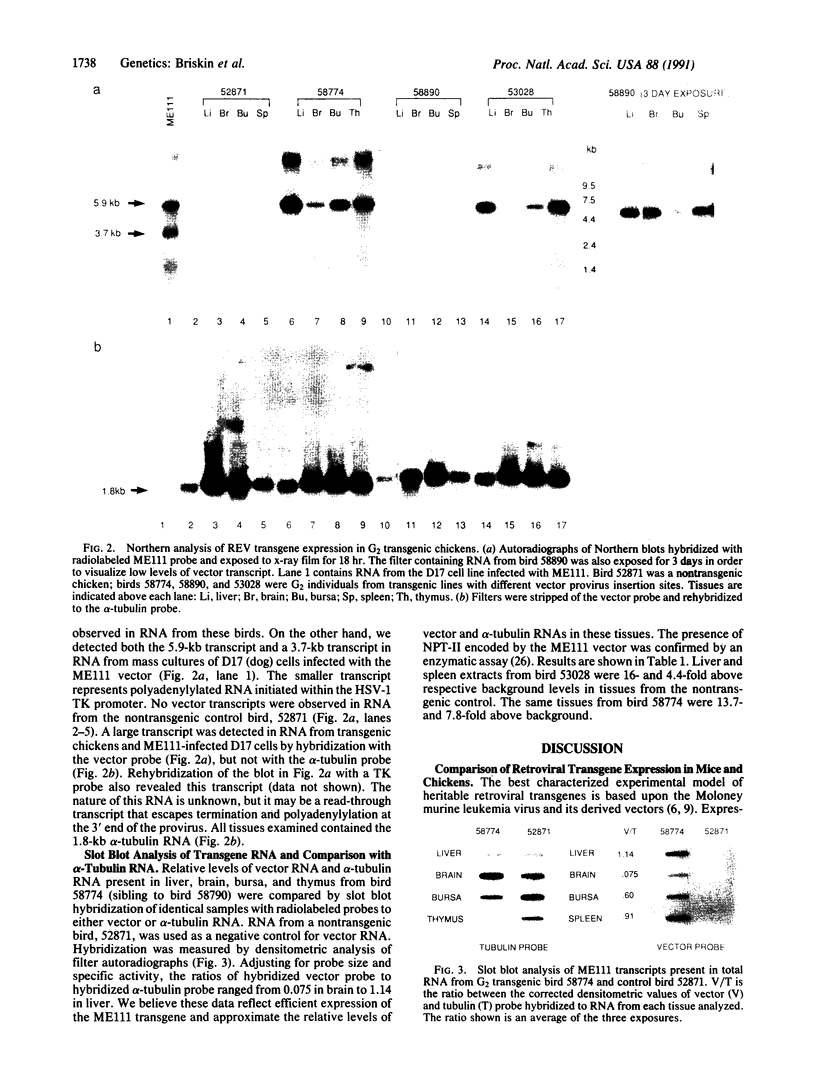
Abstract
This report describes expression of heritable reticuloendotheliosis virus (REV) vector ME111 in 20 independent lines of transgenic chickens. The results are strikingly different from studies of Moloney virus in transgenic mice, where restricted expression of inherited proviruses has led to their use primarily as insertional mutagens rather than general agents for gene transfer. In contrast, the REV ME111 provirus is actively transcribed in a variety of tissues from transgenic chickens, is expressed from transcriptional control elements present in the long terminal repeat of the provirus, and codes for active neomycin phosphotransferase II. The REV vector system as applied to the chicken represents a departure from the long-established paradigm of retroviral transgenes in mice and provides a new approach to the study of avian biology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosselman R. A., Hsu R. Y., Boggs T., Hu S., Bruszewski J., Ou S., Kozar L., Martin F., Green C., Jacobsen F. Germline transmission of exogenous genes in the chicken. Science. 1989 Jan 27;243(4890):533–535. doi: 10.1126/science.2536194. [DOI] [PubMed] [Google Scholar]
- Bosselman R. A., Hsu R. Y., Boggs T., Hu S., Bruszewski J., Ou S., Souza L., Kozar L., Martin F., Nicolson M. Replication-defective vectors of reticuloendotheliosis virus transduce exogenous genes into somatic stem cells of the unincubated chicken embryo. J Virol. 1989 Jun;63(6):2680–2689. doi: 10.1128/jvi.63.6.2680-2689.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 1976 Apr;49(2):321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Hruby D. E. Quantitative assay of recombinant vaccinia virus-encoded neomycin phosphotransferase in infected eukaryotic cell lysates. J Virol Methods. 1987 May;16(1-2):147–154. doi: 10.1016/0166-0934(87)90039-5. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985 Jun 13;315(6020):594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kochav S., Ginsburg M., Eyal-Giladi H. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. II. Microscopic anatomy and cell population dynamics. Dev Biol. 1980 Oct;79(2):296–308. doi: 10.1016/0012-1606(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Peckham I., Sobel S., Comer J., Jaenisch R., Barklis E. Retrovirus activation in embryonal carcinoma cells by cellular promoters. Genes Dev. 1989 Dec;3(12B):2062–2071. doi: 10.1101/gad.3.12b.2062. [DOI] [PubMed] [Google Scholar]
- Pratt L. F., Cleveland D. W. A survey of the alpha-tubulin gene family in chicken: unexpected sequence heterogeneity in the polypeptides encoded by five expressed genes. EMBO J. 1988 Apr;7(4):931–940. doi: 10.1002/j.1460-2075.1988.tb02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Crittenden L. B. Transgenic chickens: insertion of retroviral genes into the chicken germ line. Virology. 1987 Mar;157(1):236–240. doi: 10.1016/0042-6822(87)90334-5. [DOI] [PubMed] [Google Scholar]
- Seliger B., Kollek R., Stocking C., Franz T., Ostertag W. Viral transfer, transcription, and rescue of a selectable myeloproliferative sarcoma virus in embryonal cell lines: expression of the mos oncogene. Mol Cell Biol. 1986 Jan;6(1):286–293. doi: 10.1128/mcb.6.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Fadly A. M., Crittenden L. B. Interactions between endogenous virus loci ev6 and ev21. 1. Immune response to exogenous avian leukosis virus infection. Poult Sci. 1990 Aug;69(8):1244–1250. doi: 10.3382/ps.0691244. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Fadly A. M., Crittenden L. B. Interactions between endogenous virus loci ev6 and ev21. 2. Congenital transmission of EV21 viral product to female progency from slow-feathering dams. Poult Sci. 1990 Aug;69(8):1251–1256. doi: 10.3382/ps.0691251. [DOI] [PubMed] [Google Scholar]
- Soriano P., Cone R. D., Mulligan R. C., Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986 Dec 12;234(4782):1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Schuetze S., Vanek M., Wagner E. F. Expression of retroviral vectors in transgenic mice obtained by embryo infection. EMBO J. 1987 Feb;6(2):383–388. doi: 10.1002/j.1460-2075.1987.tb04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jaenisch R., Mulligan R. C. Transfer of a mutant dihydrofolate reductase gene into pre- and postimplantation mouse embryos by a replication-competent retrovirus vector. J Virol. 1989 Nov;63(11):4857–4865. doi: 10.1128/jvi.63.11.4857-4865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Weiher H., Barklis E., Ostertag W., Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987 Sep;61(9):2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., Noda T., Gray D. A., Sharpe A. H., Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990 Aug 10;62(3):425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]