Abstract
Postcopulatory sexual selection through sperm competition may be an important evolutionary force affecting many reproductive traits, including sperm morphometrics. Environmental factors such as pollutants, pesticides, and climate change may affect different sperm traits, and thus reproduction, in sensitive bird species. Many sperm-handling processes used in assisted reproductive techniques may also affect the size of sperm cells. The accurately measured dimensions of sperm cell structures (especially the head) can thus be used as indicators of environmental influences, in improving our understanding of reproductive and evolutionary strategies, and for optimizing assisted reproductive techniques (e.g., sperm cryopreservation) for use with birds. Computer-assisted sperm morphometry analysis (CASA-Morph) provides an accurate and reliable method for assessing sperm morphometry, reducing the problem of subjectivity associated with human visual assessment. Computerized systems have been standardized for use with semen from different mammalian species. Avian spermatozoa, however, are filiform, limiting their analysis with such systems, which were developed to examine the approximately spherical heads of mammalian sperm cells. To help overcome this, the standardization of staining techniques to be used in computer-assessed light microscopical methods is a priority. The present review discusses these points and describes the sperm morphometric characteristics of several wild and domestic bird species.
Keywords: avian, computer-assisted assay, morphometry, spermatozoa
INTRODUCTION
Avian spermatogenesis
Avian spermatozoa are filiform, which limits their examination by computer-assisted sperm morphology analysis (CASA-Morph) systems, as these systems were designed to investigate the approximately spherically headed spermatozoa of mammalian species. Filiform spermatozoa are the product of a complicated process involving a chronological sequence of cellular interactions modulated by endocrine, autocrine, and paracrine hormones, cytokines, and growth factors. The main stages of spermatogenesis include the proliferation and renewal of spermatogonia, the meiotic events in primary spermatocytes, and their morphological transformation during spermiogenesis. The spermatogonia are found at the periphery of the seminiferous tubules, in contact with the latters’ basement membranes; elliptical in shape, they contain large, round nuclei.1 The primary spermatocytes are the largest germ cells present in the testis and contain chromatin in the form of dispersed, thin filaments.1 The secondary spermatocytes are smaller than primary spermatocytes, but slightly larger than spermatogonia;2 their nuclei contain thick clumps of chromatin.1 Spermatids, which emerge after the second meiotic division, are comparatively small cells with a small and spherical nucleus3 located above or between bundles of filiform spermatids.1 Spermiogenesis also involves the formation of a pro-acrosomal granule that attaches to the anterior pole of the nucleus, forming the acuminate spine of the mature sperm cell. At the opposite end of the nucleus, the proximal and distal centrioles form the flagellum. The formation of a microtubule system (or “manchette”) around the nucleus allows its elongation into the narrow, cylindrical, worm-like shape characteristic of mature sperm cells. The improper formation of this microtubule system results in malformed spermatids. The final step in spermiogenesis includes the sloughing of the cytoplasmic remnant. Abnormal spermatids are destroyed by the Sertoli cells via induced apoptosis, followed by the engulfing and digestion of the debris. In turkeys suffering from yellow semen syndrome, abnormal spermatids appear in the semen;4 in partridges and roosters, spermatids are not seen in normal ejaculates,5 but in falcons, nonapoptotic spherical spermatids (immature spermatids) are habitually seen in the lumen of the seminiferous tubule and in normal ejaculates, although their physiological role in reproduction is unknown.
The duration and characteristics of spermatogenetic activity vary with season. Gonadotropins and testosterone levels show seasonal alterations that determine cellular changes in the testes. During the breeding period, when the levels of these hormones rise, the testes undergo a proliferative phase. In contrast, when gonadotropin and testosterone levels fall, testicular size decreases via the inhibition of cell proliferation and the apoptosis of the germinal cells.6,7 In very seasonal species (the majority of wild birds), spermatogenetic activity is abolished when basal levels of testosterone are reached (azoospermic conditions).8 However, in species such as the chicken (Gallus gallus domesticus) and turkey (Meleagris gallopavo), spermatogenesis does not stop. Nonetheless, it is strongly affected by low gonadotropin and testosterone levels, leading to ejaculates with low sperm concentrations9 and morphologically abnormal spermatozoa.10
Assisted reproduction in birds
Assisted reproduction techniques for use with birds have largely been developed for poultry. The design of sperm recovery techniques, the formulation of semen diluent, and the establishment of artificial insemination procedures with fresh and chilled spermatozoa, all initially developed for chicken and turkey production.11,12,13 Recently, however, the risk of extinction of numerous wild species and even domestic breeds of poultry has encouraged the scientists and governments to look for ways of using assisted reproduction technologies developed for mammals with threatened birds.
Around the world, over 1300 bird species are threatened (www.birdlife.org). Captive breeding and assisted reproduction can both be used to help avert extinction.14 However, given the space limitations faced by zoos and animal parks, long-term germplasm storage would appear to offer a means of preserving genetic diversity while minimizing space requirements. A number of national programs (e.g., in France, the USA, The Netherlands, and Spain) are currently trying to cryopreserve chicken spermatozoa.15,16 Although the conservation of primordial germ cells (PGCs) would open up new possibilities, a number of technical limitations have prevented this being feasible. Thus, to date, it has only been possible to cryopreserve male gametes – a drawback since in birds the female is the heterogametic sex.17 Spermatozoa are, of course, the most accessible sex cells.18 Unfortunately, the fertility rates of cryopreserved avian spermatozoa are dramatically lower than those recorded for domestic mammalian species. For example, cryopreserved rooster sperm may retain <2% of the fertilizing capacity of fresh semen.19 Sperm quality assays can, however, be used to predict the fertilizing capacity of spermatozoa and to evaluate the damage caused to their function by cryoprotectants and the freezing-thawing process. In such testing, the variables usually assayed include motility (either subjectively or objectively via computerized sperm motion analysis), sperm viability, membrane function, acrosomal integrity, sperm morphological abnormalities, and sperm-egg interactions.20,21,22 Recently, the analysis of DNA integrity has also been reported as feasible.23 Other variables, such as sperm head size, and the size of sperm subpopulations categorized by sperm cell dimensions, are beginning to be used in birds, as in mammals.24,25
The ability of spermatozoa to survive a freeze/thaw cycle and the value of different freezing-thawing protocols may be predicted by examining morphometric variables before and after cryopreservation.24,25,26 The sperm head size influences the volume of water carried by the cell, as well as the permeability of the cell membrane to water and cryoprotectant, in turn affecting the survival rates of the cells. Thus, the expected cryodamage to spermatozoa might be directly related to sperm head dimensions,25 and a better ability of spermatozoa to survive the freezing process should be expected for the chicken (sperm head area 13.9 μm2) than for that of species with larger sperm heads (e.g., king penguins, 19.7 μm2). In other species with small sperm heads, such as falcons, the response to the freeze-thaw process appears to be affected by the presence of large numbers of immature sperm cells.27 Guinea fowl28 and gander29 semen contain many more pleomorphic cells than that of chickens, turkeys, and ducks (although not as many as in falcon semen; see30 for a review); this could reduce the cryopreservation efficacy associated with their spermatozoa.
SPERM SIZE AND REPRODUCTIVE FUNCTION IN BIRD SPECIES
Knowledge of sperm head dimensions may provide important information on the evolutionary adaptation and function of the spermatozoa from different species. Variation in the size and shape of cells or their nuclei (pleomorphism) in ejaculates may be related to the presence of different subpopulations of mature spermatozoa with direct roles in fertilizing capacity, as reported for mammals.31 Pleomorphism may also be related to the presence of cells showing different degrees of maturity (primary spermatocytes, secondary spermatocytes, spermatids in their different stages of maturation).27 External factors such as environmental pollutants and pesticides might also modify sperm head dimensions via disturbances in the chromatin package or by affecting acrosomal integrity.32
Sperm size and sperm competence
Sexual strategies such as polygyny and monogamy also influence the morphological and functional characteristics of spermatozoa. In polygynous avian species, such as the majority of galliformes, sperm competition dictates ejaculates be of high quality, i.e., of high motility and with low sperm abnormality. In contrast, the spermatozoa of monogamous species (e.g., falcons, eagles, and certain penguin species) usually exhibit high sperm abnormality.33 The high incidence of immature spermatozoa (about 60%) in falcons suggests that these creatures may show a degree of monogamy unusual even among birds. Differences in the degree of pleomorphy are also seen in smaller birds. In the Eurasian bullfinch (Pyrrhula pyrrhula), a more monogamous species in which sperm competition is low, 8%–18% of sperm cells may be immature or show head abnormalities, while in the polygamous dunnock (Prunella modularis), in which sperm competition is intense, only 4%–5% may be so affected.34 Pleomorphism is easily observed by standard microscopy and is related to the presence of spermatocytes or spermatids that have reached different points in their transformation. Sometimes, subtle variations in the head size may comprise subpopulations identifiable only by methods that can accurately record sperm morphometry. It has been suggested that morphometric characteristics are regulated genetically.31
Postcopulatory sexual selection via sperm competition may be an important evolutionary force affecting different reproductive traits including sperm morphometrics.35 Sperm competition is a strong selective force that promotes, among other things, a larger and faster-swimming gamete. Although larger sperm heads might be considered a handicap to rapid swimming,36 recent data show that red-legged partridge spermatozoa with longer heads swim faster.8 Other authors have reported similar observations in other species.37,38 The length and area of the sperm head of the red-legged partridge are smaller in pure (Alectoris rufa) than in hybrid (A. rufa × Alectoris chukar) birds. A. chukar is more promiscuous than A. rufa,39 and thus more polyandry might be expected of the hybrids than of pure birds. Similarly, several authors report that the males of species with polyandrous females have longer and faster spermatozoa than species with sexually monogamous females.33,40,41 Female reproductive biology (i.e., clutch size and spread of laying) has been suggested to affect sperm size and the evolution of sperm morphometrics in several pheasant species.35 In the latter study, sperm size traits were negatively associated with the duration of sperm storage. In other species, such as the Gouldian finch (Erythrura gouldiae), sperm morphometric values may show plasticity within the same breeding season if the social environment is modified. Males may produce an increased sperm midpiece size when in a highly competitive environment and an increased sperm tail length when competition is weak.42
External factors affecting sperm size
Sperm characteristics may be used as biological markers of environmental influences (pollutants, pesticides, and climate change) since germinal cells are very sensitive to them. Birds are found in forest, urban, mountain, marine, desert, and even polar habitats, with some species migrating between continents. Bird spermatozoa, therefore, provide an interesting model for evaluating the impact of environmental stresses.
Toxins and pollutants may disturb different stages of spermatogenesis and, thus, the final size of avian sperm cells. In mammals, it is well established that high concentrations of estrogenic chemical residues (e.g., DDT) in water, sediment, and tissue impair spermatogenesis in humans and certain wild animals,43 and certain pesticides are known to have harmful effects on sperm function and DNA.32 The pesticide phosphamidon has been reported to increase the proportion of sperm cells with aberrant head morphology in mice,44 and organophosphorus pesticides can cause the formation of diploid or even polyploid spermatids, which have abnormal head sizes.45,46 In humans, exposure to ethylene dibromide – an active component of several pesticide fumigants – has been related to a narrowing of sperm heads.47 Although far fewer studies of their effects in birds have been undertaken, it might be expected that such pesticides have similar effects on them.
Birds have shown clear ecological and evolutionary responses to recent climate change,48 and further changes may be expected in their sexual behavior, breeding activity and migratory behavior in the medium and long term. It may be that these responses affect future reproductive function related to polygyny, polyandry, and monogamy, and in turn sperm morphological characteristics. Further, bird species can be affected by heat stress.49 This might impair spermatogenesis by reducing testicular germ cell proliferation and increasing apoptosis.50 Certainly, environmental heat stress has been reported to affect sperm head ellipticity in rams.51
The manipulation of spermatozoa in the laboratory may also affect sperm cell morphometry. For example, the choice of fixative (e.g., methanol, glutaraldehyde) employed when stains are used is known to modify the size of avian spermatozoa.5 The osmotic stress resulting from the use of hyper- or hypo-tonic solutions to dilute semen may also affect sperm size via the dehydration or swelling of cells. The freezing-thawing of spermatozoa can also influence sperm cell size; in falcons, this is caused by the loss of the acrosome or changes in chromatin structure.5
Finally, diseases (e.g., yellow semen syndrome in turkeys) can directly or indirectly affect the morphometric values of sperm cells.4
SPERM MORPHOMETRY
Conventional method
Sperm morphology and morphometry have been deemed important in sperm evaluation since artificial reproduction techniques were first developed. Studies on the function of the different structures in sperm cells and their importance in sperm quality and fertilization capacity have long been reported. The first were mainly descriptive. For example, in 1888, Ballowitz52 described sperm cells to be composed of a head, midpiece, and tail, and described an intensely stained region in the apex of the head which would be identified later as the acrosome. In the 1940s, the electron microscope was used to examine the spermatozoa of several species including roosters.53 The authors described the different regions of these cells after treating them enzymatically or with distilled water, and measured their length and width. Detailed electron microscope-based information is available on the appearance, measurements, and classification of normal and defective chicken spermatozoa,53 as well as on their ultrastructure.54 Scanning and transmission electron microscopy have since been used to analyze the ultrastructure of ostrich, guinea fowl, turkey, and Japanese quail sperm cells.55,56,57 However, this usually requires the use of phosphate-buffered glutaraldehyde solutions to fix the sample, followed by dehydration in ascending concentrations of ethanol,57 and this can strongly affect sperm morphometric variables. Indeed, shrinkage often occurs when biological material is prepared for electron microscopy.58 This may explain why both chicken and turkey sperm morphometric variables are usually recorded as smaller when examined in the electron microscope53,54 than in the light microscope.5,57
Morphometric analyses of avian spermatozoa by light microscopy are, however, relatively scarce.59 Most studies have involved conventional techniques and the subjective assessment of sperm variables;60,61 unfortunately, these methods can return significantly different results when performed in different laboratories or by different operators.62,63,64 Eosin-nigrosin staining has been used to measure sperm traits in duck spermatozoa,65 and amido black, Spermac®57 and other stains55,66 in the morphometric analysis of quail and rooster sperm, but they provide conflicting results.
Computer-assisted sperm morphometry (CASA-Morph)
A number of reliable and accurate CASA-Morph systems have been developed for the assessment of sperm morphometry.24,67,68 The problem of subjectivity associated with visual assessment methods is significantly reduced by these systems,69 which have been standardized for use with semen from different animal species, including humans.70,71 In each case, the most appropriate staining and sampling techniques have been established.72,73 CASA-Morph systems are now commonly used to examine mammalian spermatozoa,74 and the head morphometry results they provide have been used to predict fertilization rates75,76,77,78 and sperm cryodamage.25,26,79 However, they have been little used with avian semen, a consequence of the filiform shape of bird spermatozoa.
For mammalian spermatozoa, the most commonly used stains employed with CASA-Morph systems are Hemacolor®, Diff-Quick®, Haematoxylin, and SpermBlue®.68,80,81 Hemacolor® is the most suitable for sperm head morphometry assessment in ibexes (Capra pyrenaica)68 and humans.82 A recent study compared the performance of Hemacolor® and aniline blue staining as part of a computer-assessed, light microscopic method for measuring avian sperm head characteristics.5 Semen from roosters (G. g. domesticus) and red-legged partridges (A. rufa) was used. Both stains clearly distinguished the end of the head from the midpiece and flagellum: accurate measurements of the sperm head were therefore guaranteed. The percentage of measurable spermatozoa was higher with the Hemacolor® staining technique than with the aniline blue technique, both for the rooster and partridge spermatozoa. The reasons that some spermatozoa could not be measured included the background particles around them preventing their certain identification, and the stain being too faint to differentiate some cells from the background (this occurred only with the aniline blue technique). Unfortunately, in those samples with large numbers of particles, the Hemacolor® technique was problematical since these particles were also stained, making it difficult to identify the sperm cells. However, the Hemacolor® technique might be deemed more appropriate for computerized sperm assessment systems since it provides larger percentages of measurable cells and shows greater repeatability.5
Aniline blue staining has been used to assess chromatin condensation status in human spermatozoa83,84,85 because the stain indicates the persistence of histones. It is commonly used to stain avian spermatozoa since it reveals morphological abnormalities well and renders acrosomal integrity easy to examine.9 However, it has never been used for morphometric purposes since its stain intensity is rather low, although studies conducted with rooster, partridge,5,8 and golden eagle86 spermatozoa have shown an intensity of staining sufficient to be detected by the computerized system. Again, the proportion of measurable spermatozoa was lower than that with Hemacolor®. Aniline blue staining, however, allows sperm abnormalities to be recognized easily, and facilitates acrosomal imaging when using phase contrast microscopy.9 The better definition of the acrosome provided by the latter stain may explain the difference in rooster and partridge sperm head lengths recorded with it and with Hemacolor®.
When using CASA-Morph, staining can influence the sperm morphometric results obtained.64,87 The different fixatives required (i.e., methanol for Hemacolor® and 2% (v/v) glutaraldehyde for aniline blue) may be the reason, dehydrating or swelling the sperm cells to different extents.68,81 Certainly, sperm head size would seem to subject to such effects.69 Formaldehyde,88 Hancock's solution,89 and glutaraldehyde59 have all been used in sperm assessment, with the last of these reported to reduce the cell shrinkage observed in air-dried semen smears, thus allowing greater structural detail to be observed by phase contrast microscopy.90,91 In roosters, Hemacolor® returns significantly larger sperm head widths and areas than the aniline blue technique does while the latter results in greater sperm head lengths.5 In the red-legged partridge, no differences are seen in the results for sperm head width and area provided by these two techniques, but aniline blue staining is associated with larger length measurements.5 These findings may reflect a species-specific response to the methanol fixative. Similar results have been observed in other species.10,80,92
Morphometric characteristics in domestic and wild species
Sperm morphometric data have been reported for only a few bird species, with most recorded for roosters, turkeys, and quails.53,57,93 Only, recently, some studies have focused on wild species. Although all bird sperm heads are filiform, the exact shape varies between Orders, and there are appreciable differences in the shape of spermatozoa from galliformes, passeriformes, and falconiformes. A vermiform sperm head morphology (long and narrow) is very apparent in galliformes (chicken, quail, and partridges), while in falconiformes (eagles and falcons), the sperm heads are wider (Figure 1). Morphologically, avian spermatozoa have been classified as of the “sauropsid” and “spiral shaped” types.57 Sauropsid-type spermatozoa, which include those of galliformes and most nonpasserine birds, are smooth and elongated. The spiral type shows an external, helically wound, undulating membrane, which is very characteristic of passerines.94 The length of bird spermatozoa is very variable (30–300 μm), with no correlation between sperm dimensions and species size and weight.95 For example, within Galliformes, roosters (with a body weight of 3.0–3.5 kg for native breeds) have smaller spermatozoa than red legged-partridges (with a body weight of 0.4–0.5 kg) do (Table 1). The size of the different parts of the sperm (head, midpiece [especially], total tail, etc.) is also very variable between species (Table 1).
Figure 1.
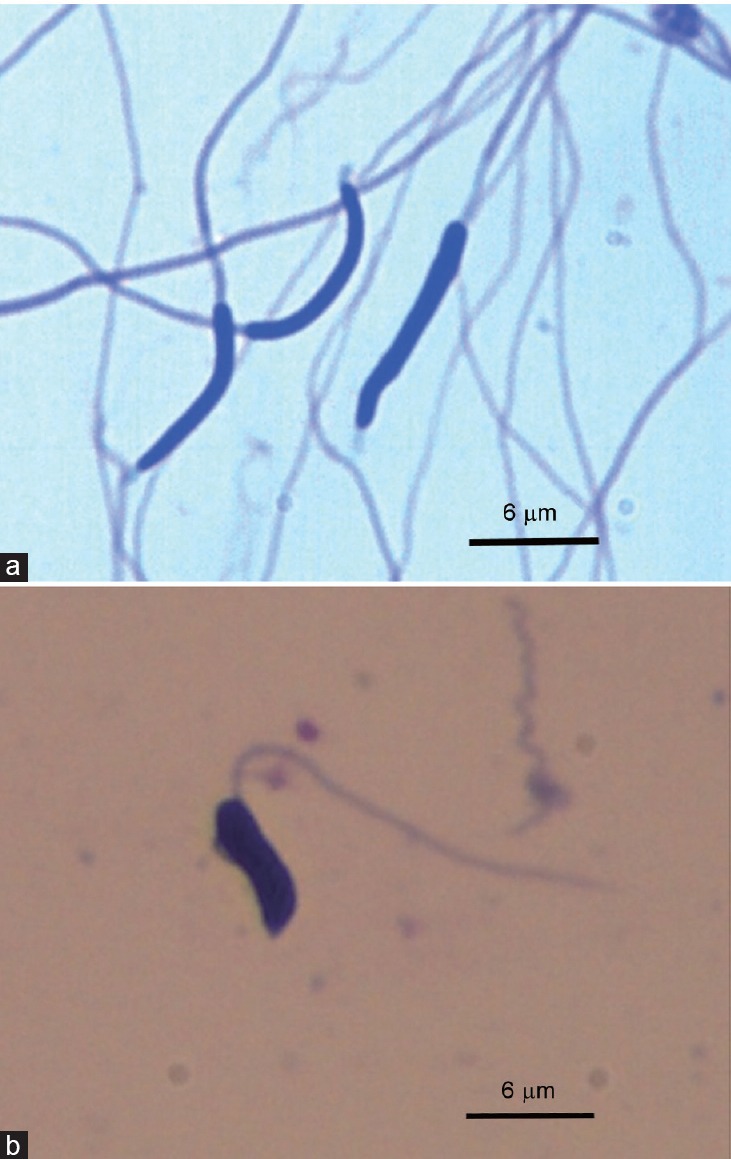
Chicken sperm (vermiform sperm head morphology – sauropsid type [a]) and peregrine falcon sperm (short and wide sperm head morphology [b]). Sperm stained with Hemacolor®. Scale bar = 6 μm.
Table 1.
Summary of sperm morphometric measurements (expressed as mean±s.d. when available) in avian species
Falcons have smaller spermatozoa than galliformes, with the sperm heads shorter but wider. Sperm head length in fowl ranges from 11 to 21 μm, while in falcons, it is usually 6–7 μm (Table 1). Falcon ejaculates characteristically contain pleomorphic spermatozoa whereas sperm morphology in the golden eagle is very heterogeneous. The most common abnormal sperm morphology is a rounded head with a triangular acrosome although commonly observed wasp-stinger-shaped acrosomes are deemed normal on the basis of the intactness of the organelle. The percentage of abnormal spermatozoa in golden eagle ejaculates is also higher than that in ostriches and the white-backed vulture.97,98 Pleomorphy, a characteristic of falcon spermatozoa, is particularly notable in fresh gyrfalcon (Falco rusticolus) sperm cells. The immature sperm cells in such ejaculates include type I or type II spermatocytes, spermatids in different phases of development, and quasi-mature spermatozoa still with the residual body attached to the head.27 No spermatogonia are observed, however, unlike that reported earlier for the peregrine falcon (Falco peregrinus).99 The mean percentage of immature spermatozoa is about 55%–65% in peregrine falcon and gyrfalcon ejaculates (Villaverde-Morcillo et al., unpublished data). This high percentage agrees with that reported earlier for peregrine falcon ejaculates99 although the latter authors identified the immature sperm cells they saw as spermatogonia. It may be that the high percentage of spermatocytes and spermatids in falcon ejaculates has a physiological role in the reproduction of these species. Indeed, four sperm subpopulations, based on sperm head size, have been identified in falcon ejaculates.27 The proportion of each is similar in the brookei peregrine falcon (F. peregrinus brookei), Scottish falcon (F. p. peregrinus), and gyrfalcon, but the role of these subpopulations in their reproductive biology and fertility remains unknown.
Penguin species are southern hemisphere birds found from the Equator to Antarctica; they are thus subjected to different ecological constraints on the reproductive strategy followed. Breeding activity is very sensitive to environmental conditions, and therefore highly responsive to climate change.100 For example, in the equatorial Galapagos penguin (Spheniscus mendiculus), the onset of breeding is closely linked to mean sea surface temperature.101 The breeding patterns and reproductive characteristics (e.g., functional and morphometric sperm parameter values) of penguins may, therefore, provide useful indicators of oceanographic conditions. With the use of Hemacolor® and a CASA-Morph system,5 morphometry of sperm samples from gentoo (Pygoscelis papua) and king penguins (Aptenodytes patagonicus) revealed similar sperm head lengths to those of the rooster, but greater head widths than those recorded for the galliformes (and therefore more like those of falcons) (Table 1). The sperm head area was, in fact, greater than that of most bird sperm cells studied. Although penguins are monogamous, interannual fidelity varies according to species,100 and polygynous trios (one male with two females) have been observed in emperor penguins (A. patagonicus). The larger sperm heads of the king penguin, and a lower count of immature cells, suggest a certain degree of polyandry is possible.
CONCLUSIONS
Given the many factors that affect the morphology and morphometrics of avian sperm cells, the standardization of staining techniques to be used in computer-assessed light microscopy methods is a priority.
COMPETING INTERESTS
None of the authors have any financial or personal relationship that could inappropriately influence or bias the content of the paper.
AUTHOR CONTRIBUTIONS
JSM conceived the study and wrote the basis of the article; MCE designed experiments, performed data analysis, and drafted the manuscript; SVM performed data analysis and drafted the manuscript; ATD and CC conducted bird management and sperm collection; ALS and RV performed data analysis; ALG and JGM took charge of penguin sperm collection and sperm analysis.
ACKNOWLEDGMENTS
This research was funded by the INIA grant RZ2012-00013-C02-01 and Fundación Parques Reunidos - INIA agreement CC16-001. The authors would like to thank Gabriel Alcántara, Lino Perez, Yolanda Martin, Cristian Rodríguez, and Sara San Pedro of Faunia, Madrid, for their help in obtaining semen samples from penguins.
REFERENCES
- 1.Noirault J, Brillard JP, Bakst MR. Spermatogenesis in the turkey (Meleagris gallopavo): quantitative approach in immature and adult males subjected to various photoperiods. Theriogenology. 2006;65:845–59. doi: 10.1016/j.theriogenology.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik I. The cytoplasmic components of germ-cells during spermatogenesis in the domestic fowl. Q J Microsc Sci. 1947;88:353–66. [Google Scholar]
- 3.Aire TA, Olowo-okorun MO, Ayeni JS. The seminiferous epithelium in the Guinea fowl (Numida melagris) Cell Tissue Res. 1980;205:319–25. doi: 10.1007/BF00234690. [DOI] [PubMed] [Google Scholar]
- 4.Thurston RJ, Korn N. Semen quality in the domestic turkey: the yellow semen syndrome. Poult Avian Biol Rev. 1997;8:109–21. [Google Scholar]
- 5.Villaverde-Morcillo S, Esteso MC, Castaño C, Toledano Díaz A, López-Sebastián A, et al. Influence of staining method on the values of avian sperm head morphometric variables. Reprod Domest Anim. 2015;50:750–5. doi: 10.1111/rda.12574. [DOI] [PubMed] [Google Scholar]
- 6.Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 7.Thurston RJ, Korn N. Spermiogenesis in commercial poultry species: anatomy and control. Poult Sci. 2000;79:1650–68. doi: 10.1093/ps/79.11.1650. [DOI] [PubMed] [Google Scholar]
- 8.Santiago-Moreno J, Castaño C, Toledano-Díaz A, Esteso MC, López-Sebastián A, et al. Characterization of red-legged partridge (Alectoris rufa) sperm: seasonal changes and influence of genetic purity. Poult Sci. 2015;94:80–7. doi: 10.3382/ps/peu020. [DOI] [PubMed] [Google Scholar]
- 9.Santiago-Moreno J, Castaño C, Coloma MA, Gomez-Brunet A, Toledano-Diaz A, et al. Use of the hypo-osmotic swelling test and aniline blue staining to improve the evaluation of seasonal sperm variation in native Spanish free-range poultry. Poult Sci. 2009;88:2661–9. doi: 10.3382/ps.2008-00542. [DOI] [PubMed] [Google Scholar]
- 10.Wakely WJ, Kosin IL. A study of the morphology of the Turkey spermatozoa with special reference to a seasonal prevalence of abnormal types. Am J Vet Res. 1951;12:240–5. [PubMed] [Google Scholar]
- 11.Brown KI, Graham EF. Effect of semen quality on fertility in turkeys. Poult Sci. 1971;1:295–7. doi: 10.3382/ps.0500295. [DOI] [PubMed] [Google Scholar]
- 12.Burrows WH, Quinn JP. The collection of spermatozoa from the domestic fowl and turkey. Poult Sci. 1937;16:19–24. [Google Scholar]
- 13.Burrows WH, Quinn JP. Artificial Insemination of Chickens and Turkeys. Circular 525. Washington, D.C: US Department of Agriculture; 1939. p. 12. [Google Scholar]
- 14.Prieto MT, Sanchez-Calabuig MJ, Hildebrandt TB, Santiago-Moreno J, Saragusty J. Sperm cryopreservation in wild animals. Eur J Wildl Res. 2014;60:851–64. [Google Scholar]
- 15.Blesbois E, Brillard JP. Specific features of in vivo and in vitro sperm storage in birds. Animal. 2007;1:1472–81. doi: 10.1017/S175173110700081X. [DOI] [PubMed] [Google Scholar]
- 16.Santiago-Moreno J, Castaño C, Toledano-Díaz A, Coloma MA, Lopez-Sebastian A, et al. Semen cryopreservation for the creation of a Spanish poultry breeds cryobank: optimization of freezing rate and equilibration time. Poult Sci. 2011;90:2047–53. doi: 10.3382/ps.2011-01355. [DOI] [PubMed] [Google Scholar]
- 17.Glover JD, McGrew MJ. Primordial germ cell technologies for avian germplasm cryopreservation and investigating germ cell development. Poult Sci. 2012;49:155–62. [Google Scholar]
- 18.Blesbois E, Seigneurin F, Grasseau I, Limouzin C, Besnard J, et al. Semen cryopreservation for ex situ management of genetic diversity in chicken: creation of the French avian cryobank. Poult Sci. 2007;86:555–64. doi: 10.1093/ps/86.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wishart GJ. Quantitation of the fertilizing ability of fresh compared with frozen and thawed fowl spermatozoa. Br Poult Sci. 1985;26:375–80. doi: 10.1080/00071668508416825. [DOI] [PubMed] [Google Scholar]
- 20.Abouelezz FM, Castaño C, Toledano-Díaz T, Esteso MC, López-Sebastián A, et al. Sperm-egg penetration assay assessment of the contraceptive effects of glycerol and egg yolk in rooster sperm diluents. Theriogenology. 2015;83:1541–7. doi: 10.1016/j.theriogenology.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Abouelezz FM, Castaño C, Toledano-Díaz A, Esteso MC, López-Sebastián A, et al. Effect of the interaction between cryoprotectant concentration and cryopreservation method on frozen/thawed chicken sperm variables. Reprod Domest Anim. 2015;50:135–41. doi: 10.1111/rda.12464. [DOI] [PubMed] [Google Scholar]
- 22.Santiago-Moreno J, López-Sebastián A, Castaño C, Coloma MA, Gómez-Brunet A, et al. Sperm variables as predictors of fertilizing capacity in black Castellana roosters for donor's selection in Genome Resource Banking. Span J Agric Res. 2009;7:555–62. [Google Scholar]
- 23.Gliozzi TM, Zaniboni L, Cerolini S. DNA fragmentation in chicken spermatozoa during cryopreservation. Theriogenology. 2011;75:1613–22. doi: 10.1016/j.theriogenology.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Esteso MC, Fernández-Santos MR, Soler AJ, Garde JJ. Head dimensions of cryopreserved red deer spermatozoa are affected by thawing procedure. Cryo Letters. 2003;24:261–8. [PubMed] [Google Scholar]
- 25.Esteso MC, Soler AJ, Fernández-Santos MR, Quintero-Moreno AA, Garde JJ. Functional significance of the sperm head morphometric size and shape for determining freezability in Iberian red Deer (Cervus elaphus hispanicus) epididymal sperm samples. J Androl. 2006;27:662–70. doi: 10.2164/jandrol.106.000489. [DOI] [PubMed] [Google Scholar]
- 26.Peña FJ, Saravia F, García-Herreros M, Núñez-Martín I, Tapia JA, et al. Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to post-thaw quality. J Androl. 2005;26:716–23. doi: 10.2164/jandrol.05030. [DOI] [PubMed] [Google Scholar]
- 27.Villaverde-Morcillo S. Spain: Universidad Complutense de Madrid; 2016. Collection, Storage and Morphometry of Avian Sperm: Use for Sperm Characterization and Cryopreservation in Wild Species. Doctoral Thesis. Faculty of Veterinary Medicine; p. 137. [Google Scholar]
- 28.Barna J, Wishart GJ. Excess nuclear DNA in spermatozoa of Guinea fowl. Theriogenology. 2003;59:1685–91. doi: 10.1016/s0093-691x(02)01237-2. [DOI] [PubMed] [Google Scholar]
- 29.Lukaszewicz E. Characteristics of fresh gander semen and its susceptibility to cryopreservation in six generations derived from gees inseminated with frozen-thawed seme. Cryo Letters. 2006;27:51–7. [PubMed] [Google Scholar]
- 30.Wishart GL. Semen quality and semen storage. In: Hocking PM, editor. Biology of Breeding Poultry. Science Symposium Series. Vol. 29. Oxfordshire: CAB International; 2009. p. 464. [Google Scholar]
- 31.Maroto-Morales A, Ramón M, García-Álvarez O, Soler AJ, Fernández-Santos MR, et al. Morphometrically-distinct sperm subpopulations defined by a multistep statistical procedure in ram ejaculates: intra- and interindividual variation. Theriogenology. 2012;77:1529–39. doi: 10.1016/j.theriogenology.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Urióstegui-Acosta M, Hernández-Ochoa I, Sánchez-Gutiérrez M, Piña-Guzmán B, Rafael-Vázquez L, et al. Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice. Toxicol Appl Pharmacol. 2014;279:391–400. doi: 10.1016/j.taap.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Birkhead T. Promiscuity: An Evolutionary History of Sperm Competition. London: Harvard University Press; 2000. p. 280. [Google Scholar]
- 34.Birkhead TR, Immler S. Making sperm: design, quality control and sperm competition. Soc Reprod Fertil Suppl. 2007;65:175–81. [PubMed] [Google Scholar]
- 35.Immler S, Saint-Jalme M, Lesobre L, Sorci G, Roman Y, et al. The evolution of sperm morphometry in pheasants. J Evol Biol. 2007;20:1008–14. doi: 10.1111/j.1420-9101.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 36.Lüpold S, Calhim S, Immler S, Birkhead TR. Sperm morphology and sperm velocity in passerine birds. Proc Biol Sci. 2009;276:1175–81. doi: 10.1098/rspb.2008.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomendio M, Roldan ER. Sperm competition influences sperm size in mammals. Proc R Soc Lond B. 1991;243:181–5. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, et al. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc Natl Acad Sci U S A. 2009;106:1128–32. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal AF, Colominas JA. Genetic quality of red legged partridge in Spain and proposals for its conservation and improvement. Selecciones Avícolas. 2007;10:667–72. [In Spanish] [Google Scholar]
- 40.Briskie JV, Montgomerie R, Birkhead TR. The evolution of sperm size in birds. Evolution. 1997;51:937–45. doi: 10.1111/j.1558-5646.1997.tb03674.x. [DOI] [PubMed] [Google Scholar]
- 41.Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, et al. Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution. 2009;63:2466–73. doi: 10.1111/j.1558-5646.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 42.Immler S, Pryke SR, Birkhead TR, Griffith SC. Pronounced within-individual plasticity in sperm morphometry across social environments. Evolution. 2010;64:1634–43. doi: 10.1111/j.1558-5646.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 43.Riana Bornman MS, Bouwman H. Environmental pollutants and diseases of sexual development in humans and wildlife in South Africa: harbingers of impact on overall health? Reprod Domest Anim. 2012;47(Suppl 4):327–32. doi: 10.1111/j.1439-0531.2012.02094.x. [DOI] [PubMed] [Google Scholar]
- 44.Khan PK, Sinha SP. Ameliorating effect of Vitamin C on murine sperm toxicity induced by three pesticides (endosulfan, phosphamidon and mancozeb) Mutagenesis. 1996;11:33–6. doi: 10.1093/mutage/11.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Carothers AD, Beatfy RA. The recognition and incidence of haploid and polyploid spermatozoa in man, rabbit and mouse. J Reprod Fertil. 1975;44:487–500. doi: 10.1530/jrf.0.0440487. [DOI] [PubMed] [Google Scholar]
- 46.Eastmond DA, Rupa DS, Reddy PP. Detection of chromosomal alterations in the sperm of pesticide-exposed workers using fluorescence in situ hybridisation (FISH) Environ Mol Mutagen. 1997;29(Suppl 28):44. [Google Scholar]
- 47.Ratcliffe JM, Schrader SM, Steenland K, Clapp DE, Turner T, et al. Semen quality in papaya workers with long term exposure to ethylene dibromide. Br J Ind Med. 1987;44:317–26. doi: 10.1136/oem.44.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–69. [Google Scholar]
- 49.Narayan EJ. Evaluation of physiological stress in Australian wildlife: embracing pioneering and current knowledge as a guide to future research directions. Gen Comp Endocrinol. 2015 doi: 10.1016/j.ygcen.2015.12.008. pii: S0016-6480(15)30043-5. doi: 10.1016/j.ygcen. 2015.12.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Kanter M, Aktas C, Erboga M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: an immunohistochemical and ultrastructural study. Toxicol Ind Health. 2013;29:99–113. doi: 10.1177/0748233711425082. [DOI] [PubMed] [Google Scholar]
- 51.Armengol MF, Sabino GA, Forquera JC, de la Casa A, Aisen EG. Sperm head ellipticity as a heat stress indicator in Australian Merino rams (Ovis aries) in Northern Patagonia, Argentina. Theriogenology. 2015;83:553–9. doi: 10.1016/j.theriogenology.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Ballowitz E. Untersunchungen über die Struktur der Spermatozóen, zugleich ein Beitrag zur Lehr von feineren Bau der contraktilen Elemente. Arch Mikr Anat. 1888;32:401–73. [Google Scholar]
- 53.Grigg GW, Hodge AJ. Electron microscopic studies of spermatozoa: I. The morphology of the spermatozoon of the common domestic fowl (Gallus domesticus) Aust J Sci Res B. 1949;2:271–86. [Google Scholar]
- 54.Bakst MR, Howarth B. The head, neck and midpiece of cock spermatozoa examined with the transmission electron microscope. Biol Reprod. 1975;12:632–40. doi: 10.1095/biolreprod12.5.632. [DOI] [PubMed] [Google Scholar]
- 55.Thurston RJ, Hess RA. Ultrastructure of spermatozoa of domesticated birds: comparative study of turkey, chicken and guinea fowl. Scanning Microsc. 1987;1:1829–38. [PubMed] [Google Scholar]
- 56.Soley JT, Roberts DC. Ultrastructure of ostrich (Struthio camelus) spermatozoa. II, Scanning electron microscopy. Onderstepoort J Vet Res. 1994;61:239–46. [PubMed] [Google Scholar]
- 57.Korn N, Thurston RJ, Pooser BP, Scott TR. Ultrastructure of spermatozoa from Japanese quail. Poult Sci. 2000;79:86–93. doi: 10.1093/ps/79.1.86. [DOI] [PubMed] [Google Scholar]
- 58.Van der Horst G, Curry PT, Kitchin RM, Burgess W, Thorne ET, et al. Quantitative light and scanning electron microscopy of ferret sperm. Mol Reprod Dev. 1991;30:232–40. doi: 10.1002/mrd.1080300311. [DOI] [PubMed] [Google Scholar]
- 59.Du Plessis L, Soley JT. Light microscopic features and morphometry of sperm in the emu (Dromaius novaehollandiae) Theriogenology. 2014;81:203–9. doi: 10.1016/j.theriogenology.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Baker HW, Clarke GN. Sperm morphology: consistency of assessment of the same sperm by different observers. Clin Reprod Fertil. 1987;5:37–43. [PubMed] [Google Scholar]
- 61.Verstegen J, Iguer-Ouada M, Onclin K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology. 2002;57:149–79. doi: 10.1016/s0093-691x(01)00664-1. [DOI] [PubMed] [Google Scholar]
- 62.Ombelet W, Pollet H, Bosmans E, Vereecken A. Results of a questionnaire on sperm morphology assessment. Hum Reprod. 1997;12:1015–20. doi: 10.1093/humrep/12.5.1015. [DOI] [PubMed] [Google Scholar]
- 63.Cooper TG. Experience with external quality control in spermatology. Hum Reprod. 1999;14:765–9. doi: 10.1093/humrep/14.3.765. [DOI] [PubMed] [Google Scholar]
- 64.Boersma A, Braun J. Computer-assisted analysis of sperm morphology in veterinary medicine. Berl Munch Tierarztl Wochenschr. 1999;112:81–5. [PubMed] [Google Scholar]
- 65.Zawadzka J, Łukaszewicz E. Sperm morphometry of six Polish duck conservative flocks. Zesz Nauk UP Wroc Biol Hod Zwierz. 2012;LXVII, 591:41–8. [Google Scholar]
- 66.Dobrescu O, Marandici A, Marcu S. Semen collection and assay in Coturnix coturnix japonica (Japanese quail) Rev Zootech Med Vet. 1971;21:40–3. [Google Scholar]
- 67.Gravance CG, Vishwanath R, Pitt C, Casey PJ. Computer automated morphometric analysis of bull sperm heads. Theriogenology. 1996;46:1205–15. doi: 10.1016/s0093-691x(96)00291-9. [DOI] [PubMed] [Google Scholar]
- 68.Esteso MC, Rodríguez E, Toledano-Díaz A, Castaño C, Pradiee J, et al. Descriptive analysis of sperm head morphometry in Iberian ibex (Capra pyrenaica): optimum sampling procedure and staining methods using Sperm-Class Analyzer®. Anim Reprod Sci. 2015;155:42–9. doi: 10.1016/j.anireprosci.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Hidalgo M, Rodríguez I, Dorado J. Influence of staining and sampling procedures on goat sperm morphometry using the Sperm Class Analyzer. Theriogenology. 2006;66:996–1003. doi: 10.1016/j.theriogenology.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 70.Sancho M, Pérez-Sánchez F, Tablado L, de Monserrat J, Soler C. Computer assisted morphometric analysis of ram sperm heads: evaluation of different fixative techniques. Theriogenology. 1998;50:27–37. doi: 10.1016/s0093-691x(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 71.Vicente-Fiel S, Palacín I, Santolaria P, Hidalgo CO, Silvestre MA, et al. A comparative study of the sperm nuclear morphometry in cattle, goat, sheep, and pigs using a new computer-assisted method (CASMA-F) Theriogenology. 2003;79:436–42. doi: 10.1016/j.theriogenology.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Davis RO, Gravance CG. Standardization of specimen preparation, staining, and sampling methods improves automated sperm-head morphometry analysis. Fertil Steril. 1993;59:412–7. doi: 10.1016/s0015-0282(16)55686-6. [DOI] [PubMed] [Google Scholar]
- 73.García-Herreros M, Aparicio IM, Barón FJ, García-Marín LJ, Gil MC. Standardization of sample preparation, staining and sampling methods for automated sperm head morphometry analysis of boar spermatozoa. Int J Androl. 2006;29:553–63. doi: 10.1111/j.1365-2605.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 74.Yániz JL, Soler C, Santolaria P. Computer assisted sperm morphometry in mammals: a review. Anim Reprod Sci. 2015;156:1–12. doi: 10.1016/j.anireprosci.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Sekoni VO, Gustafsson BK. Seasonal variations in the incidence of sperm morphological abnormalities in dairy bulls regularly used for artificial insemination. Br Vet J. 1987;143:312–7. doi: 10.1016/0007-1935(87)90064-9. [DOI] [PubMed] [Google Scholar]
- 76.Chandler JE, Painter CL, Adkison RW, Memon MA, Hoyt PG. Semen quality characteristics of dairy goats. J Dairy Sci. 1988;71:1638–46. doi: 10.3168/jds.S0022-0302(88)79728-3. [DOI] [PubMed] [Google Scholar]
- 77.Ramón M, Soler AJ, Ortiz JA, García-Alvarez O, Maroto-Morales A, et al. Sperm population structure and male fertility: an intraspecific study of sperm design and velocity in red deer. Biol Reprod. 2013;89:110. doi: 10.1095/biolreprod.113.112110. [DOI] [PubMed] [Google Scholar]
- 78.Maroto-Morales A, Ramón M, García-Álvarez O, Montoro V, Soler AJ, et al. Sperm head phenotype and male fertility in ram semen. Theriogenology. 2015;84:1536–41. doi: 10.1016/j.theriogenology.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 79.Hidalgo M, Rodríguez I, Dorado JM. The effect of cryopreservation on sperm head morphometry in Florida male goat related to sperm freezability. Anim Reprod Sci. 2007;100:61–72. doi: 10.1016/j.anireprosci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Gago C, Pérez-Sánchez F, Yeung CH, Tablado L, Cooper TG, et al. Standardisation of sampling and staining methods for the morphometric evaluation of sperm heads in the cynomolgus monkey (Macaca fascicularis) using computer-assisted image analysis. Int J Androl. 1998;21:169–76. doi: 10.1046/j.1365-2605.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 81.Soler C, Gadea B, Soler AJ, Fernández-Santos MR, Esteso MC, et al. Comparison of three different staining methods for the assessment of epididymal red deer sperm morphometry by computerized analysis with ISAS®. Theriogenology. 2005;64:1236–43. doi: 10.1016/j.theriogenology.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 82.Maree L, du Plessis SS, Menkveld R, Van der Horst G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum Reprod. 2010;25:1369–82. doi: 10.1093/humrep/deq075. [DOI] [PubMed] [Google Scholar]
- 83.Terquem A, Dadoune JP. Aniline blue staining of human spermatozoa chromatin. Evaluation of nuclear maturation. In: André J, editor. The Sperm Cell. The Hague: Nijhoff Publishers; 1983. p. 465. [Google Scholar]
- 84.Dadoune JP, Mayaux MJ, Guihard-Moscato ML. Correlation between defects in chromatin condensation of human spermatozoa stained by aniline blue and semen characteristics. Andrologia. 1988;20:211–7. [PubMed] [Google Scholar]
- 85.Auger J, Mesbah M, Huber C, Dadoune JP. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13:452–62. doi: 10.1111/j.1365-2605.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 86.Villaverde-Morcillo S, García-Sánchez R, Castaño C, Rodríguez E, Gonzalez F, et al. Characterization of natural ejaculates and sperm cryopreservation in a golden eagle (Aquila chrysaetos) J Zoo Wildl Med. 2015;46:335–8. doi: 10.1638/2013-0293R1.1. [DOI] [PubMed] [Google Scholar]
- 87.Gravance C, Champion Z, Casey P. Computer-assisted sperm head morphometry analysis (ASMA) of cryopreserved ram spermatozoa. Theriogenology. 1998;49:1219–30. doi: 10.1016/s0093-691x(98)00069-7. [DOI] [PubMed] [Google Scholar]
- 88.Alkan S, Baran A, Özdas ÖB, Evecen M. Morphological defects in turkey semen. Turk J Vet Anim Sci. 2002;26:1087–92. [Google Scholar]
- 89.Ferdinand A. Light and electron microscopical studies on the morphology of gander spermatozoa.PhD Thesis. Hannover: University of Veterinary Medicine; 1992. [Google Scholar]
- 90.Gee GF, Bertschinger H, Donoghue AM, Blanco J, Soley J. Reproduction in nondomestic birds: physiology, semen collection, artificial insemination and cryopreservation. Avian Poult Biol Rev. 2004;15:47–101. [Google Scholar]
- 91.Bertschinger HJ, Burger WP, Soley JT, de Lange JH. Semen Collection and Evaluation of the Male Ostrich. Proceedings of the Biennial Congress of the South African Veterinary Association. Grahamstown. 1992:154–8. [Google Scholar]
- 92.Buendía P, Soler C, Paolicchi F, Gago G, Urquieta B, et al. Morphometric characterization and classification of alpaca sperm heads using the Sperm-Class Analyzer® computer-assisted system. Theriogenology. 2002;57:1207–18. doi: 10.1016/s0093-691x(01)00724-5. [DOI] [PubMed] [Google Scholar]
- 93.Marquez BJ, Ogasawara FK. Scanning electron microscope studies of turkey sperm. Poult Sci. 1975;54:1139–43. doi: 10.3382/ps.0541139. [DOI] [PubMed] [Google Scholar]
- 94.Asa CS, Phillips DM. Ultrastructure of avian spermatozoa: a short review. In: Mohri H, editor. New Horizons in Sperm Cell Research. New York: Gordon and Breach Science Publishers; 1987. p. 516. [Google Scholar]
- 95.McFalarne RW. Proceedings of the XIIIth International Ornithological Congress. Ithaca, New York: 1963. The Taxonomic Significance of Avian Sperm; pp. 91–102. [Google Scholar]
- 96.Laskemoen T, Albrecht T, Bonisoli-Alquati A, Cepak J, de Lope F, et al. Variation in spern morphometry and sperm competition among barn swallow (Hirundo rustica) populations. Behav Ecol Sociobiol. 2013;67:301–9. [Google Scholar]
- 97.Wishart GJ, Lindsay C, Staines HJ, McCormick P. Semen quality in captive houbara bustard, Chlamydotis undulate undulata. Reprod Fertil Dev. 2002;14:401–5. doi: 10.1071/rd02014. [DOI] [PubMed] [Google Scholar]
- 98.Umapathy G, Sontakke S, Reddy A, Ahmed S, Shivaji S. Semen characteristics of the captive Indian white-backed vulture (Gyps bengalensis) Biol Reprod. 2005;73:1039–45. doi: 10.1095/biolreprod.105.043430. [DOI] [PubMed] [Google Scholar]
- 99.Blanco JM, Wildt DE, Gee GF, Donoghue AM. Spermatogonia in Eagle and Peregrine Falcon Ejaculates: Interspecies Variation, Cryopreservation Tolerance and Implications to Cryobanking. Eurasian Congress on Raptors. Seville, Spain. 2001:24. [Google Scholar]
- 100.Ancel A, Beaulieu M, Gilbert C. The different breeding strategies of penguins: a review. C R Biol. 2013;336:1–2. doi: 10.1016/j.crvi.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Boersma PD. Breeding patterns of Galápagos penguins as an indicator of oceanographic conditions. Science. 1978;200:1481–3. doi: 10.1126/science.200.4349.1481. [DOI] [PubMed] [Google Scholar]


