser criterion). The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA, for the establishment of morphometric subpopulations.4
All data were analyzed using InfoStat Software (version 2008, University of Córdoba, Córdoba Argentina) for Windows.5
RESULTS
No differences were observed between data obtained from either epididymis (data not shown), and for this reason, data were combined for subsequent calculations.
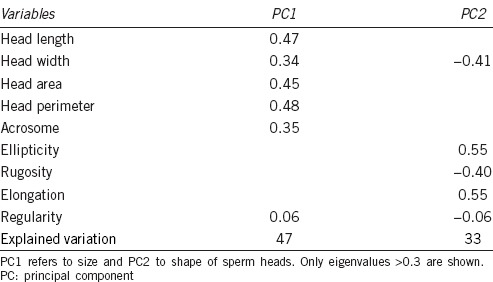
When all morphometric variables were considered (including those from the midpiece), the two PC obtained explained only the 51% of the variance; for this reason, we decided to exclude some of the data for this calculation. The definitive principal components analysis included only head parameters and showed that two PC explained 40% and 33% of the variance. The first one was related with parameters of size (length, area, and perimeter) and the second of shape (Width, Ellipticity, Rugosity, and Elongation) (Table 1).
Table 1.
Principal components obtained from puma head sperm parameters from a population of 581 spermatozoa
The subsequent subpopulation analysis distributed the 581 analyzed cells into three subpopulations (SP) (Figure 1 and Table 2). SP1 was characterized by an elongated shape of intermediate size (Figure 2a and Table 2); SP2 comprised the larger cells with a high proportion of acrosome (Figure 2b and Table 2); and SP3 was composed of short and small cells (Figure 2c and Table 2).
Figure 1.
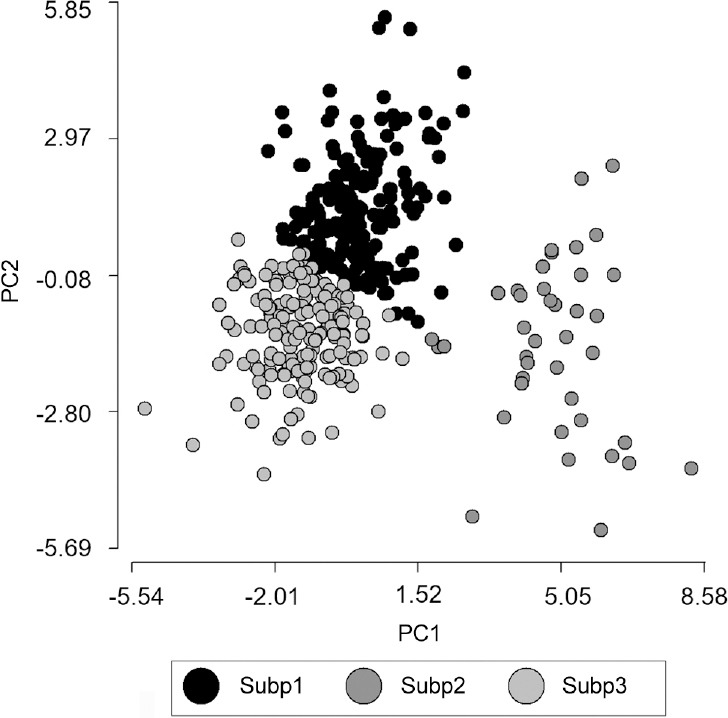
Distribution of cells in subpopulations (Subp) based on PC (principal component) values.
Table 2.
Values (mean±s.d.) of morphometric parameters of puma sperm according with their SP assignment
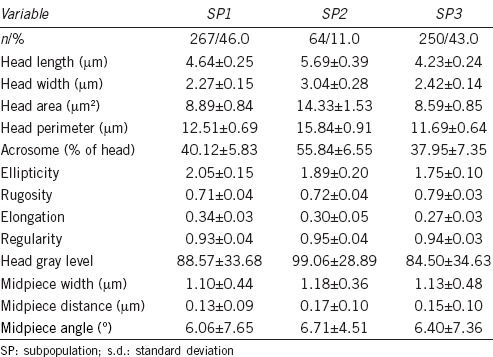
Figure 2.
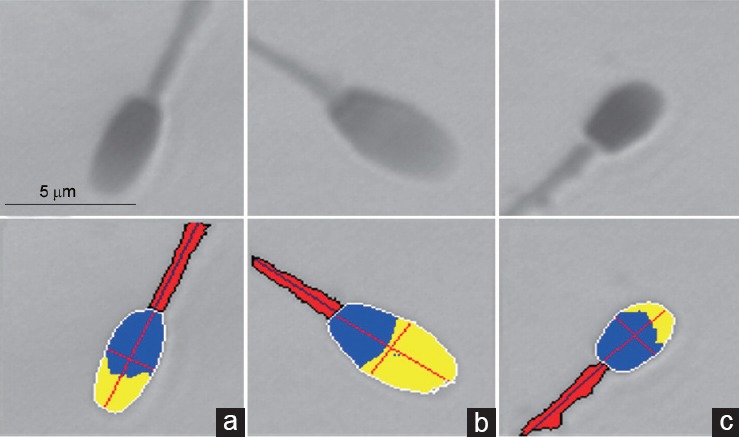
Puma spermatozoa representing each subpopulation. (a) SP1, elongated shape of intermediate size; (b) SP2, large cells with a high proportion of acrosome; (c) SP3, short and small cells. Upper row, images captured from the microscope; Lower row, digitized images: yellow, acrosome; blue, postacrosomal region (values for head are the sum of both areas); red, midpiece. SP: subpopulation. Scale bar = 5 μm applicable to all images.
DISCUSSION
This is the first approach to the study of puma sperm morphometry and to the subpopulation structure of the sperm population. Certainly, it was generated from only one case, which is a great limitation, but the extreme difficulty in obtaining these kinds of samples, alone justifies publication of these initial data. This limitation is common to similar data from other wild species.5,6
Previous works on puma semen have been limited to a general seminogram, lacking sperm morphometry. It is curious that both of these authors analyzed the percentage of “normal” cells, from the morphological point of view,1,3,7 but how it is possible to speak about “normal” cells in this species? If it is difficult, if not impossible, to be certain of what “normal morphology” is in species such as humans, bulls, or stallions, where seminograms are made daily, it may seem nonsensical to determine this from the few studies on the puma. Nevertheless, we have found, taking into consideration the low sample size, the presence of three well-defined sperm subpopulations. It is difficult to make an assertion about them, because we had only one sample, but the SP2 resembles that of ghost cells observed in primates epididymal sperm samples.8,9,10 It has been hypothesized that these cells increase their size and thus appear pale, as a consequence of osmotic differences between different epididymal zones and the medium used for sperm collection, indicating a more osmotically sensitive sperm population. In the present case, it is difficult to know if this corresponds to a really large acrosome or to the osmotic entrance of water under the conditions of sperm preparation. Whatever the cause, the three morphometric subpopulations described here for cauda epididymidal spermatozoa are clearly different and thus be important to determine which cells should be selected for a germplasm bank establishment.
It has been observed that mammalian sperm morphometric differences can have an evolutionary significance11 in different species’ diversity (dog,12 rodents,13,14 South America camelids15). Similar works could also be done on the Felidae family.
AUTHOR CONTRIBUTIONS
HC and CS conceived and designed the experiments; VA, CO, and EA performed the experiments; AM, CS, and HC analyzed the data; CS wrote the paper.
COMPETING INTERESTS
CS is Professor at Valencia University and acts as Scientific Director of Proiser R+D S.L Research and Development Laboratory. Neither he nor the other authors have interests that influenced the results presented in this paper.
ACKNOWLEDGMENTS
The authors would like to thank Anthony Valverde for helping in the statistical calculations and Jesús Contell for the composition of Figure 2.
REFERENCES
- 1.Miller AM, Roelke ME, Goodrowe KL, Howard JG, Wildt DE. Oocyte recovery, maturation and fertilization in vitro in the puma (Felis concolor) J Reprod Fertil. 1990;88:249–58. doi: 10.1530/jrf.0.0880249. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Rodriguez M, Alvarez M, López-Ureña E, Martínez-Rodríguez C, Borragan C, et al. Brown bear sperm double freezing: effect of elapsed time and use of PureSperm gradient between freeze-thawing cycles. Cryobiology. 2013;76:339–46. doi: 10.1016/j.cryobiol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Wildt DE, Phillips LG, Simmons LG, Chakrabotry PK, Brown JL, et al. A comparative analysis of ejaculate and hormonal characteristics of the captive male cheetah, tiger, leopard, and puma. Biol Reprod. 1988;38:245–55. doi: 10.1095/biolreprod38.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Vicente-Fiel S, Palacin I, Santolaria P, Yániz JL. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F) Anim Reprod Sci. 2013;139:182–9. doi: 10.1016/j.anireprosci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Balzarini MG, Gonzalez L, Tablada M, Casanoves F, Di Rienzo JA, et al. 2008. Infostat. User's guide, Brujas Editorial, Córdoba, Argentina. 2015 [Google Scholar]
- 6.Anel L, Alvarez M, Anel E, Martínez-Pastor F, Martinez F, et al. Evaluation of three different extenders for use in emergency salvaging of epididymal spermatozoa from a Cantabric brown bear. Reprod Domest Anim. 2011;46:e85–90. doi: 10.1111/j.1439-0531.2010.01646.x. [DOI] [PubMed] [Google Scholar]
- 7.Swanson WF, Johnson WE, Cambre RC, Citino SB, Quigley KB, et al. Reproductive status of endemic felid species in Latin American zoos and implications for ex situ conservation. Zoo Biol. 2003;22:421–41. [Google Scholar]
- 8.Yeung CH, Pérez-Sánchez F, Soler C, Poser D, Kliesch S, et al. Maturation of human spermatozoa (from selected epididymides of prostatic carcinoma patients) with respect to their morphology and ability to undergo the acrosome reaction. Hum Reprod Update. 1997;3:205–13. doi: 10.1093/humupd/3.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Gago C, Soler C, Pérez-Sánchez F, Yeung CH, Cooper TG. Effect of cetrorelix on sperm morphology duting migration through the epididymis in the cynomolgus macaque (Macaca fascicularis) Am J Primatol. 2000;51:103–17. doi: 10.1002/(SICI)1098-2345(200006)51:2<103::AID-AJP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Soler C, Pérez-Sánchez F, Schulze H, Bergmann M, Oberpenning F, et al. Objective evaluation of morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MJ, Dixson AS, Dixson AF. Mammalian sperm and oviducts are sexually selected: evidence for co-evolution. J Zool. 2006;270:682–6. [Google Scholar]
- 12.Soler C, Alambiaga A, Martí MA, García-Molina A, Valverde A, et al. Dog sperm head morphometry and subpopulation structure: its diversity and evolution. Asian J Androl. 2016 doi: 10.4103/1008-682X.189207. DOI: 10.4103/1008-682X.189207. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallardo MH, Mondaca FC, Ojeda RA, Kóhler N, Garrido O. Morphological diversity in the sperms of caviomorph rodents. J Neotrop Mammal. 2002;9:159–70. [Google Scholar]
- 14.Breed WG. The spermatozoon of Eurasian murine rodents: its morphological diversity and evolution. J Morphol. 2004;261:52–69. doi: 10.1002/jmor.10228. [DOI] [PubMed] [Google Scholar]
- 15.Soler C, Sancho M, García-Molina A, Núñez J, Parráguez VH, et al. Llama and alpaca comparative sperm head morphometric analysis. J Camelid Sci. 2014;7:48–58. [Google Scholar]


