Abstract
The main aims of this research were to study possible differences in objective morphometric sperm characteristics, establish normative sperm morphometry standards, and evaluate the presumed different subpopulation distribution of avian spermatozoa from the rooster (Gallus domesticus) and Guinea fowl (Numida meleagris) as model avian species. Seventy-two ejaculates (36 per species studied) were obtained manually, following a training period involving gently combined dorso-abdominal and lumbo-sacral massage of the birds. Ejaculates were processed for volume, sperm concentration, viability, motility, and morphology. Moreover, samples were submitted for sperm morphometric assessment using objective Computer-Assisted Semen Analysis for Morphometry (CASA-Morph) methods, with sperm morphometric descriptors evaluated by Principal Component Analysis (PCA) and multivariate clustering analyses. There were several differences observed between the avian species in values obtained for ejaculate volume and sperm concentration (P < 0.001). Irrespective of species, PCA revealed two Principal Components (PCs) explaining more than 80% of the variance. In addition, the number of subpopulations differed with species (three and five subpopulations for rooster and Guinea fowl, respectively). Moreover, the distribution of the sperm subpopulations was found to be structurally different between species. In conclusion, our findings from using CASA-Morph methods indicate pronounced sperm morphometric variation between these two avian species. Because of the strong differences observed in morphometric parameter values and their subpopulation distribution, these results suggest that application of objective analytical methods such as CASA-Morph could substantially improve the reliability of comparative studies and help establish valid normative sperm morphological values for avian species.
Keywords: Gallus domesticus, Numida meleagris, principal component analysis, sperm morphometry, sperm subpopulations
INTRODUCTION
Traditionally, Assisted Reproductive Technologies (ARTs) have been associated with mammalian species; however, ARTs have been introduced around the world to the poultry industry during recent decades.1,2 This has led to new research related to the reproductive physiology of different avian species, filling the void created by the lack of information on the fertility characteristics in these species.1 Nowadays, the chicken (Gallus domesticus) and the Guinea fowl (Numida meleagris) are important sources of eggs and meat. However, the intensification of the poultry industry has increased the interest in artificial insemination (AI) techniques and, therefore, the sperm fertilizing capacity of these species.3,4
Little research has been done on avian seminal characteristics since the application of AI technology to poultry. In addition to this, lack of knowledge, age, housing, breeding management, artificial incubation, nutrition, photoperiod, sex determination, environment, and many other factors severely limit the reproductive capacity of these avian species.1,2,3,4,5 Thus, semen quality and quantity could be the important limiting factors for an AI program, especially because all efforts related to the increase in reproductive performance should be focused on improving reproductive ability.6 The success of the AI technique is directly dependent on the quality of the semen collected, and therefore, an accurate evaluation of semen quality before AI is of the utmost importance.6
Sperm morphology, motility, and concentration are important qualitative semen parameters to be considered.7,8 In this regard, there is some research on the subjective evaluation of semen characteristics in avian species; however, there is scant information on the objective assessment of bird sperm characteristics. Moreover, bird sperm evaluation has been limited to subjective assessment despite the very different morphological shapes of avian spermatozoa. This makes it difficult to improve the genetic quality of farmed poultry efficiently, in which birds subjected to intense breeding conditions have exhibited a rapid decrease in genetic diversity.9 Several studies have detailed the morphological features of both normal and defective spermatozoa in the chicken10,11 and Guinea fowl;12,13,14 however, the basic reproductive biology related to sperm morphometry has received little attention.
Computer-Assisted Semen Analysis for Morphometry (CASA-Morph) is an effective technique that has been used to investigate the sperm morphology, being an objective method for the assessment of sperm quality applied to several mammalian species, including the human.15,16,17,18,19 However, despite the importance of avian species in the animal industry, no studies have provided information on ejaculated avian sperm subpopulations related to the morphometric features of the sperm head, which are very different between species.14 Biophysical factors may be fertility indicators; sperm head morphometry influences the cell's hydrodynamics, and inter- and intra-species variations in cholesterol/phospholipid content affect membrane permeability and fluidity that underlie sperm fertilizing potential.14
In view of the importance attached to sperm head morphometry as one of the factors used in the assessment of fertility in poultry, and the potential selection of roosters and Guinea fowl males for AI programs, it is crucial that a detailed classification of sperm morphometric features and the sperm subpopulation distribution are made available for these species. This information is required to provide comparative data for the accurate identification of abnormal forms and the differential subpopulation structure in different birds. One of my interests is to determine the characteristics of individual sperm head dimensions and shape that may account for phenotypic differences in sperm subpopulations observed in whole ejaculates.20,21 Unfortunately, there are no data in literature on the objective evaluation of differential sperm subpopulation structure in avian species, so any difference in the distribution of avian sperm subpopulations both within and between species is unknown. This lack of knowledge prompted the aims of the present research: (1) to study possible differences in objective sperm morphometric characteristics between the rooster (G. domesticus) and Guinea fowl male (N. meleagris); (2) to evaluate presumed differential subpopulation distributions using these species as avian model species.
MATERIALS AND METHODS
Chemicals and media
All reagents and media used (unless stated otherwise) were of high purity and were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All solutions and media were prepared by adjusting the temperature, and pH with sterile deionized water.
Animals and ejaculate collection
Ejaculates were collected manually following a training period by gently combined dorso-abdominal and lumbo-sacral massage methods to trigger a reflex response from 12 mature roosters (G. domesticus) and from 12 mature Guinea fowl males (N. meleagris) (randomly selected) once every two weeks over a 6-month period (January to June). All were healthy controls (age 12–18 months; weight 2.0–3.0 kg), caged individually for easy management, and maintained under the same nutritional conditions (standard commercial mash and water ad libitum), ambient temperature (20–22°C), and regular lighting regimen (12L:12D). Each ejaculate (3/male) was collected with care taken to avoid contamination with cloacal products. Contaminated ejaculates were discarded if males were producing yellow, watery, or no semen.
Semen processing, sperm quality evaluation, and sample staining
After collection in a Petri dish, the ejaculate was collected in a micropipette. The collected semen was pooled and immediately (within 10 min of collection) evaluated for the following sperm quality parameters: ejaculate volume (using a graduated micropipette); sperm concentration (expressed as the number of cells ml−1 determined by the use of a Neubauer hemocytometer after dilution with an isotonic solution of sodium citrate hydrate); the total number of spermatozoa per ejaculate determined from the ejaculate volume and the sperm concentration, sperm viability (live and dead spermatozoa were differentiated using nigrosin-eosin vital staining and by counting 200 spermatozoa and classifying them as stained or unstained on duplicate smears), sperm motility (the percentage of motile spermatozoa, subjectively assessed by visual estimation from contrast phase microscopy), and sperm morphology (smears fixed in 2.5% [v/v] glutaraldehyde to assess sperm head, midpiece, and principal piece sperm abnormalities) from average counts used for percentage calculation.
For sperm morphometric assessment, sperm smears were prepared by placing 5 μl of the sperm suspension on the clear end of a frosted slide and dragging the drop across the slide to create a thin feathered smear (duplicated smears). After 15 min, the air-dried microscopic slides were stained with Hemacolor (staining for 15 min in Coplin jars). Thereafter, the smears were gently rinsed with distilled water to remove excess stain. Samples were subsequently allowed to air dry, and then mounted and permanently sealed with Permount™ Mounting Media (Fisher Scientific, NJ, USA) (22 mm × 60 mm coverslips). At least 250 spermatozoa were randomly analyzed per slide, and all evaluations were made by the same person.
Computerized sperm morphometric analysis
Hemacolor-stained smears were routinely used for computerized morphometric analysis using a commercially available system (Motic Corporation Ltd., Hong Kong, China) equipped with a Nikon Eclipse E200 (Nikon, Tokyo, Japan) microscope with a ×100 oil-immersion bright-field objective lens. The video signal was acquired by a MotiCam 2000 digital camera (CMOS ½”; Motic Corporation Ltd., Hong Kong, China) mounted over the microscope and connected to a Pentium P8400 4 GB processor (Intel Corporation, USA). The configuration of the computer system included the interface Motic Images Plus 2.0 ML (Motic China Group, Xiamen, China) imaging analysis software. Digitized images were made up of 1 920 000 pixels (picture elements) and 256 gray levels.
At least 250 sperm cells per sample were randomly captured. Data were compiled and stored for further analysis. Only cells that did not overlap with debris or other cells were considered for analysis. The search, capture, and morphometric analysis of all slides were carried out by the same person. Each sperm head was measured for four primary dimensional parameters: area (A, μm2), Perimeter (P, μm), Length (L, μm), and Width (W, μm); and three dimensionless head shape-derived parameters: ellipticity (EL, [L/W]), Elongation (EO, [(L − W)/(L + W)]), and Rugosity (R, [4лA/P2]). These morphometric descriptors were chosen to provide maximal statistical information with a minimal number of parameters.16 Measurements of each sperm cell were saved in an Excel© (Microsoft Corporation, Redmond, WA, USA)-compatible database by the software for further analysis.
Principal component analysis and sperm subpopulation determination
The variation in sperm morphometric characteristics between the avian species was evaluated by using the general linear model (GLM) for repeated measures. The same procedure was used with the ejaculate volume, sperm concentration, sperm viability, sperm motility, and sperm morphology to classify the measured spermatozoa. The differences between species, allowing for all the morphometric variables, were evaluated by discriminant analysis. The data matrix from all the spermatozoa analyzed represented more than 10 400 observations, each one defined by the seven morphometric descriptors specified above. To evaluate the presumed differential subpopulation distribution of avian spermatozoa, Principal Component Analysis (PCA) was performed for each species. Here, each variable is weighted with its eigenvector, which generates a small number of linear combinations (Principal Components) that retain maximally the information in the original variables. The VARIMAX method with Kaiser Normalization was used as the rotation method and the next step was a nonhierarchical analysis using the k-means model, which uses Euclidean distances from the quantitative variables after standardization of the data, so that the cluster centers were the means of the observations assigned to each cluster. The multivariate k-means cluster analysis was used to classify the spermatozoa into a small number of subpopulations (clusters) according to their morphometric descriptors, as previously described.17 All analyses were performed with the statistical package SPSS version 15.0 for Windows software (SPSS Inc., Chicago, IL, USA).
Statistical analysis
ANOVA and χ2-test procedures were applied to evaluate statistical differences in the distributions of observations (individual spermatozoa) within species and subpopulations (percentages of spermatozoa assigned), and then a general linear model (GLP) procedure was used to determine the effects of species, as well as their variation, on the relative distribution frequency of spermatozoa within subpopulations. The GLM procedure was also used to evaluate the influence of the two independent variables on the mean morphometric parameters defining the different sperm subpopulations (i.e., the cluster centers). Differences between means were analyzed by Tukey's test. The level of significance was set at P < 0.05.
RESULTS
Sperm quality parameters in Gallus domesticus and Numida meleagris
Sperm quality characteristics derived from the species are shown in Table 1. In general, all sperm quality parameter values were higher in G. domesticus than N. meleagris. Except for ejaculate volume and sperm concentration (P < 0.001), our results indicate that no statistical differences were found for sperm quality parameters in G. domesticus and N. meleagris (P ≥ 0.05). However, for sperm motility, although there were differences, they were not statistically significant (P = 0.05) differences.
Table 1.
Sperm quality parameter values in Gallus domesticus and Numida meleagris

Sperm morphometric parameters in Gallus domesticus and Numida meleagris
Table 2 shows the sperm morphometric descriptors assessed in both species. Interestingly, after analysis, statistically significant differences in sperm dimensional and shape parameters were found for both species (P < 0.01).
Table 2.
Sperm head morphometric dimensional and shape parameter values in Gallus domesticus and Numida meleagris

PCA and sperm morphometric data derived from Gallus domesticus and Numida meleagris
The data matrix comprised 4516 and 5898 observations for G. domesticus and N. meleagris, respectively. PCA rendered two PCs in each species, with eigenvalues above one, which accounted for more than 80% of the variability in both cases from the seven initial morphometric descriptors (80.5% for rooster and 81.3% for Guinea fowl) (Table 3). Both PCs (PC1 and PC2) were constant in each species and were used to characterize and classify each spermatozoon in the subsequent cluster analysis.
Table 3.
Results from the PCA performed on the computerized sperm morphometric data obtained from Gallus domesticus and Numida meleagris
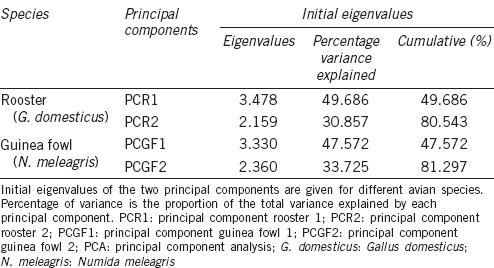
For roosters, PCR1 was positively related to the dimensional parameters A, P, and L, and to shape parameters EL and EO; however, it was negatively related to the dimensional parameter W and R. PCR2 was negatively related to the shape parameters (except R) and positively related to all dimensional parameters. The corresponding equations were, for PCR1: (0.3624 × A) + (0.4527 × P) + (0.4986 × L) − (0.0259 × W) + (0.4280 × EL) + (0.3930 × EO) − (0.2767 × R), and for PCR2: (0.457 × A) + (0.2594 × P) + (0.1420 × L) + (0.6553 × W) − (0.3119 × EL) − (0.3815 × EO) + (0.1876 × R).
For Guinea fowl, PCGF1 was positively related to the dimensional parameters A, P, and L, and to the shape parameters EL and EO; however, it was negatively related to the dimensional parameter W and shape parameter R. PCGF2 was positively related to the shape parameters EL and EO and negatively related to all dimensional parameters and the shape parameter R. The corresponding equations were, for PCGF1: (0.3832 × A) + (0.4676 × P) + (0.5132 × L) − (0.0308 × W) + (0.4062 × EL) + (0.3627 × EO) − (0.2711 × R), and for PCGF2: (−0.4204 × A) − (0.2888 × P) − (0.1186 × L) − (0.6269 × W) + (0.3627 × EL) + (0.4226 × EO) − (0.2099 × R).
Sperm subpopulation structure after PCA and clustering analysis in Gallus domesticus and Numida meleagris
After the PCA and cluster analysis for both species, three sperm subpopulations were identified in G. domesticus and five in N. meleagris in the data matrices of 4516 and 5898 elements, respectively. A representation of the different model cluster distributions of sperm heads in the different species according to the subpopulation is given in Figure 1. The disclosed subpopulations were characterized by different proportions of sperm head clusters (P < 0.001). Morphometric characteristics of those subpopulations and their distribution in each species are shown in Table 4. Different sperm head types (standard measurements) from rooster (G. domesticus) and Guinea fowl (N. meleagris) ejaculates, from the different sperm subpopulations obtained after Principal Component Analysis (PCA) and Clustering Analyses, are represented in Figure 2. The frequency of sperm distribution (percentage) within each cluster, as defined by the clustering analysis for each species, is represented in Figure 3.
Figure 1.
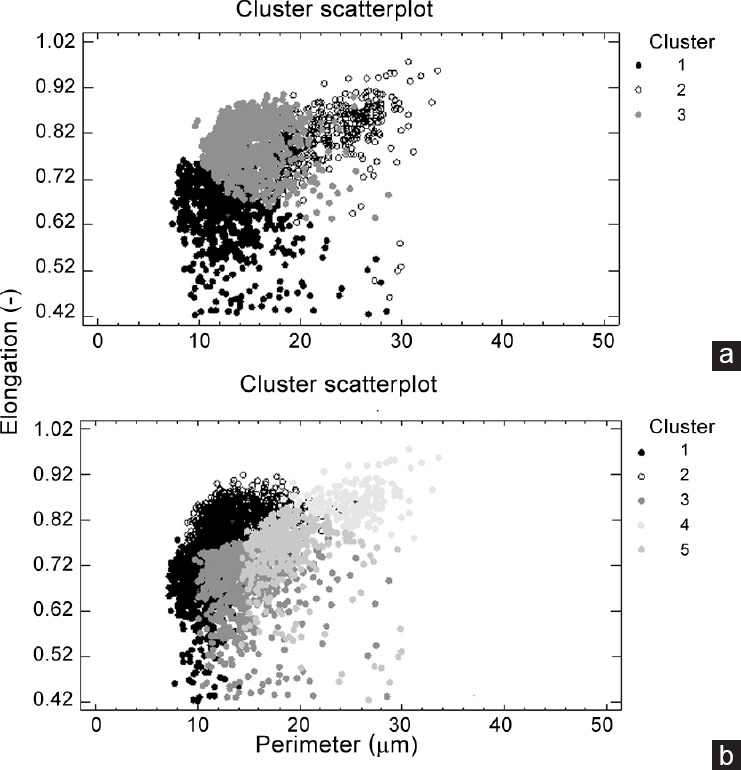
Dot plots of the clusters obtained after the Principal Component and Clustering Analyses derived from the sperm head morphometric data matrix from the rooster (Gallus domesticus) and Guinea fowl (Numida meleagris). Each event represents an individual spermatozoon. Upper plot of the figure (a) represents the three sperm subpopulations found in the rooster (Gallus domesticus) and Lower plot of the figure (b) represents the five subpopulations in the Guinea fowl (Numida meleagris).
Table 4.
Distribution of sperm morphometric subpopulations (CLs) from Gallus domesticus and Numida meleagris
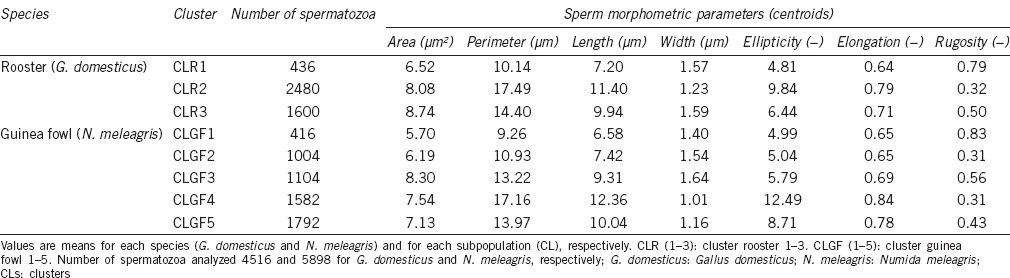
Figure 2.
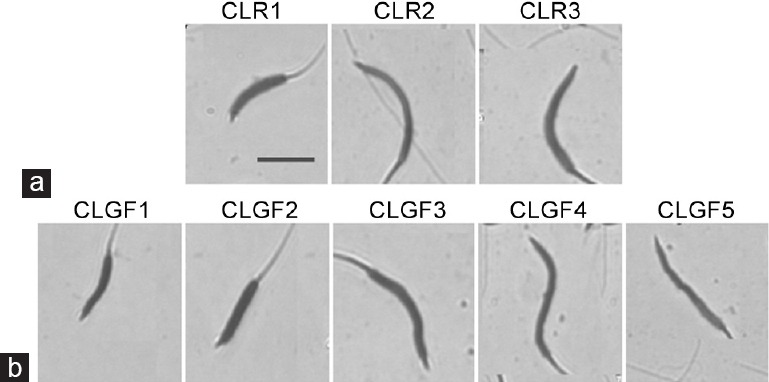
Representation of sperm head types (standard measurements) from rooster (Gallus domesticus) and Guinea fowl (Numida meleagris) ejaculates according to the different sperm subpopulations obtained after Principal Component Analysis (PCA) and Clustering Analyses. Representative micrographs showing bright-field images (from a × 100 oil immersion objective) of rooster (Gallus domesticus) and Guinea fowl (Numida meleagris) sperm heads (scale bar, 5 μm) characterising each subpopulation (CLR1 to CLR3 and CLGF1 to CLGF5 for rooster and Guinea fowl, respectively): (a) CLR1: small, wide and slightly elliptical; CLR2: average size, long, narrow and very elliptical; CLR3: very large, wide and elliptical; (b) CLGF1: very small, wide, very short and slightly elliptical; CLGF2: small, very short, very wide and slightly elliptical; CLGF3: very large, very wide, short and slightly elliptical; CLGF4: average sized, very long, very narrow and very elliptical; CLGF5: average sized, long, narrow and elliptical spermatozoa.
Figure 3.
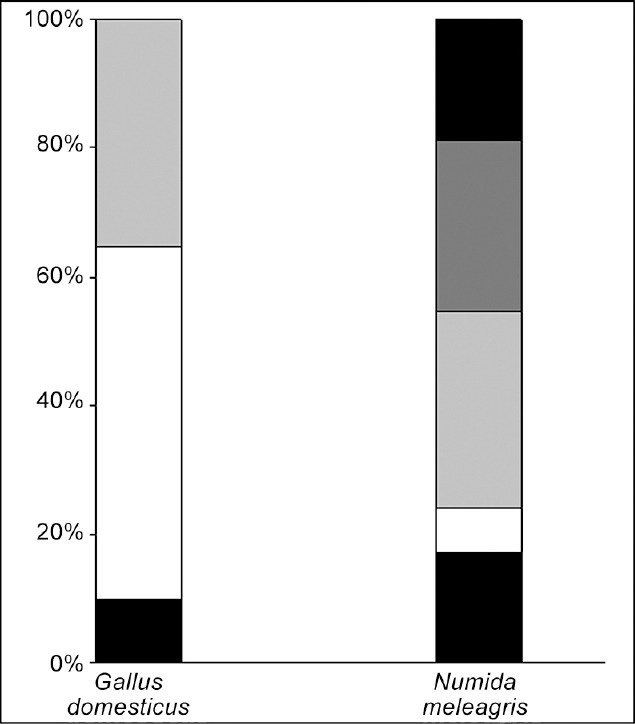
Frequency of distribution (percentage of spermatozoa) within each cluster, as defined after the Clustering and Discriminant Analyses in the rooster (Gallus domesticus) and Guinea fowl (Numida meleagris). Pattern codes (from bottom to top) for each cluster (subpopulation) are: black for CLR1; white for CLR2; light grey for CLR3; black for CLGF1; white for CLGF2; light grey for CLGF3; grey for CLGF4; dark grey for CLGF5.
DISCUSSION
The existence of quantitative morphological differences in avian spermatozoa is an important determinant of the characterization of spermatozoa from different species.14 However, very little information on species-specific avian sperm morphometric differences has been presented. Although there have been a number of studies on semen characteristics, we report here the first research of avian sperm morphometry with the ultimate goal of describing the sperm subpopulation ejaculate structure to generate some avian models for determining the differences, in this case, between G. domesticus and N. meleagris. One important distinction relative to this approach was the assessment of pooled, rather than individual ejaculates, to avoid the potential male-to-male variation and within-male variation that is often associated with single ejaculate assessments, and the examination of individual species, not only individual male variation, to increase the power of the assessment for species-specific semen characteristics.22
The data presented in this study demonstrate that there is an inherent factor related to ejaculate volume and sperm concentration in ejaculates from both G. domesticus and N. meleagris, possibly due to physiological species differences during spermatogenesis and sperm maturation.9,23 Similarly, the sperm morphometric phenotype can be attributed to species-specific differences by the use of the CASA-Morph system. Previous sperm studies in other species have shown morphometric dimensional and shape differences related to sperm membrane damage.17 Because both species here showed similar percentages of sperm membrane damage, the morphometric differences may be related to other biological factors, such as differences in the spermatogenic process in the species. Because of the sensitivity of CASA-Morph, this analysis system could be an alternative method for evaluating avian spermatozoa, by evaluating both sperm dimensions and shape. In this research, objective sperm analysis was more discriminatory than traditional subjective analyses, such as ejaculate volume, sperm concentration, sperm viability, sperm motility, and especially sperm morphology.
The ability to select males on the basis of objective semen characteristics that are consistent throughout the reproductive activity would be valuable to the avian industry.24 Traditional subjective sperm evaluation techniques used for the selection of avian males have been either not feasible or not predictive of fertility.25,26 However, the CASA-Morph system shows promise as a valuable tool for male selection and fertility determination owing to: (1) the possible correlation of avian sperm morphometry with fertility, (2) the detection of different morphometric phenotypes characterizing a given male or species, (3) the management of each sperm morphometric phenotype for male selection, (4) the detection of different sperm pathologies, and (5) the possibility of performing new studies for fertility determination, such as the objective analysis of sperm morphometric subpopulations. Therefore, avian males might be screened efficiently at the onset of semen production for certain sperm phenotypes and then be selected or culled depending on their reproductive potential.27 Although the filiform morphology of rooster and Guinea fowl sperm is similar, the results described in the present study suggest that rooster and male Guinea fowl appear to have species-specific differences in sperm morphometry and sperm subpopulation structure. These intraspecific differences may limit the extension of animal genetic resources to determine males with higher fertility characteristics.27,28 This fact is important because of the lack of published information on sperm morphometric features in these species, and the resultant absence of standardized systems for their morphological classification. Thus, the CASA-Morph system might help identify males within species with specific seminal characteristics more accurately than traditional light microscopy, where a technician's subjective criteria can bias results.28 That is why there is a strong evidence that the application of modern assessment methods improves reproductive management, and that the characterization of spermatozoa in avian species is important for understanding the biological basis of avian species fertility differences.6,7,24,29
In mammals, it has been suggested that inter- and intra-species differences in sperm morphology are linked to membrane lipid composition and its fluidity.30,31,32,33 Previous studies have demonstrated large differences in the membrane composition of avian spermatozoa, including cholesterol content and the membrane phospholipids.34 The cholesterol proportion of rooster sperm cells is lower than that in the Guinea fowl and could reflect the differences in sperm membrane fluidity.35 As found for mammals, this could influence sperm morphology in these avian species. Other studies have reported that differences in the quality of nuclear DNA and its compaction may influence the dimension and shape of the sperm head in the Guinea fowl.36 The current study showed higher A, P, L, and W values in rooster spermatozoa than those for Guinea fowl, which could be associated with the ultrastructural characteristics of the nucleus formed during spermiogenesis. However, macrocephalic spermatozoa are commonly found in Guinea fowl, which may explain the greater number of sperm subpopulations observed in this species.36
These experimental results of differences among avian species in sperm quality and sperm morphometry, as well as obvious differences in number and structure of sperm subpopulations in each species, have several implications. The morphometric characteristics observed of individual spermatozoa within ejaculates differ significantly and contribute to the phenotypes expressed. The rooster sperm subpopulation structure is more homogeneous and stable than that in the Guinea fowl, most likely due to genetic selection pressures to which roosters have been subjected over the last 50 years.37 This may be biologically significant because the sperm morphometric phenotype, as in mammals, may be predictive of fertility potential. Thus, the small sperm phenotype subpopulations, present in ejaculates from both roosters and Guinea fowl, differed not only in number but also in size, with a greater percentage of cells belonging to this phenotype in the Guinea fowl. In contrast, the large sperm phenotype percentages were higher in the rooster. Whereas spermatozoa contained in the small and large cell clusters of both species could be morphometrically suboptimal for fertilization if related to microcephalic, macrocephalic, and immature cells derived from a rapid, defective, or incomplete maturation, the average size spermatozoa in both species could represent the morphometrically optimal subpopulations containing standard fertilizing cells, if expressing optimal membrane structural and biochemical characteristics. In both species, the differences between small phenotype percentages were almost 3-fold higher in the Guinea fowl male than rooster, and the differences between big phenotype percentages were 2-fold higher in the rooster than the Guinea fowl. Thus, rooster spermatozoa tended to be larger than Guinea fowl spermatozoa, and such differences might themselves be the consequence of species-specific differences.14
In conclusion, the present study demonstrated that the sperm dimensional and shape characteristics were accurately assessed using a CASA-Morph system in G. domesticus and N. meleagris, and therefore, this analysis system can be recommended as a useful tool for sperm analysis in other avian species. Analyzing the sperm data matrix obtained using computerized sperm analysis methods reflects morphometric traits that differ markedly between the two species and that may be related to species-specific differences in galliformes. Moreover, this research provides conclusive evidence that sperm subpopulation distribution differs among species, and supports the idea that G. domesticus and N. meleagris can be used as galliform models to study phenotypic differences and other factors affecting sperm fertilizing ability. Further studies are needed in other avian species to explain how sperm morphometry is related to fertility, how the species-specific sperm subpopulation distribution could be a determinant to characterize other avian species, and how knowledge of these subpopulations could increase the efficacy and accuracy of male selection for AI programs.
AUTHOR CONTRIBUTIONS
MG-H carried out the experiments including the ejaculate collection, sperm sample preparation, analysis, and contributed to the concept and study design, experiments, data analysis, drafting, and critical revision of the manuscript.
COMPETING INTERESTS
The author of this paper has no financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
ACKNOWLEDGMENTS
The author would like to thank Agustín Sánchez-Domínguez for excellent technical assistance. Dr. García-Herreros was funded by Ecuadorian Government (Sponsor Grant: SENESCYT, Prometeo Project, Ecuador).
REFERENCES
- 1.Brillard JP. Sperm storage and transport following natural mating and artificial insemination. Poult Sci. 1993;72:923–8. doi: 10.3382/ps.0720923. [DOI] [PubMed] [Google Scholar]
- 2.Donoghuea AM, Wishart GJ. Storage of poultry semen. Anim Reprod Sci. 2000;62:213–32. doi: 10.1016/s0378-4320(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 3.Das SC, Nagasaka N, Yoshimura Y. Changes in the expression of estrogen receptor mRNA in the utero-vaginal junction containing sperm storage tubules in laying hens after repeated artificial insemination. Theriogenology. 2006;65:893–900. doi: 10.1016/j.theriogenology.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Hemmings N, Birkhead TR, Brillard JP, Froment P, Briere S. Timing associated with oviductal sperm storage and release after artificial insemination in domestic hens. Theriogenology. 2015;83:1174–8. doi: 10.1016/j.theriogenology.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Pierson EE, Krista LM, McDaniel GR. Effect of age and physiological status on sperm storage 24 hours after artificial insemination in broiler breeder hens. Br Poult Sci. 1988;29:193–7. doi: 10.1080/00071668808417043. [DOI] [PubMed] [Google Scholar]
- 6.McDaniel CD, Hannah JL, Parker HM, Smith TW, Schultz CD, et al. Use of a sperm analyzer for evaluating broiler breeder males. 1. Effects of altering sperm quality and quantity on the sperm motility index. Poult Sci. 1998;77:888–93. doi: 10.1093/ps/77.6.888. [DOI] [PubMed] [Google Scholar]
- 7.Parker HM, McDaniel CD. Semen dilution prior to analysis influences the ability of the sperm quality analyzer to predict fertility whether inseminating with a constant number of sperm or a constant volume of semen. Poult Sci. 2003;82:1808–15. doi: 10.1093/ps/82.11.1808. [DOI] [PubMed] [Google Scholar]
- 8.Holsberger DR, Donoghue AM, Froman DP, Ottinger MA. Assessment of ejaculate quality and sperm characteristics in turkeys: sperm mobility phenotype is independent of time. Poult Sci. 1998;77:1711–7. doi: 10.1093/ps/77.11.1711. [DOI] [PubMed] [Google Scholar]
- 9.Froman DP, Pizzari T, Feltmann AJ, Castillo-Juarez H, Birkhead TR. Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc Biol Sci. 2002;269:607–12. doi: 10.1098/rspb.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby JD, Froman DP, Engel HN, Jr, Bernier PE, Hess RA. Decreased spermatozoal survivability associated with aberrant morphology of the ductuli efferentes proximales of the chicken (Gallus domesticus) Biol Reprod. 1990;42:383–9. doi: 10.1095/biolreprod42.2.383. [DOI] [PubMed] [Google Scholar]
- 11.Lukaszewicz E, Jerysz A, Partyka A, Siudzińska A. Efficacy of evaluation of rooster sperm morphology using different staining methods. Res Vet Sci. 2008;85:583–8. doi: 10.1016/j.rvsc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RJ, Hess RA, Hughes BL, Froman DP. Ultrastructure of the Guinea fowl (Numida meleagris) spermatozoon. Poult Sci. 1982;61:1738–43. doi: 10.3382/ps.0611738. [DOI] [PubMed] [Google Scholar]
- 13.Hess RA, Hughes BL, Thurston RJ. Frequency and structure of macrophages and abnormal sperm cells in Guinea fowl semen. Reprod Nutr Dev. 1986;26:39–51. doi: 10.1051/rnd:19860104. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RJ, Hess RA. Ultrastructure of spermatozoa from domesticated birds: comparative study of turkey, chicken and Guinea fowl. Scanning Microsc. 1987;1:1829–38. [PubMed] [Google Scholar]
- 15.García-Herreros M, Barón FJ, Aparicio IM, Santos AJ, García-Marín LJ, et al. Morphometric changes in boar spermatozoa induced by cryopreservation. Int J Androl. 2008;31:490–8. doi: 10.1111/j.1365-2605.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Martí JI, Aparicio IM, García-Herreros M. Head morphometric changes in cryopreserved ram spermatozoa are related to sexual maturity. Theriogenology. 2011;75:473–81. doi: 10.1016/j.theriogenology.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 17.García-Herreros M, Leal CL. Sperm morphometry: a tool for detecting biophysical changes associated with viability in cryopreserved bovine spermatozoa. Andrologia. 2014;46:820–2. doi: 10.1111/and.12141. [DOI] [PubMed] [Google Scholar]
- 18.Valle RR, Nayudu PL, Leal CL, García-Herreros M. Sperm head morphometry in ejaculates of adult marmosets (Callithrix jacchus): a model for studying sperm subpopulations and among-donor variations. Theriogenology. 2012;78:1152–65. doi: 10.1016/j.theriogenology.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 19.van der Horst G, Maree L. SpermBlue: a new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech Histochem. 2009;84:299–308. doi: 10.3109/10520290902984274. [DOI] [PubMed] [Google Scholar]
- 20.Martí JI, Aparicio IM, García-Herreros M. Sperm morphometric subpopulations are differentially distributed in rams with different maturity age in cryopreserved ejaculates. Theriogenology. 2011;76:97–109. doi: 10.1016/j.theriogenology.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Martí JI, Aparicio IM, Leal CL, García-Herreros M. Seasonal dynamics of sperm morphometric subpopulations and its association with sperm quality parameters in ram ejaculates. Theriogenology. 2012;78:528–41. doi: 10.1016/j.theriogenology.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Barnes DA, Thurston RJ, Scott TR, Korn N. Effect of added spermiophages in pooled turkey semen on fertility, embryonic mortality, and hatchability. Poult Sci. 1996;75:943–8. doi: 10.3382/ps.0750943. [DOI] [PubMed] [Google Scholar]
- 23.Brillard JP, de Reviers M. Testis development and daily sperm output in Guinea-fowl raised under constant daily photoperiods. Reprod Nutr Dev. 1981;21:1105–12. doi: 10.1051/rnd:19810809. [DOI] [PubMed] [Google Scholar]
- 24.Parker HM, Yeatman JB, Schultz CD, Zumwalt CD, McDaniel CD. Use of a sperm analyzer for evaluating broiler breeder males. 2. Selection of young broiler breeder roosters for the sperm quality index increases fertile egg production. Poult Sci. 2000;79:771–7. doi: 10.1093/ps/79.5.771. [DOI] [PubMed] [Google Scholar]
- 25.Ansah GA, Segura JC, Buckland RB. Semen production, sperm quality, and their heritabilities as influenced by selection for fertility of frozen-thawed semen in the chicken. Poult Sci. 1985;64:1801–3. doi: 10.3382/ps.0641801. [DOI] [PubMed] [Google Scholar]
- 26.Nwakalor LN, Okeke GC, Njoku DC. Semen characteristics of the Guinea fowl Numida meleagris meleagris. Theriogenology. 1988;29:545–54. doi: 10.1016/s0093-691x(88)80003-7. [DOI] [PubMed] [Google Scholar]
- 27.Barbato GF. Genetic relationships between selection for growth and reproductive effectiveness. Poult Sci. 1999;78:444–52. doi: 10.1093/ps/78.3.444. [DOI] [PubMed] [Google Scholar]
- 28.Bilgili SF, Renden JA, Sexton KJ. The influence of staining techniques and examiners on evaluation of the morphology of fowl spermatozoa. Poult Sci. 1985;64:2358–61. doi: 10.3382/ps.0642358. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri D, Wishart GJ, Lake PE, Ravie O. Predicting the fertilising ability of avian semen: comparison of a simple colourimetric test with other methods for predicting the fertilising ability of fowl semen. Br Poult Sci. 1988;29:847–51. doi: 10.1080/00071668808417113. [DOI] [PubMed] [Google Scholar]
- 30.Bearer EL, Friend DS. Morphology of mammalian sperm membranes during differentiation, maturation, and capacitation. J Electron Microsc Tech. 1990;16:281–97. doi: 10.1002/jemt.1060160403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parks JE, Lynch DV. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology. 1992;29:255–66. doi: 10.1016/0011-2240(92)90024-v. [DOI] [PubMed] [Google Scholar]
- 32.Gadella BM, Flesch FM, van Golde LM, Colenbrander B. Dynamics in the membrane organization of the mammalian sperm cell and functionality in fertilization. Vet Q. 1999;21:142–6. doi: 10.1080/01652176.1999.9695009. [DOI] [PubMed] [Google Scholar]
- 33.Jones R, James PS, Howes L, Bruckbauer A, Klenerman D. Supramolecular organization of the sperm plasma membrane during maturation and capacitation. Asian J Androl. 2007;9:438–44. doi: 10.1111/j.1745-7262.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Douard V, Hermier D, Blesbois E. Changes in turkey semen lipids during liquid in vitro storage. Biol Reprod. 2000;63:1450–6. doi: 10.1095/biolreprod63.5.1450. [DOI] [PubMed] [Google Scholar]
- 35.Blesbois E, Grasseau I, Seigneurin F. Membrane fluidity and the ability of domestic bird spermatozoa to survive cryopreservation. Reproduction. 2005;129:371–8. doi: 10.1530/rep.1.00454. [DOI] [PubMed] [Google Scholar]
- 36.Barna J, Wishart GJ. Excess nuclear DNA in spermatozoa of Guinea fowl. Theriogenology. 2003;59:1685–91. doi: 10.1016/s0093-691x(02)01237-2. [DOI] [PubMed] [Google Scholar]
- 37.Froman DP. Sperm motility in birds: insights from fowl sperm. Soc Reprod Fertil Suppl. 2007;65:293–308. [PubMed] [Google Scholar]


